Key Points
Plasminogen is glycated in diabetes, resulting in reduced plasmin generation and impaired protein activity.
Impaired plasminogen function and the consequent hypofibrinolysis in diabetes are reversible by modest improvement in glycemia.
Abstract
Diabetes is associated with hypofibrinolysis by mechanisms that are only partially understood. We investigated the effects of in vivo plasminogen glycation on fibrinolysis, plasmin generation, protein proteolytic activity, and plasminogen-fibrin interactions. Plasma was collected from healthy controls and individuals with type 1 diabetes before and after improving glycemia. Plasma-purified plasmin(ogen) functional activity was evaluated by chromogenic, turbidimetric, and plasmin conversion assays, with surface plasmon resonance employed for fibrin-plasminogen interactions. Plasminogen posttranslational modifications were quantified by mass spectrometry and glycation sites located by peptide mapping. Diabetes was associated with impaired plasma fibrin network lysis, which partly normalized upon improving glycaemia. Purified plasmin(ogen) from diabetic subjects had impaired fibrinolytic activity compared with controls (723 ± 16 and 317 ± 4 s, respectively; P < .01), mainly related to decreased fibrin-dependent plasmin generation and reduced protease activity (Kcat/KM 2.57 ± 1.02 × 10−3 and 5.67 ± 0.98 × 10−3 M−1s−1, respectively; P < .05). Nε-fructosyl-lysine residue on plasminogen was increased in diabetes compared with controls (6.26 ± 3.43 and 1.82 ± 0.95%mol, respectively; P < .01) with preferential glycation of lysines 107 and 557, sites involved in fibrin binding and plasmin(ogen) cleavage, respectively. Glycation of plasminogen in diabetes directly affects fibrinolysis by decreasing plasmin generation and reducing protein-specific activity, changes that are reversible with modest improvement in glycemic control.
Introduction
Patients with diabetes have an increased risk of atherothrombotic events1,2 with poor short- and long-term prognosis following myocardial infarction compared with the nondiabetic population.3-5 The formation of an obstructive platelet-rich fibrin clot in a coronary artery represents the final step in the atherothrombotic process, resulting in myocardial infarction.6 The structure of the fibrin network and resistance to fibrinolysis can modulate predisposition to vascular occlusive events.7-9
Diabetes is associated with a hypofibrinolytic state, representing one mechanism for increased cardiovascular risk in this population. A number of factors have been implicated in impaired fibrin clot lysis in diabetes, including altered structure of the fibrin network, increased incorporation of antifibrinolytic proteins, and inhibition of the fibrinolytic process. Quantitative and qualitative changes in fibrinogen, including glycation and oxidation, have key roles in altered clot structure in diabetes, resulting in the formation of compact clots with increased branching that are more difficult to lyse.10,11 Diabetes is also associated with increased incorporation of plasminogen inhibitor and complement C3 into the clot,12,13 with both proteins displaying antifibrinolytic activities. Moreover, plasma concentrations of plasminogen activator inhibitor-1 are elevated in diabetes and insulin-resistant states, which compromises the fibrinolytic process through limiting plasmin generation.14 All these changes promote inhibition of clot lysis and potentially contribute to the development and severity of ischemic cardiovascular disease in individuals with diabetes.14
Tissue plasminogen activator (tPA) mediates plasminogen conversion to plasmin, the protein responsible for fibrin clot degradation. Binding of tPA to fibrin increases the catalytic conversion of plasminogen to plasmin 1000-fold while concomitantly localizing plasmin generation to the site of thrombus formation, thus preventing systemic plasmin generation. Despite the hypofibrinolytic state in diabetes and the key role of plasminogen in fibrin clot lysis, the direct effects of glycaemia on plasminogen function have not been investigated. Posttranslational modification of plasminogen by in vitro nitration impairs plasmin activity and fibrinolysis,15 and in vivo studies report that elevated glucose levels result in increased plasminogen glycation, which affects protein clearance.16 These findings support the potential for posttranslational modifications of plasminogen in diabetes, which may affect protein activity, representing an additional mechanism for modulating cardiovascular risk in this population.
The aim of the current study was to investigate the effects of glycemic control and glycation on the fibrinolytic properties of plasminogen purified from the plasma of patients with type 1 diabetes. Our results reveal impaired plasminogen function and increased glycation in diabetes, mainly affecting lysine residues close to the plasmin(ogen) cleavage site. These changes are partially reversible by modest improvements in glycemic control, highlighting a previously unrecognized and modifiable mechanism for impaired fibrinolysis in hyperglycemia.
Materials and methods
Patient recruitment and blood sampling
Blood samples were collected from 20 subjects with poorly controlled type 1 diabetes mellitus (T1DM; HbA1c >8.5% or >69 mmol/mol) whose control was improved through regular contact with the attending physician and/or diabetes nurse specialist. The frequency of contact was tailored to the need of each individual and ranged from weekly to monthly through phone, e-mail, or clinic attendance. None had evidence of vascular complications and any patient on treatment other than insulin was excluded from the study. Subjects were age and sex matched to a healthy control group (n = 18). Blood samples were taken mid-morning after a light breakfast into citrated tubes, without the use of a tourniquet, at baseline and after improving glycemic control. Plasma was separated within 2 hours of collection and stored at −40°C. Informed consent was obtained from participants in accordance with the Declaration of Helsinki, and ethical approval for the study was provided by the local ethics committee.
Plasminogen and other materials
Human plasminogen, fibrinogen, tPA, urokinase plasminogen activator (uPA), thrombin, and plasminogen-depleted plasma were purchased from Enzyme Research Laboratories (ERL; Swansea, United Kingdom). Protease inhibitor cocktail (4-(2-aminoethyl) benzenesulfonyl fluoride, 104 mM; aprotinin, 80 μM; bestatin, 4 mM; E-64, 1.4 mM; leupeptin, 2 mM, and pepstatin A, 1.5 mM) and tosyl phenylalanyl chloromethyl ketone-treated trypsin (bovine pancreas) were from Sigma-Aldrich (Poole, United Kingdom).
Plasminogen purification
Plasminogen was purified by lysine-sepharose affinity chromatography17 from: 1) 10 random samples of the diabetes group, with mean pre- and postintervention HbA1c of 10.7% (93 mmol/mol) and 8.2% (66 mmol/mol), respectively; P < .01; 2) 12 random healthy control samples; and 3) control plasma pooled from 20 individuals. The integrity of purified plasminogen was assessed by polyacrylamide gel electrophoresis on 4% to 12% bis-(2-hydroxy-ethyl)-amino-tris(hydroxymethyl)-methane gels. Plasminogen concentration was assessed by enzyme-linked immunosorbent assay using 10 µg/mL of polyclonal sheep anti-plasminogen antibody for capture and horseradish peroxidase-labeled goat anti-plasminogen (ERL) (1:10 000 dilution) for detection. Commercially available plasminogen (ERL) was used to establish a standard curve.
Measurement of fibrinolysis in plasma samples
Previous work has shown that fibrinolytic efficiency is compromised in diabetes and is affected by glycemic control. We used the validated turbidimetric assay to investigate lysis in a diabetic plasma sample, before and after improving glycemic control, as well as in a healthy control group using the conditions previously described.13 Briefly, 25-µL plasma samples were mixed with 75 µL lysis mix (100 mM NaCl, 50 mM Tris, 83 ng/mL tPA, pH 7.4) at ambient temperature in a 96-well plate. Fibrin polymerization was initiated by addition of 50 µL of activation mix (50 mM Tris, 100 mM NaCl, 0.09 U/mL thrombin, 22.5 mM CaCl2, pH 7.4) and increase in turbidity at 340 nm continuously monitored every 12 s on a ELx-808 IU ultramicroplate reader (BIO-TEK Instruments Inc) over a period of 60 minutes, then every 2 minutes for 9 hours. Each experiment was performed in duplicate and repeated at least twice. Lysis time was measured as time from full clot formation to 50% lysis.
Assessment of purified plasminogen fibrinolytic activity
Studies using plasma-purified plasminogen were carried out as previously described,12 employing 2.94 µmol/L commercially available fibrinogen, 100 nmol/L plasma-purified plasminogen, and 15 nmol/L tPA.
Assessment of plasmin generation
These were investigated using chromogenic and western-blot analyses.
Chromogenic analyses.
The rate of plasmin generation by tPA and uPA at the fibrin surface and/or plasmin proteolytic activity was measured using a modification of the method by Bobbink.18 Microtiter plates were coated with 235 nmol/L fibrinogen (ERL) in tris(hydroxymethyl)aminomethane (Tris)-buffered saline (TBS; 50 mmol/L NaCl, 100 mmol/L Tris-HCl, pH 7.4) for 40 minutes at room temperature prior to being blocked with 250 µL TBS containing 3% bovine serum albumin and 0.01% (vol/vol [v/v]) Tween-20 for 90 minutes at 37°C. Plates were washed with 250 µL 50 mmol/L Tris-HCl, 110 mmol/L NaCl, 0.01% (v/v) Tween-20, pH 7.4 prior to incubation with 5 U/mL thrombin and 5 mmol/L CaCl2 in 50 mmol/L Tris-HCl, 110 mmol/L NaCl, pH 7.4 for 45 minutes at room temperature to convert fibrinogen to fibrin-bound thrombin, and fibrinopeptide (Fp) A/FpB fibrinogen fragments were removed by a 250-µL high-salt wash (1 mol/L NaCl, 50 mmol/L Tris-HCl, pH 7.4) followed by a 250-µL TBS-Tween wash (50 mmol/L Tris, 100 mmol/L NaCl, 0.01% [v/v] Tween-20, pH 7.4). Finally, 75 nmol/L purified plasminogen, 0.8 mmol/L plasmin-specific chromogenic substrate S2251 (Chromogenix, Lexington, MA) and either 12 nmol/L tPA or 50 nmol/L uPA were added and the rate of S2251 hydrolysis monitored via kinetic absorbance readings at 405 nM.
Western blot.
Conversion of plasminogen to plasmin by tPA cleavage was also assessed by incubating 75 nmol/L plasma-purified plasminogen with 200 nmol/L tPA in TBS in the presence of 235 nmol/L fibrinogen, 2.5 mM CaCl2, and 2 U/mL thrombin at 37°C. Aliquots were removed at 50-s time intervals and frozen in dry ice before sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE), western blot, and densitometry analysis using the Kodak ImageStation 440 and 1D Image Analysis Software, Kodak, version 3.5.5.
Assessment of plasmin proteolytic activity
To assess the catalytic efficiency of plasmin, plasma-purified plasminogen was incubated with tPA to ensure complete conversion to plasmin had taken place prior to experiments. This was carried out by incubating 75 nmol/L plasma-purified plasminogen with 200 nmol/l tPA in TBS at 37°C. Aliquots were removed at 1-h intervals to identify the point at which complete conversion to plasmin had occurred in the absence of fibrin. The samples were snap-frozen in dry ice, followed by SDS-PAGE, western blot, and densitometry as described above. To determine if plasminogen degradation occurred during the experimental period, plasminogen was incubated in TBS in the absence of tPA. A time course showed that 4-hour incubation of plasma-purified plasminogen (from patients or controls) with tPA ensured complete conversion to plasmin, and this incubation period was used throughout. Plasmin proteolytic activity was then measured using the S2251 chromogenic assay as detailed above. Rates of chromogenic substrate S2251 conversion were determined for 6 concentrations of plasminogen per patient (doubling dilution from 50 to 1600 μmol/L) followed by calculating Kcat/KM.
Binding of plasminogen to fibrinogen
The binding affinity of plasminogen purified from patients and controls to fibrin was analyzed by surface plasmon resonance using a Biacore 3000 system (GE Healthcare, United Kingdom). Amine coupling was employed to immobilize fibrinogen to carboxymethyl dextran-coated biosensor chips (CM5), yielding ∼1000 response units. Thrombin (5 U/mL) in 140 mmol/L NaCl, 10 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4 was passed over the sensor chip at 2 µL/min for 20 minutes to convert fibrinogen to fibrin. This was followed by regeneration with 1 mol/L NaCl, 10 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5 mmol/L CaCl2, 0.05% (v/v) P20, pH 7.4 (2 injections of 90 µL, flow rate 30 µL/min) to remove thrombin, and FpA/FpB fibrinogen fragments. Plasminogen binding experiments were performed at 25°C for a defined concentration range (6.25-400 nmol/L). Plasma-purified plasminogen from either control samples or pre-/postintervention diabetic samples (n = 9) was passed over the sensor chip surface at 30 µL/min (contact time 3 minutes) to analyze protein association, followed by a 3-minute dissociation phase. Sensor surfaces were regenerated as above, after which reequilibration with running buffer was performed for 15 minutes. Kinetic binding analysis, using a 1:1 binding model and fitting kd and ka separately, was performed using double subtraction of the ligand-free reference cell signal and a zero plasminogen concentration (BIAevaluation software, version 4.1 Biacore; GE Healthcare).
Functional studies described above were repeated on at least 4 occasions with mean values presented.
Assessment of plasminogen posttranslational modifications
Estimation of vivo plasminogen glycation.
The Nε-Fructosyl-lysine (FL) residue content of plasminogen isolated from plasma samples was determined by exhaustive enzymatic hydrolysis followed by stable isotopic dilution analysis liquid chromatography-tandem mass spectrometry, as previously described,19 with minor modifications. Plasminogen (40–100 µg) was suspended in 20 mM HCl (50 μL) followed by the addition of pepsin (2 mg/mL in 20 mM HCl; 5 µL) and thymol solution (2 mg/mL in 20 mM HCl; 5 µL). The sample was incubated at 37°C for 24 hours, then neutralized and buffered at pH 7.4 by addition of 25 µL 0.5 M potassium phosphate, pH 7.4, and 5 µL 260 mM KOH. Pronase E (20 μL, 2 mg/mL in 10 mM KH2PO4 buffer, pH 7.4), 5 μL penicillin (1000 U/mL), and streptomycin (1 mg/mL) were added and samples incubated at 37°C for a further 24 hours. Thereafter, 10 μL each of prolidase and aminopeptidase solutions (2 mg/mL in 10 mM KH2PO4, pH 7.4) was added and samples incubated for 48 hours. All the above steps were performed under argon. FL, the major advanced glycation end-product methylglyoxal-derived hydroimidazolone MG-H1 and major oxidative marker methionine sulfoxide, and amino acids lysine, arginine, and methionine were determined in enzymatic hydrolysates by stable isotopic dilution analysis liquid chromatography tandem mass spectrometry.20 Autohydrolysis of hydrolytic enzymes was corrected by concurrent digestion of 40 to 100 µg poly-l-threonine (a polypeptide devoid of glycation adducts).
Identification of glycation sites on plasminogen.
For tentative assignment of the location of the FL residues in plasminogen, we glycated commercially obtained human plasminogen by incubation in phosphate-buffered saline and 1% (v/v) protease inhibitor cocktail with and without 50 mM β-d-glucose at 37°C for 5 days under aseptic conditions. The pH was adjusted to 3.0 to stabilize plasminogen21 and washed over a microspin ultrafilter (3-kDa cutoff, 4°C, 10 000 g) by 4 cycles of concentration and dilution in 10 mM formic acid. Aliquots of 20 μg plasminogen were lyophilized to dryness. For limited proteolysis, plasminogen was reconstituted in water (10 µL), 5 mM dithiothreitol added (1.0 µL), and incubated in the dark at 37°C for 30 minutes, followed by the addition of 10 mM iodoacetamide (1.0 µL) and incubation for a further 30 minutes. Subsequently, trypsin (200 µg/mL in 100 mM ammonium bicarbonate with 1 mM calcium chloride, pH 8.5; 5 µL) was added and incubated in the dark at 37°C for 10 hours. Samples were lyophilized to dryness, reconstituted in 0.1% trifluoroacetic acid (10 µL), and de-salted by the octadecylsilica ZipTip method according to the manufacturer’s instructions (Millipore, Watford, United Kingdom). Finally, the tryptic digest was reconstituted in 0.1% formic acid and analyzed by liquid chromatography-mass spectrometry using an Acquity UPLC-Quattro Premier XE system in single mass analyzer mode (Waters, Watford, United Kingdom). Cumulative mass spectra were analyzed for tryptic peptide parent ions against a theoretical peptide map of human plasminogen tryptic digest using the Biolynx software and single ion response chromatograms generated from accumulated data. Peptide ion responses were normalized to long-retained, aromatic, and lysine-free peptide T83 (selected ion response and absorbance at 280 nm gave similar outcomes); coefficients of variance for normalized peptide responses were 8% to 13% (n = 3). Glycation sites were identified by decreased peptide responses in glycated plasminogen, determined by comparing the mean of 3 normalized peptide responses in peptide digests modified and unmodified, and detection of related glycated, uncleaved dipeptide, as previously described.22
Results
Subject demographics
Twenty T1DM subjects with poor glycemic control were selected on the basis of improved glucose levels upon treatment. Mean age was 22.2 ± 1.1 years and 10 were males. Time since diagnosis was 11.2 ± 1.2 years and 4 had background retinopathy with no other micro- or macrovascular complications. A total of 18 individuals with T1DM were treated with basal bolus insulin and 2 were on an insulin pump. The overall change in HbA1c in the whole group was significant, dropping from 10.1 ± 0.3% (87 mmol/mol) at baseline to 8.8 ± 0.3% (73 mmol/mol) postintervention (P < .01) after a mean period of 18.7 ± 2.2 weeks. Intervention consisted of intensification of insulin therapy by altering the basal bolus insulin doses or pump basal rate and/or bolus insulin. Healthy controls (n = 18) with no previous medical history and not taking any medication were matched for age and sex.
Fibrinolysis of plasma samples
In agreement with previous work, the time to 50% fibrin clot lysis (mean ± SEM) was prolonged in individuals with diabetes compared with controls (516 ± 16 and 334 ± 7 seconds, respectively; P < .01). A drop of HbA1c by 1.3% (14 mmol/mol) was associated with a significant reduction in plasma clot lysis in the diabetes group to 400 ± 16 seconds (P < .01 compared with baseline), although this remained longer than the healthy control group (334 ± 7 seconds; P < .01).
Functional studies of plasminogen
Plasmin(ogen) purified from diabetes patients displays impaired fibrinolysis, which is modulated by improving glycemic control.
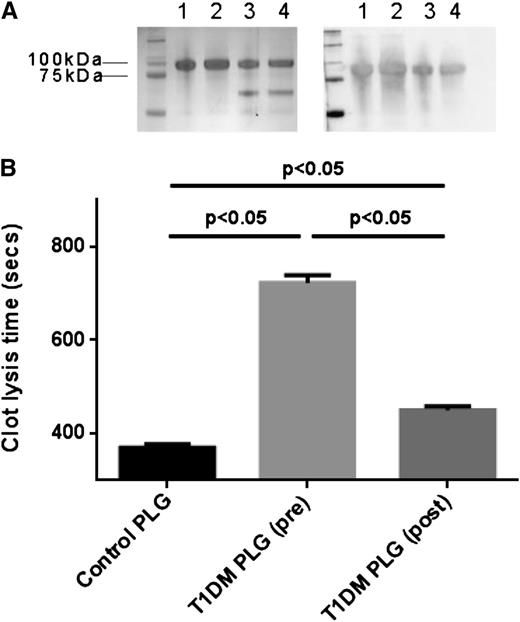
Integrity and confirmation of identity of purified plasminogen were assessed by SDS-PAGE and western-blot analysis as shown in Figure 1A. Lysis times of fibrin clots using 10 random plasminogen purified from subjects with diabetes at baseline was significantly longer compared with pooled (n = 20) healthy controls (723 ± 16 and 371 ± 4 seconds, respectively; P < .01). Paired samples of plasminogen purified before and after improving glycemic control demonstrated partial recovery of fibrinolytic activity (450 ± 8 seconds, P < .05 compared with baseline; Figure 1B).
Plasma-purified plasminogen integrity and analysis of fibrinolytic activity. (A) SDS-PAGE (left) and western blot using anti-plasminogen antibody (right). Lanes 1 and 2: commercial plasminogen. Lane 3: plasma-purified plasminogen from a healthy control. Lane 4: plasma-purified plasminogen from a subject with T1DM. (B) Turbidimetric analysis of clot lysis using commercial fibrinogen and plasminogen purified from 10 T1DM samples pre- and postimprovement of glycemia (HbA1c 10.7 and 8.2%, respectively). Plasmin(ogen) fibrinolytic activity from pooled healthy control plasma (n = 20) is also shown. PLG, plasminogen.
Plasma-purified plasminogen integrity and analysis of fibrinolytic activity. (A) SDS-PAGE (left) and western blot using anti-plasminogen antibody (right). Lanes 1 and 2: commercial plasminogen. Lane 3: plasma-purified plasminogen from a healthy control. Lane 4: plasma-purified plasminogen from a subject with T1DM. (B) Turbidimetric analysis of clot lysis using commercial fibrinogen and plasminogen purified from 10 T1DM samples pre- and postimprovement of glycemia (HbA1c 10.7 and 8.2%, respectively). Plasmin(ogen) fibrinolytic activity from pooled healthy control plasma (n = 20) is also shown. PLG, plasminogen.
Diabetes is associated with decreased plasminogen cleavage to plasmin.
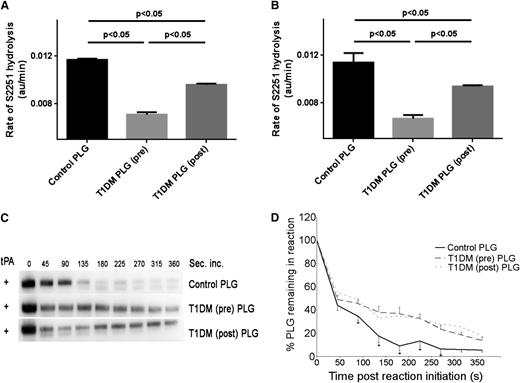
As plasminogen purified from diabetic subjects showed impaired fibrinolytic activity, we investigated whether plasminogen conversion to plasmin represented one mechanism for our findings. Plasminogen purified from preintervention diabetic samples when incubated with tPA in the presence of fibrin demonstrated impaired rates of S2251 substrate hydrolysis (n = 10, 0.0071 ± 0.00015 au/min) compared with pooled control plasma (0.0117 ± 0.00014 au/min, P < .05). Postintervention, the rate of S2251 substrate hydrolysis increased (n = 10, 0.0096 ± 0.000117 au/min, P < .05 compared with baseline; Figure 2A). Fibrin-independent uPA conversion of plasma-purified plasminogen to plasmin followed a similar pattern, with pre- and postintervention samples displaying 0.0067 ± 0.000334 au/min and 0.0094 ± 0.00015 au/min, respectively (P < .05), and both were significantly longer compared with healthy control protein (0.0114 ± 0.000833 au/min, P < .05; Figure 2B).
Effects of glycemia on plasminogen conversion to plasmin using chromogenic substrate assay. Plasminogen conversion to plasmin initiated by tPA (A) or uPA (B) by monitoring plasmin-induced cleavage of chromogenic substrate S2251. Plasma-purified plasminogen was used from a healthy control pool (n = 20) or individual subjects with T1DM pre- and postintervention (n = 10 each). Results are presented as rate of S2251 hydrolysis (au/min). (C) Time course of conversion of plasminogen to plasmin in the presence of fibrin detected by SDS-PAGE and immunoblotting using anti-plasminogen antibody. (D) Densitometry analysis of bands in (C) representing remaining percentage zymogen plasminogen in sample (n = 10). *P < .05. PLG, plasminogen.
Effects of glycemia on plasminogen conversion to plasmin using chromogenic substrate assay. Plasminogen conversion to plasmin initiated by tPA (A) or uPA (B) by monitoring plasmin-induced cleavage of chromogenic substrate S2251. Plasma-purified plasminogen was used from a healthy control pool (n = 20) or individual subjects with T1DM pre- and postintervention (n = 10 each). Results are presented as rate of S2251 hydrolysis (au/min). (C) Time course of conversion of plasminogen to plasmin in the presence of fibrin detected by SDS-PAGE and immunoblotting using anti-plasminogen antibody. (D) Densitometry analysis of bands in (C) representing remaining percentage zymogen plasminogen in sample (n = 10). *P < .05. PLG, plasminogen.
We also performed an experiment of tPA-mediated cleavage of plasminogen in the presence of fibrin and used western-blot analyses with densitometry measurements to determine the rate of plasminogen conversion. At 180 seconds, 90 ± 5% of total plasminogen purified from controls was converted to plasmin compared with 61 ± 5% and 65 ± 4% of plasminogen purified from patients with poor or improved glycemic control, respectively (Figure 2C-D). These data indicate that rates of both uPA- and fibrin-enhanced tPA-mediated cleavage of plasminogen to plasmin are less efficient in diabetes.
Diabetes reduces the specific activity of plasmin.
To differentiate the effect of glycemia on plasminogen conversion to plasmin and the specific activity of plasmin, plasminogen purified from individual diabetic subjects (n = 9) and control plasma (n = 9) was incubated with tPA alone for 4 hours to ensure complete conversion of plasminogen to plasmin (Figure 3A). The proteolytic activity of plasmin, measured as rate of plasmin-specific hydrolysis of S2251, was significantly reduced in diabetic plasmin at baseline compared with plasmin from healthy controls (0.0167 ± 0.0004 and 0.0068 ± 0.0007 min, respectively; P < .05). Improving glycemic control caused a significant increase in plasmin proteolytic activity to 0.0124 ± 0.0006 min (P < .05), which was still reduced compared with healthy controls (P < .05; Figure 3B). An analysis of plasmin protease catalytic efficiency (Kcat/KM, M−1s−1) indicated that plasmin isolated from preintervention samples demonstrated significantly impaired protease efficiency compared with normal controls (2.57 ± 1.02 × 10−3 and 5.67 ± 0.98 × 10−3 M−1s−1, respectively; P < .05). Postintervention, the plasmin enzymatic activity was 4.43 ± 1.34 × 10−3 M−1s−1, which failed to show a statistical difference compared with baseline (P > .1; Figure 3C).
Effects of glycemia on plasmin activity. (A) Time course of incubation of plasma-purified plasminogen with tPA in the absence of fibrin was performed to ensure complete conversion to plasmin. Samples were analyzed by western blot using an anti-plasminogen antibody for detection. (B) S2251 chromogenic substrate (800 μm/L) analysis of plasmin protease activity using plasmin generated by preincubation with tPA of control plasminogen (n = 9), protein purified from T1DM plasma at baseline (pre, n = 9), or after improving glycemic control (post, n = 9). (C) Analysis of plasmin protease kinetics with Kcat/Km values using substrate concentrations between 50 and 1600 μmol/L. PLM, plasmin; PLG, plasminogen.
Effects of glycemia on plasmin activity. (A) Time course of incubation of plasma-purified plasminogen with tPA in the absence of fibrin was performed to ensure complete conversion to plasmin. Samples were analyzed by western blot using an anti-plasminogen antibody for detection. (B) S2251 chromogenic substrate (800 μm/L) analysis of plasmin protease activity using plasmin generated by preincubation with tPA of control plasminogen (n = 9), protein purified from T1DM plasma at baseline (pre, n = 9), or after improving glycemic control (post, n = 9). (C) Analysis of plasmin protease kinetics with Kcat/Km values using substrate concentrations between 50 and 1600 μmol/L. PLM, plasmin; PLG, plasminogen.
Binding of plasminogen to fibrin
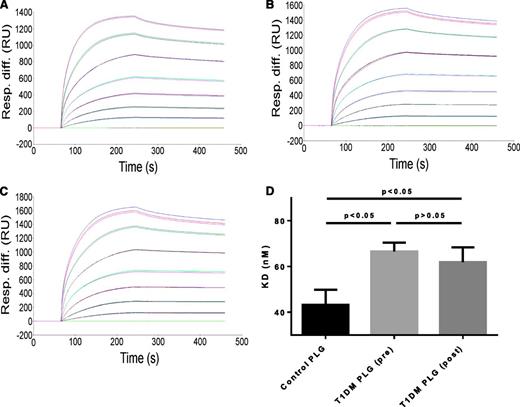
Plasminogen binding to immobilized fibrin occurred in a dose-dependent manner (Figure 4A-C). Plasminogen purified from controls (n = 9) had a slightly higher affinity for fibrin than plasminogen purified from diabetic samples (n = 9) (KD of 43.4 ± 6.6 nM and KD 66.5 ± 3.9 nM, respectively; P < .05). The binding affinity for plasminogen purified after improving glycemia was not significantly different from preglycemic control (KD of 61.9 ± 6.5 nM; P > .1).
Effects of diabetes on binding interactions between fibrin and plasminogen purified from healthy controls or subjects with T1DM. A total of 9 controls and 9 T1DM samples, before and after improving glycemia, were analyzed. Representative data analysis plot of surface plasmon resonance binding studies using plasminogen purified from controls (A) and from diabetic samples before (B) or after (C) improving glycemic control. (D) Summary of surface plasmon resonance study data. PLG, plasminogen.
Effects of diabetes on binding interactions between fibrin and plasminogen purified from healthy controls or subjects with T1DM. A total of 9 controls and 9 T1DM samples, before and after improving glycemia, were analyzed. Representative data analysis plot of surface plasmon resonance binding studies using plasminogen purified from controls (A) and from diabetic samples before (B) or after (C) improving glycemic control. (D) Summary of surface plasmon resonance study data. PLG, plasminogen.
Posttranslational modifications of plasminogen
Diabetes is associated with increased plasminogen FL adducts.
In patients with T1DM (n = 10), the percentage of FL plasminogen increased to 6.26 mol% compared with 1.82 mol% in controls (n = 12) without a significant increase in the extent of modification by advanced glycation endproducts or methionine sulfoxide. Results are summarized in Table 1.
Glycation of plasminogen occurs at lysine residues 107 and 557.
Glycated plasminogen was subjected to limited proteolysis with trypsin followed by peptide mapping studies. Liquid chromatography-mass spectrometry of tryptic digests of glycated and control plasminogen preparations gave very similar total ion response chromatograms (Figure 5A-B). Global inspection of all individual ion response chromatograms (Figure 5C-E), normalized to a non-lysine–containing peptide internal standard, gave evidence of a significant decrease of response in glycated plasminogen, with respect to control, of 2 peptides: T17, detected as the doubly charged ion, mass to charge ratio (m/z) = 410.7, and T64, detected as singly charged ion, m/z = 489.2. An analysis of 3 independent glycated and control preparations suggested peptide T17 was decreased by 40% (P < .01) and T64 was decreased by 30% (P < .05) with a total FL residue content of 70 mol%. Glycation of lysine residues by glucose blocks trypsin cleavage and hence uncleaved glycated dipeptide is expected. Glycated tryptic dipeptide T16-(FL)T17 was detected as both doubly and triply charged ions (m/z = 1087.5 and 725.3, respectively), accounting for one missed arginine residue cleavage next to a proline residue. Glycated dipeptide T63-T64 was not detected. This suggests that sites of glucose glycation in plasminogen are lys-107 of T17 and lys-557 of T63. There was no evidence of glycation at other sites (Figure 5; Table 2).
Mass spectrometry peptide mapping of in vitro glycated human plasminogen. Data are presented as total ion response chromatograms (m/z 100-2000) from 2 to 90 min. (A-B) Plasminogen before and after glycation by glucose, respectively. (C-E) Selected ion response chromatograms: C, m/z = 410.7 [T17, (M+2)/2] of control plasminogen; D, peptide m/z = 1087.5 [T16-FL-T17, (M+2)/2] of glycated plasminogen; and E, m/z = 489.2 [T64, (M+1)] of control plasminogen. Chromatograms of 3 typical independent replicates.
Mass spectrometry peptide mapping of in vitro glycated human plasminogen. Data are presented as total ion response chromatograms (m/z 100-2000) from 2 to 90 min. (A-B) Plasminogen before and after glycation by glucose, respectively. (C-E) Selected ion response chromatograms: C, m/z = 410.7 [T17, (M+2)/2] of control plasminogen; D, peptide m/z = 1087.5 [T16-FL-T17, (M+2)/2] of glycated plasminogen; and E, m/z = 489.2 [T64, (M+1)] of control plasminogen. Chromatograms of 3 typical independent replicates.
Discussion
The fibrinolytic system has a key role in determining thrombosis potential and predisposition to cardiovascular events.23 Our findings indicate that glycation of plasminogen leads to decreased plasmin generation and impaired functional protein activity and, as a consequence, impaired fibrinolysis. This occurs through 2 main mechanisms: decreased conversion of plasminogen to plasmin and reduced catalytic efficiency of generated plasmin. These findings appear to be related to the preferential glycation sites on plasminogen identified in this study and can be modulated by in vivo improvements of glycemia. The results highlight an important, novel mechanism for impairment of fibrinolysis in subjects with diabetes and provide evidence suggesting that relatively modest tightening of glycemic control ameliorates cardiovascular risk by improving efficiency of the fibrinolytic system.
Type 2 diabetes is characterized by a range of metabolic abnormalities associated with underlying insulin resistance, including changes in lipid levels, oxidative stress, and glycation with inhibition of fibrinolysis.14,24,25 To minimize the confounding effects of these complex metabolic changes, we studied purified plasminogen from young uncomplicated type 1 patients. Our data demonstrate that specific interactions between glycemia/glycation and the fibrinolytic system further contribute to inhibition of fibrinolysis. We show that plasmin generation by uPA (fibrin independent) and fibrin-enhanced tPA cleavage of plasminogen are impaired in individuals with diabetes. In addition to impaired plasmin generation in diabetes, we demonstrate that the catalytic efficiency of the generated plasmin is decreased almost 2-fold compared with protein purified from individuals with normal glucose metabolism. We demonstrate 2 sites of fructosamine modification on plasminogen at positions lys-107 and lys-557. Lys-107 is located in kringle 1 of plasminogen, which represents a specific binding site for fibrin.26 We show small differences in glycated plasminogen binding to fibrin(ogen), which may have a minor role in the observed reduced conversion to plasmin by tPA in the presence of fibrin. More importantly, however, is the second glycation site at lys-557, which lies 4 amino acids upstream from the Arg561-Val562 cleavage site that converts plasminogen to plasmin.27 This glycation site seems to interfere with hydrolysis of the Arg561-Val562 bond by uPA and possibly tPA. Moreover, it may alter the overall folding of the active site of plasmin, thereby altering protein activity. This would be consistent with our finding that diabetic plasminogen preconverted to plasmin is associated with a lower specific activity compared with control.
Our findings have important implications for the role of glycemia in the modulation of atherothrombotic risk. The observation that posttranslational modifications of plasmin(ogen) alter both the generation of plasmin and its specific activity provides information that might help to explain some of what is known in this field. First, in some patients, it is difficult to establish immediately following a vascular ischemic event whether hyperglycemia is the result of long-standing insulin resistance or of a short-term acute stress response. In the latter case, exposure to hyperglycemia may not be of sufficient duration to impair plasmin(ogen) function, and short-term improvements in glycemia may not be associated with changes in fibrinolysis. Second, it is notable that marked improvements in plasmin generation and clot lysis times were seen ex vivo following relatively modest in vivo reduction in HbA1c and well before reaching the recommended clinical therapeutic targets. Placed in the context of current concerns regarding the potentially deleterious effects of overzealous glycemic control on cardiovascular risk,28 our findings indicate that the fibrinolytic aspects of glycemia-related atherothrombotic risk can be ameliorated to a significant extent without risk of hypoglycemia. This is particularly important, as hypoglycemia may itself be prothrombotic.29,30
The current study has revealed novel mechanisms of hypofibrinolysis in diabetes by which glycation of plasminogen leads to both decreased plasmin generation and lower catalytic efficiency of plasmin activity. Analysis of plasminogen glycation sites indicates 2 fructosamine-modified lysine residues that could be responsible for these findings. Future work is warranted to investigate this aspect of the work, perhaps involving site-directed mutagenesis of the appropriate residues to elucidate the effects on fibrin binding and plasmin activity. Our results indicate that modest, medium-term improvements in glycemic control ameliorate the hypo-fibrinolytic state in diabetes related to reversal of the detrimental effects of plasminogen glycation. The importance of this mechanism in clinical settings requires further studies, investigating interactions between improved glycemic control, plasmin activity, and cardiovascular outcome in subjects with diabetes.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was primarily supported by project grant BDA:RD06/0003226 from Diabetes UK. The authors recognize and thank the National Institute for Health Research UK for the generous support.
Authorship
Contribution: R.A.A. and T.G. researched the data, wrote the manuscript, and made equal contributions to the study; K.F.S. researched the data and edited the manuscript; S.M., K.H., K.A.S., E.J.D., and M.M.A. researched the data; N.R. and P.J.T. refined the study, researched the data, and edited the manuscript; H.P. and P.J.G. designed the study, wrote the manuscript, and made equal contributions to the study; and R.A., H.P., and P.J.G. are the guarantors and take responsibility for the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter J. Grant and Helen Philippou, Department of Cardiovascular and Diabetes Research, The LIGHT Laboratories, University of Leeds, Leeds, LS2 9JT, United Kingdom; e-mail: p.j.grant@leeds.ac.uk and h.philippou@leeds.ac.uk.
References
Author notes
R.A.A. and T.G. contributed equally to this study.
H.P. and P.J.G. contributed equally to this study.


![Figure 5. Mass spectrometry peptide mapping of in vitro glycated human plasminogen. Data are presented as total ion response chromatograms (m/z 100-2000) from 2 to 90 min. (A-B) Plasminogen before and after glycation by glucose, respectively. (C-E) Selected ion response chromatograms: C, m/z = 410.7 [T17, (M+2)/2] of control plasminogen; D, peptide m/z = 1087.5 [T16-FL-T17, (M+2)/2] of glycated plasminogen; and E, m/z = 489.2 [T64, (M+1)] of control plasminogen. Chromatograms of 3 typical independent replicates.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/1/10.1182_blood-2013-04-494641/4/m_134f5.jpeg?Expires=1769680173&Signature=Ke6VJde5mnFyst5PU2po9UWcG2-X4HknP8PSJEXldhHPfPWbfgfDlp-s7r~xAmM7uQyU2sBaf9v708lYWBtIr2QEmCSdCRy8C9q5YDVmHfkvL-4gcpOIGAadCacPZVorr8NRhDF3cVDbH0QJcJ7fdKA1ddjL8iyrQxqjQUSClGOLG7RFFucw6J4ugPv4lddAv5SNbxLEuUeE-CLoCt6rk1J7FCDUkgvywXcFr6mhAcTWyVaiMQyqfNwZlBsUpROzTQkQnw3XRwubfw77MrenkOFOfxuiA2wJcaRAWyVvZmRW8i7mHPCwqIbOQIX-0NGF8nulK0hdqlugu18G7OgPAQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
