Abstract
Invariant natural killer (iNKT) T cells and mucosal-associated invariant T (MAIT) cells represent peculiar T-lymphocyte subpopulations with innate-like properties that differ from conventional T cells. iNKT are reduced in the primary immunodeficiency caused by mutations in the X-linked inhibitor of apoptosis (XIAP). By studying the mechanism of this depletion, we herein report that iNKT cells exhibit a high susceptibility to apoptosis that is not observed with conventional T cells. Elevated expression of caspases 3 and 7 accounts for the proapoptotic phenotype of iNKT cells, which is inhibited by XIAP although it exerts a moderate effect in conventional T cells. Similarly, MAIT cells exhibit a proapoptotic propensity with elevated expression of activated caspases and are decreased in XIAP-deficient individuals. Knockdown of the transcription factor PLZF/ZBTB-16, which is involved in the effector program of iNKT cells, diminishes their proapoptotic phenotype. Conversely, overexpression of PLZF/ZBTB-16 in conventional T cells leads to a proapoptotic phenotype. Our findings identify a previously unknown pathway of regulation of innate-like T-cell homeostasis depending on XIAP and PLZF. The proapoptotic feature of iNKT cells also gives a reliable explanation of their exhaustion observed in different human conditions including the XIAP immunodeficiency.
Key Points
Human innate-like lymphocytes, iNKT and MAIT cells exhibit a proapoptotic phenotype associated with increased levels of caspases.
The proapoptotic feature of iNKT and MAIT cells depends on the transcription factor PLZF and is regulated by the anti-apoptotic factor XIAP.
Introduction
Invariant natural killer T (iNKT) lymphocytes represent a distinct T-cell lineage with innate-like traits differing from conventional T cells.1 In humans, iNKT cells express an invariant (or semi-invariant in mice) TCR made of the Vα24/Jα18/Vβ11 rearrangements, which recognize glycosphingolipids from foreign and self origin presented by the MHC class I–like monomorphic molecule CD1d.2 Several factors required for the development of iNKT cells but not of conventional T cells have been identified including the SLAM family receptors, the SLAM-associated protein (SAP) and transcription factors, such as NF-κB, Egr2, HEB, and PLZF.3 Functionally, iNKT cells are characterized by their ability to rapidly release large amounts of T helper (Th) type 1 (Th1), Th2, and Th17 cytokines and chemokines when activated. In accordance with these unique features, many studies in mice have demonstrated their involvement in a variety of immune responses and immunopathologic conditions.1 Recently, a second invariant T-cell subpopulation, the mucosal associated invariant T (MAIT) cells was identified both in humans and mice.4-6 These cells express a semi-invariant TCR made of Vα7.2/Jα33 rearrangements and share with iNKT cells several developmental, functional, and phenotypical features.7,8
In humans, our knowledge of the biology of iNKT cells is more limited. Indeed, iNKT cells are present in very variable albeit low proportions in contrast to mice, rendering studies in humans difficult. iNKT cell defects in humans have been reported in several conditions, hence providing an insight into their possible functions in humans.9 However, it is not known whether these defects precede the development of the disease or are a consequence of it. Primary immunodeficiencies have provided more obvious clues to the biology of human iNKT cells revealing important and unexpected factors involved in their development, such as SAP, XIAP, and WASP.10-14 The X-linked lymphoproliferative (XLP) syndrome was the first identified inherited immunodeficiency associated with an iNKT cell defect. The main clinical feature of XLP is a peculiar susceptibility to Epstein-Barr virus (EBV) infection, subsequent hemophagocytic lymphohistiocytosis (HLH), and hypogammaglobulinemia.15 On a molecular basis, XLP is divided into 2 distinct diseases, XLP type 1 (XLP-1) and XLP type 2 (XLP-2) caused by mutations in SH2D1A and XIAP, respectively.10,16-18 The SH2D1A gene product, the SLAM-associated protein (SAP) is a small adaptor that couples SLAM family receptors to downstream signaling pathways. In the absence of SAP, CD1d-restricted iNKT-cell development is blocked in the thymus at the positive selection step.19 The XIAP gene product, the X-linked inhibitor of apoptosis (XIAP) is known to be a physiologic inhibitor of caspases 3, 7, and 9.20 In addition to its anti-apoptotic activity, XIAP is also involved in other signaling pathways, such as copper metabolism, NF-κB and MAP kinase, and NOD2 pathways.21,22 In contrast to SAP-deficiency, the mechanism of the depletion of iNKT cells in humans with a XIAP deficiency was still unknown until now.
Here, we report that iNKT and MAIT cells are characterized by a high propensity to apoptosis which is not associated with conventional T cells. This unique proapoptotic phenotype is exacerbated in the absence of XIAP expression explaining why XIAP-deficient patients exhibited low numbers of iNKT and MAIT cells. PLZF, known to direct the effector program of iNKT cells, was shown to be involved in the acquisition of the iNKT cell proapoptotic phenotype.
Methods
Detailed procedures of the materials and methods are provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cell cultures
Informed consent was obtained from each donor and patients and study protocols are conformed to the 1975 declaration of Helsinki and the current French legislation and ethical guidelines. For expansions of iNKT cells and Vβ2T cells, peripheral blood mononuclear cells (PBMCs; 500 000/mL) were cultured in RPMI 1640 GlutaMax medium (Invitrogen) with penicillin (100 U/mL), streptomycin (100 mg/mL), IL-2 (1000UI/mL), and 10% heat-inactivated fetal calf serum (Gibco) in the presence of 100 ng/mL α-galactosylceramide (αGalCer; Alexis) or 0.5 μg/mL toxic shock syndrome toxin (TSST-1; Toxin Technology), respectively. After 8 days of culture, the proportions of iNKT cells and Vβ2 T cells in the cultures varied from 1.5% to 50% and from 10% to 70%, respectively. For expansions from thymus, 2.5 × 106/mL of thymocytes were cultured.
Flow cytometry
Cells were labeled according to standard protocols. iNKT in PBMCs were detected by staining with anti-Vβ11TCR, anti-Vα24TCR, and anti-CD3. In apoptosis and phenotyping experiments, iNKT cells were labeled with anti-CD3, anti–TCR-iNKT (anti–human invariant NKT cell antibody, clone 6B11) or with PBS-57–loaded CD1d tetramers (TT+), Vβ2 T cells with anti-TCRVβ2 and anti-CD3, and MAIT cells with anti-CD161, anti-TCRVα7.2, and anti-CD3. Apoptotic cells were detected by staining with annexin V alone or in combination with 7-AAD Viaprobe (BD Bioscience) according to standard protocols. Active caspases were detected using FLICA reagent (Sigma-Aldrich) following the manufacturer's protocol. For intracellular staining of XIAP and PLZF, cells were permeabilized using Intraprep kit (Beckman Coulter) and stained with the mouse anti–human XIAP from BD (clone 48) revealed by a pacific-blue conjugated goat anti–mouse antibody (BD), or APC-conjugated anti-PLZF (clone 6318100; R&D Systems). Samples were acquired on a FACScanto II flowcytometer (BD Bioscience). Data were analyzed using FlowJo Version 9.5.3 software (TreeStar).
Apoptosis assays
Apoptosis assays were performed as previously described.10,23 Briefly, cells were stimulated with 10 μg/mL of coated anti-CD3 (OKT3, Janssen-Cilag) or in the presence of 100 ng/mL of soluble anti-FAS (clone APO1.3; Alexis) and 5 μg/mL of rabbit anti–mouse IgG Fab′2 (Jackson ImmunoResearch Laboratories). Expanded cells and ex vivo cells were stimulated 24 hours and 8 hours, respectively. Apoptosis was based on the percentage of annexin V+ cells or annexin V+/7-AAD− to exclude necrotic cells. The percentage of induced apoptosis was calculated according to the formula: 100 × (% of experimental apoptotic cells − % of spontaneously apoptotic cells)/(100 − % of spontaneously apoptotic cells).
Transduction of cells with sh-RNA
Cell expansions were transduced at day 3 with the lentiviral vector pLKO.1 containing a sh-RNA for XIAP (Openbiosystems, n°TRCN0000003788), for PLZF (Openbiosystems, clone 1 n°TRCN0000012940, clone 2 n°TRCN0000012942) or a scramble sh-RNA, in which the puromycine resistant gene was replaced by the reporter GFP gene. Apoptosis was determined in GFP-positive and GFP-negative cells. For survey of GFP expression in long-term expansion, cells were repeatedly stimulated with 1 μg/mL OKT3 or 25 ng/mL APO1.3.
Immunoblots
Cell lysates and immunoblots were performed according to standard protocols. Quantifications of western blots were done using ImageJ 1.45 software (National Institutes of Health).
Luciferase assay for the caspase 3 promoter
Luciferase assays with a pGL3-Basic luciferase vector (Promega) containing the full-length promoter (P1) of human caspase 3 (a kind gift of Dr Bong Lee, National Institutes of Health) were done according to standard protocols.24
Statistical analysis
The statistical analysis of results was performed using Prism Version 4.0 software (GraphPad).
Results
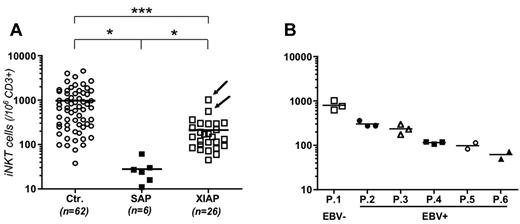
We previously reported that blood iNKT cell counts were low in 8 XIAP-deficient patients, whereas conventional T-cell populations were not affected.10 We extended these data to newly identified patients with XIAP-deficiency15 and compared them with SAP-deficient patients. iNKT cell numbers in the peripheral blood of SAP-deficient and XIAP-deficient patients were significantly reduced compared with those of healthy controls (Figure 1A). However, compared with SAP-deficient patients, the reduction in XIAP-deficient patients was significantly less pronounced (Figure 1A). In 6 investigated XIAP-deficient patients, iNKT cell numbers were stable over time (Figure 1B). All patients but 2 XIAP-deficient patients were previously infected with EBV. Remarkably, the 2 XIAP-deficient patients with no evidence of EBV infection (ie, negative viral load and absence of antibodies to EBV) exhibited the highest numbers of iNKT cells (Figure 1A arrows and P.1 in Figure 1B). The 2 patients are carriers of null mutations L184X and E118X and are still asymptomatic at the age of 11 and 12 years.
Decreased numbers of iNKT cells in PBMCs from XIAP-deficient patients. (A) Comparison of iNKT cell (TCRVα24+TCRVβ11+) counts within CD3+ lymphocytes from blood of control healthy donors (Ctrl.), SAP-deficient patients (SAP) and XIAP-deficient patients (XIAP). iNKT cells were detected by staining with anti-Vα24 and anti-Vβ11 TCR antibodies and analyzed after gating on CD3+ cells. All XIAP-patients have been infected by EBV except 2 patients who are indicated by arrows. Bars correspond to mean values of each group of iNKT cell numbers, unpaired t test (*P < .05, ***P < .001). (B) Six XIAP-deficient patients (P.1, P.2, P.3, P.4, P.5, and P.6) were tested 2 or 3 times at 6- to 12-month intervals. Patients were infected by EBV (EBV+) except P.1.
Decreased numbers of iNKT cells in PBMCs from XIAP-deficient patients. (A) Comparison of iNKT cell (TCRVα24+TCRVβ11+) counts within CD3+ lymphocytes from blood of control healthy donors (Ctrl.), SAP-deficient patients (SAP) and XIAP-deficient patients (XIAP). iNKT cells were detected by staining with anti-Vα24 and anti-Vβ11 TCR antibodies and analyzed after gating on CD3+ cells. All XIAP-patients have been infected by EBV except 2 patients who are indicated by arrows. Bars correspond to mean values of each group of iNKT cell numbers, unpaired t test (*P < .05, ***P < .001). (B) Six XIAP-deficient patients (P.1, P.2, P.3, P.4, P.5, and P.6) were tested 2 or 3 times at 6- to 12-month intervals. Patients were infected by EBV (EBV+) except P.1.
High susceptibility to apoptosis of in vitro expanded iNKT
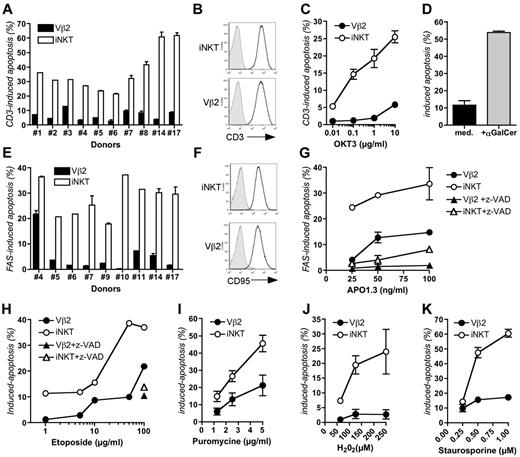
Based on these observations and the known anti-apoptotic function of XIAP, we hypothesized that human iNKT cells could be particularly prone to apoptosis after activation and, thus being more dependent on XIAP for survival than conventional T cells. To test this hypothesis, apoptosis of iNKT cells in response to various stimuli was analyzed and compared with that of conventional T cells. Vβ2–expressing conventional T cells and iNKT cells from PBMCs of 10 healthy individuals were expanded in parallel in response to their respective ligands TSST-1 and αGalCer. Of note, healthy donors exhibited variable blood iNKT cell counts ranging from low to high (supplemental Table 1). After 1 week of culture, expression of activation markers including CD45RO, CD45RA, CD25, CD69, and HLA-DR was analyzed on expanded iNKT and Vβ2 T cells (supplemental Figure 1 left panels; supplemental Figure 2 right panels). With the exception of HLA-DR, which was more expressed by iNKT cells, there was no difference showing that both cell populations had an activated-memory phenotype. Activation-induced cell death (AICD; also referred to as restimulation-induced cell death) was examined in expanded Vβ2 T cells and iNKT cells in response to anti-CD3 antibody stimulation. AICD that depends on the FAS-FAS ligand interactions is an important mechanism that drives the deletion of activated T cells in self-limiting immune responses.25 In all cases, AICD of iNKT cells was markedly increased compared with that of Vβ2 T cells (Figure 2A; supplemental Figure 3A). These results were not because of differences in CD3 expression between iNKT and conventional T cells because CD3 levels were similar (Figure 2B). Apoptotic iNKT cells were observed as early as after 6 hours of stimulation (data not shown) and with all used concentrations of anti-CD3 antibody (Figure 2C). Similar results were observed when iNKT cells were stimulated by B cells expressing CD1d loaded with αGalCer (Figure 2D).
Both intrinsic and extrinsic triggers of apoptosis induce a marked apoptosis of iNKT cells in contrast to conventional Vβ2 T cells. (A) Activation-induced cell death of expanded iNKT and Vβ2 T cells from 10 different healthy donors stimulated with anti-CD3 antibody (OKT3). Error bars (SD) correspond to assays done in duplicate. (B) Expression of CD3 on iNKT and Vβ2 cells by flow cytometry. Gray histograms represent the isotype control and the black lines represent the anti-CD3. Data correspond to 1 representative experiment of 10. (C) Same as panel A, except that cells were stimulated with increasing concentrations of anti-CD3 antibody. Data from 1 experiment with 1 healthy donor (No. 6) with assays done in duplicate (error bars correspond to SD). (D) Apoptosis of iNKT cells incubated with RAJI cells expressing CD1d with medium (med) or αGalCer for 24 hours. Data correspond to 1 representative experiment of 3 with 2 different donors (Nos. 9 and 6). (E) Apoptosis of expanded iNKT and Vβ2 T cells from 9 different healthy donors stimulated with soluble anti-FAS antibody (APO1.3). Assays were done in duplicate (error bars correspond to SD). (F) Expression of FAS (CD95) on iNKT and Vβ2 T cells by flow cytometry. Gray histograms represent the isotype control and the black lines represent the anti-CD95 antibody staining. Data correspond to 1 representative experiment of 7. (G) Same as panel E, except that cells from 4 different healthy donors were stimulated with increasing concentrations of APO1.3 in the presence or not of z-VAD. One representative experiment of 4 independent experiments with 4 healthy donors (Nos. 3, 4, 6, and 20) is showed with assays done in duplicate. (H) Etoposide-induced apoptosis of expanded cells from 1 healthy donor (No. 4). With the highest concentration of etoposide, cells were also incubated with z-VAD (right panel). (I) Puromycin-induced apoptosis. Data are mean ± SD of 3 experiments with 3 healthy donors (Nos. 5, 6, and 9) with assays done in duplicate. (J) H2O2-induced apoptosis. Data are mean ± SD of 2 experiments with 2 healthy donors (Nos. 6 and 9) with assays done in duplicate. (K) Staurosporine-induced apoptosis. Data are mean ± SD of 2 experiments in duplicate with 2 healthy donors (Nos. 7 and 9). See also supplemental Figure 3.
Both intrinsic and extrinsic triggers of apoptosis induce a marked apoptosis of iNKT cells in contrast to conventional Vβ2 T cells. (A) Activation-induced cell death of expanded iNKT and Vβ2 T cells from 10 different healthy donors stimulated with anti-CD3 antibody (OKT3). Error bars (SD) correspond to assays done in duplicate. (B) Expression of CD3 on iNKT and Vβ2 cells by flow cytometry. Gray histograms represent the isotype control and the black lines represent the anti-CD3. Data correspond to 1 representative experiment of 10. (C) Same as panel A, except that cells were stimulated with increasing concentrations of anti-CD3 antibody. Data from 1 experiment with 1 healthy donor (No. 6) with assays done in duplicate (error bars correspond to SD). (D) Apoptosis of iNKT cells incubated with RAJI cells expressing CD1d with medium (med) or αGalCer for 24 hours. Data correspond to 1 representative experiment of 3 with 2 different donors (Nos. 9 and 6). (E) Apoptosis of expanded iNKT and Vβ2 T cells from 9 different healthy donors stimulated with soluble anti-FAS antibody (APO1.3). Assays were done in duplicate (error bars correspond to SD). (F) Expression of FAS (CD95) on iNKT and Vβ2 T cells by flow cytometry. Gray histograms represent the isotype control and the black lines represent the anti-CD95 antibody staining. Data correspond to 1 representative experiment of 7. (G) Same as panel E, except that cells from 4 different healthy donors were stimulated with increasing concentrations of APO1.3 in the presence or not of z-VAD. One representative experiment of 4 independent experiments with 4 healthy donors (Nos. 3, 4, 6, and 20) is showed with assays done in duplicate. (H) Etoposide-induced apoptosis of expanded cells from 1 healthy donor (No. 4). With the highest concentration of etoposide, cells were also incubated with z-VAD (right panel). (I) Puromycin-induced apoptosis. Data are mean ± SD of 3 experiments with 3 healthy donors (Nos. 5, 6, and 9) with assays done in duplicate. (J) H2O2-induced apoptosis. Data are mean ± SD of 2 experiments with 2 healthy donors (Nos. 6 and 9) with assays done in duplicate. (K) Staurosporine-induced apoptosis. Data are mean ± SD of 2 experiments in duplicate with 2 healthy donors (Nos. 7 and 9). See also supplemental Figure 3.
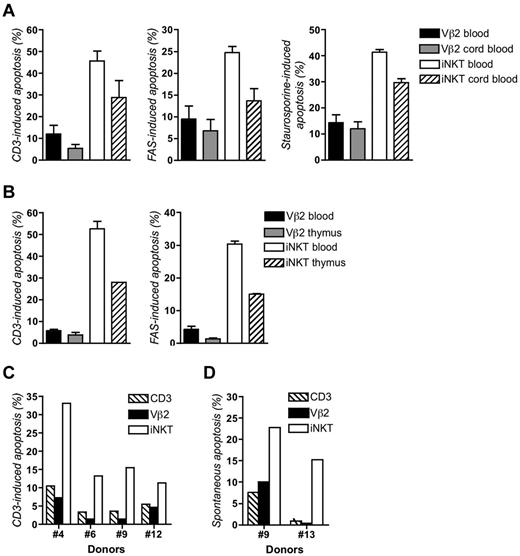
Susceptibility to apoptosis of iNKT cells was further investigated in response to stimuli inducing apoptosis either via the extrinsic pathway which depends on death receptors, including FAS, or the intrinsic pathway which targets the mitochondria.25,26 FAS stimulation of expanded iNKT cells from 9 different donors induced a substantial apoptosis that was not observed with Vβ2 T cells from the same donors (Figure 2E; supplemental Figure 3B). The intensity of apoptosis was not related to the expression level of FAS (CD95) which was similar for iNKT and Vβ2 T cells (Figure 2F). This heightened rate of apoptosis with iNKT cells was observed with different concentrations of anti-FAS antibody and was blocked by z-VAD, a broad range inhibitor of caspases (Figure 2G). The particular susceptibility of iNKT cells to apoptosis was also observed when cells were treated with etoposide, puromycin, H2O2, and staurosporine; all reagents known to activate the intrinsic apoptosis pathway (Figure 2H-K). Apoptosis was blocked by addition of z-VAD (Figure 2H and data not shown). This susceptibility to apoptosis in response to FAS, CD3, and staurosporine was also observed with iNKT cells expanded from cord blood and thymus (Figure 3A-B). Of note, cord blood and thymus-derived cells were less of memory-activated effector phenotype than cells expanded from peripheral blood based on higher CD62L expression and lower expression of CD69 and HLA-DR that correlated with a weaker level of induced apoptosis (supplemental Figure 1). Collectively, these data demonstrate that iNKT cells but not conventional T cells exhibit an intrinsic proapoptotic feature that is already detected in the thymic and cord blood iNKT cells.
iNKT cells expanded from cord blood or thymus and freshly isolated iNKT cells are more prone to apoptosis than Vβ2 conventional T cells. (A) Apoptosis of expanded iNKT and Vβ2 T cells from blood and cord blood stimulated with anti-CD3, anti-FAS, or staurosporine. Data correspond to 2 experiments done in duplicate (error bars correspond to SD) with cells from 2 different donors (Nos. 7 and 14) and 2 different cord blood donors. (B) Apoptosis of expanded iNKT and Vβ2 T cells from 1 blood donor and thymocytes of 1 donor stimulated with anti-CD3 or anti-FAS. Data correspond to 1 experiment with assays done in duplicate (error bars correspond to SD). (C) Apoptosis of freshly isolated PBMCs from 4 different healthy donors (Nos. 4, 6, 9, and 12) stimulated with anti-CD3 for 8 hours. (D) Apoptosis of freshly isolated iNKT cells and Vβ2 T cells of PBMCs from 2 healthy donors (Nos. 9 and 13) left unstimulated for 72 hours. See also supplemental Figures 1 and 2.
iNKT cells expanded from cord blood or thymus and freshly isolated iNKT cells are more prone to apoptosis than Vβ2 conventional T cells. (A) Apoptosis of expanded iNKT and Vβ2 T cells from blood and cord blood stimulated with anti-CD3, anti-FAS, or staurosporine. Data correspond to 2 experiments done in duplicate (error bars correspond to SD) with cells from 2 different donors (Nos. 7 and 14) and 2 different cord blood donors. (B) Apoptosis of expanded iNKT and Vβ2 T cells from 1 blood donor and thymocytes of 1 donor stimulated with anti-CD3 or anti-FAS. Data correspond to 1 experiment with assays done in duplicate (error bars correspond to SD). (C) Apoptosis of freshly isolated PBMCs from 4 different healthy donors (Nos. 4, 6, 9, and 12) stimulated with anti-CD3 for 8 hours. (D) Apoptosis of freshly isolated iNKT cells and Vβ2 T cells of PBMCs from 2 healthy donors (Nos. 9 and 13) left unstimulated for 72 hours. See also supplemental Figures 1 and 2.
High susceptibility to apoptosis of fresh iNKT cells
To be sure that the differences in apoptosis sensitivity between iNKT and conventional T cells did not result from in vitro culture conditions, we tested the apoptosis of fresh iNKT cells from PBMCs of 4 healthy donors without any prior in vitro expansion (Figure 3C). Analysis of phenotypical markers including CD45RO, HLA-DR, CD25, CD69, and CD95 showed that most of fresh iNKT cells and Vβ2 T cells were not activated and had a memory phenotype (supplemental Figure 2). Furthermore, fresh iNKT cells were negative for Ki67 expression indicating that they were not cycling in vivo, whereas a few Vβ2 T cells (1%-2%) were Ki67+ (supplemental Figure 2B). In contrast, expanded iNKT cells and Vβ2 T cells were cycling and displayed a memory-activated memory phenotype (supplemental Figure 2A right panels). Anti-CD3–induced apoptosis of fresh iNKT cells was more pronounced than of fresh Vβ2 T cells or total CD3+ T cells (Figure 3C). As expected for cells that had not been pre-activated, the fraction of apoptotic cells was lower than that in in vitro cultured cell counterparts (Figure 2). Spontaneous apoptosis of fresh iNKT cells and Vβ2 T cells was also examined. After 72 hours, the fraction of apoptotic cells among iNKT cells was also higher than among Vβ2 T cells and total CD3+ cells (Figure 3D). These results (1) support the assumption whereby iNKT cells are apoptosis-prone and (2) demonstrate that the high apoptosis susceptibility is a general feature of these cells irrespective of their activation status.
XIAP regulates the proapoptotic phenotype of iNKT cells
We next determined the role of XIAP in apoptosis of iNKT cells by analyzing available iNKT cells from the XIAP-deficient patient (P.1) who had a normal NKT cell count (Figure 1). After anti-CD3 or anti-FAS stimulation, higher levels of apoptosis were observed in XIAP-deficient iNKT cells compared with wild-type iNKT cells of a healthy donor (Figure 4A). The intensity of apoptosis of XIAP-deficient iNKT cells was markedly higher than one of the XIAP-deficient Vβ2 T cells, which did not differ from that of healthy donor Vβ2 T cells. The absence of XIAP protein expression thus results in a selective enhanced apoptosis in iNKT cells. To confirm these data, shRNA experiments were undertaken to down-regulate XIAP expression in controls cells and see its effect on cell survival. A lentiviral vector containing shRNA construct with a GFP reporter gene was used to transduce both iNKT and Vβ2 T cells. Both GFP-positive targeted iNKT (GFP+) and Vβ2 T cells showed a strong decreased expression of XIAP (sh-XIAP cells) that was not seen in cells targeted with a scramble shRNA (sh-control cells) or in nontargeted GFP-negative cells (GFP−; Figure 4B). Apoptosis upon anti-FAS or anti-CD3 stimulation of GFP+ and GFP− cells was then analyzed (Figure 4C-D). In iNKT cells, GFP+ sh-XIAP cells exhibited a higher apoptotic rate than GFP− and GFP+ sh-control cells. Importantly, the apoptosis of GFP+Vβ2 T cells targeted with the XIAP shRNA was not clearly increased and similar to that of nontargeted GFP−Vβ2 T cells. Cells were also repeatedly stimulated with anti-CD3 or anti-FAS antibodies and the proportion of GFP+ cells in culture analyzed at several time points (Figure 4E). The fraction of GFP+cells expressing the scramble shRNA remained stable over time. In contrast, the fraction of GFP+iNKT expressing the XIAP shRNA disappeared with a faster kinetic than iNKT cells expressing a control shRNA or Vβ2 T cells expressing the XIAP shRNA. Taken together, these data confirm that iNKT cells are much more dependent on XIAP for their survival than conventional T cells.
XIAP-deficiency worsens the proapoptotic phenotype of iNKT cells. (A) Apoptosis of expanded iNKT and Vβ2 T cells from 1 XIAP-deficient patient (P.1 in Figure 1) and 1 healthy donor (No. 16) stimulated with anti-CD3 or anti-FAS antibodies. Data are representative of 1 experiment of 2. Assays were done in duplicate (error bars correspond to SD). (B) GFP (left panels) and XIAP (right panels) expressions in iNKT and Vβ2 T cells from healthy donors infected with a lentiviral pLKO-GFP vector containing either a shRNA for XIAP (sh-XIAP) or scramble shRNA (sh-control), tested at day 4 after infection. Histograms correspond to staining with anti-XIAP antibody or isotype control in GFP-positive (GFP+) and GFP-negative (GFP−) cells. (C) Apoptosis of GFP+ and GFP− iNKT and Vβ2 T cells stimulated with anti-CD3 antibody. Data are mean ± SD of 3 independent experiments with 3 different healthy donors (Nos. 6, 9, and 13) with assays done in duplicate. Paired, 2-tailed t test (**P < .01). (D) Apoptosis of GFP+ iNKT and Vβ2 cells stimulated with anti-FAS antibody. Data correspond to 1 experiment representative of 2 with assays done in duplicate (error bars correspond to SD). (E) Same as in panels C and D, except that cells were repeatedly treated with anti-CD3 or anti-FAS antibodies every day and the proportions of GFP+iNKT and GFP+Vβ2 cells in cultures was determined by flow cytometry. Data represent the loss of GFP+ cells targeted with the shRNA for XIAP or the sh-control within the culture compared with the percentage of GFP+ cells at day 0. One representative experiment of 2.
XIAP-deficiency worsens the proapoptotic phenotype of iNKT cells. (A) Apoptosis of expanded iNKT and Vβ2 T cells from 1 XIAP-deficient patient (P.1 in Figure 1) and 1 healthy donor (No. 16) stimulated with anti-CD3 or anti-FAS antibodies. Data are representative of 1 experiment of 2. Assays were done in duplicate (error bars correspond to SD). (B) GFP (left panels) and XIAP (right panels) expressions in iNKT and Vβ2 T cells from healthy donors infected with a lentiviral pLKO-GFP vector containing either a shRNA for XIAP (sh-XIAP) or scramble shRNA (sh-control), tested at day 4 after infection. Histograms correspond to staining with anti-XIAP antibody or isotype control in GFP-positive (GFP+) and GFP-negative (GFP−) cells. (C) Apoptosis of GFP+ and GFP− iNKT and Vβ2 T cells stimulated with anti-CD3 antibody. Data are mean ± SD of 3 independent experiments with 3 different healthy donors (Nos. 6, 9, and 13) with assays done in duplicate. Paired, 2-tailed t test (**P < .01). (D) Apoptosis of GFP+ iNKT and Vβ2 cells stimulated with anti-FAS antibody. Data correspond to 1 experiment representative of 2 with assays done in duplicate (error bars correspond to SD). (E) Same as in panels C and D, except that cells were repeatedly treated with anti-CD3 or anti-FAS antibodies every day and the proportions of GFP+iNKT and GFP+Vβ2 cells in cultures was determined by flow cytometry. Data represent the loss of GFP+ cells targeted with the shRNA for XIAP or the sh-control within the culture compared with the percentage of GFP+ cells at day 0. One representative experiment of 2.
Elevated expression of proapoptotic proteins in iNKT cells
The molecular mechanism underlying susceptibility to apoptosis of iNKT cells was thus analyzed. Protein expression of several effectors of apoptosis such as members of the Bcl-2 family and executioner caspases was determined in expanded iNKT and Vβ2 T cells from 3 healthy individuals by Western blotting. BID, procaspases 3, 7, and cleaved forms of caspase 3 were found to be more strongly expressed in expanded iNKT cells compared with Vβ2 T cells (Figure 5A). Expression of XIAP was also increased in expanded iNKT cells. Consistent with this proapoptotic molecular signature, the proportion of ex vivo iNKT cells expressing activated caspases, as detected by a fluorescent inhibitor of activated caspases (FLICA), was found to be higher compared with that of Vβ2 T cells or of total CD3+ T cells (Figure 5B). Therefore, these results suggest that the up-regulation of proapoptotic molecules such as caspases 3, 7, and BID in iNKT cells accounts for their exquisite susceptibility to apoptosis triggers.
iNKT cells express high amounts of caspases in contrast to conventional Vβ2 T cells. (A) Expression of pro and anti-apoptotic proteins in expanded iNKT and Vβ2 T cell lysates from 3 healthy donors (Nos. 4, 6, and 9) analyzed by SDS-PAGE and subsequent western-blotting. Asterisk (*) corresponds to proteins overexpressed in iNKT cells, relative to Vβ2 T cells. (B) Detection of active caspases by flow cytometry in fresh isolated iNKT cells, Vβ2 T cells and CD3+ T cells from PBMCs of 3 healthy donors (Nos. 4, 6, and 9). After gating on CD3+TCR-iNKT+ (iNKT), CD3+TCRVβ2+ (Vβ2), or CD3+ T cells (CD3+), cells were stained by a fluorescent inhibitor of activated caspases (FLICA) allowing detection of activated caspases.
iNKT cells express high amounts of caspases in contrast to conventional Vβ2 T cells. (A) Expression of pro and anti-apoptotic proteins in expanded iNKT and Vβ2 T cell lysates from 3 healthy donors (Nos. 4, 6, and 9) analyzed by SDS-PAGE and subsequent western-blotting. Asterisk (*) corresponds to proteins overexpressed in iNKT cells, relative to Vβ2 T cells. (B) Detection of active caspases by flow cytometry in fresh isolated iNKT cells, Vβ2 T cells and CD3+ T cells from PBMCs of 3 healthy donors (Nos. 4, 6, and 9). After gating on CD3+TCR-iNKT+ (iNKT), CD3+TCRVβ2+ (Vβ2), or CD3+ T cells (CD3+), cells were stained by a fluorescent inhibitor of activated caspases (FLICA) allowing detection of activated caspases.
The transcription factor PLZF regulates the proapoptotic phenotype of iNKT
iNKT cells are characterized by the expression of the transcription factor PLZF (promyelocytic leukemia zinc finger, ZBTB16), which is involved in the acquisition of the effector phenotype of iNKT cells.27,28 In other cell types including tumor cells, PLZF was also shown to induce growth suppression and apoptosis with proapoptotic genes, such as caspase 3, as targets of PLZF.29-31 For these reasons, we tested whether PLZF could be involved in the proapoptotic phenotype of iNKT. Down-regulation of PLZF expression using 2 distinct shRNA in expanded iNKT resulted in a decreased apoptosis induced by staurosporine or FAS (Figures 6A-C). Repeated FAS stimulations resulted in the accumulation of targeted GFP+ iNKT cells in culture (Figure 6C). This was not observed with iNKT cells expressing a control shRNA. Conversely, overexpression of PLZF using a lentiviral plasmid containing a PLZF transgene in Vβ2 conventional T cells and in CD3+ T cell blasts increased their susceptibility to apoptosis and their loss on repeated FAS stimulations (Figure 6D-F, supplemental Figure 4). Similarly, overexpression of PLZF in Jurkat T cells increased their susceptibility to apoptosis triggered by FAS and staurosporine (Figure 6G-I). In iNKT cells, one of the candidate targets of PLZF may be the caspase 3, which was found to be overexpressed (Figure 5A). The PLZF effect on a luciferase reporter gene under the control of the CASPASE 3 promoter was tested in Jurkat TAg cells. PLZF was indeed found to increase luciferase activity (Figure 6J) also leading to caspase 3 and cleaved products overexpression (Figure 6K). Therefore, these data showed that the proapoptotic phenotype of iNKT cells is in part dependent of PLZF through caspase 3 induction.
The transcription factor PLZF controls the proapoptotic phenotype of iNKT cells. (A) Expression of PLZF in expanded GFP+iNKT targeted by a control shRNA (sh-control) or specific for PLZF (sh-PLZF) by flow cytometry. Gray histograms, isotype control; black lines, anti-PLZF. One representative experiment of 4. (B) Staurosporine-induced apoptosis of GFP+iNKT cells targeted with a control shRNA (sh-control) or 2 shRNA specific for PLZF (sh-PLZF-1-, n = 6; sh-PLZF-2-, n = 3). (C) Same as in panel B except that GFP+iNKT cells were analyzed for FAS-induced apoptosis (left panel, n = 5). In the right panel, cells were repeatedly treated with anti-FAS antibodies every 24 hours and the proportions of GFP+ cells in the cultures were determined. Data represent the ratio of the percentage of GFP+ cells in the culture to the percentage of GFP+ cells at time 0. One representative experiment of 2 with 2 donors (Nos. 4 and 9). (D) Expression of PLZF in expanded Vβ2 T cells infected by a lentiviral empty vector (empty vector) or containing a PLZF transgene (Tg-PLZF). Gray histograms, isotype control; black lines, anti-PLZF. One representative experiment of 4. (E) Staurosporine-induced apoptosis in GFP+ Vβ2 T cells expressing the empty vector (empty vector) or a PLZF transgene (Tg-PLZF; n = 3, donors Nos. 4, 6, and 9). (F) Same as panel C. FAS-induced apoptosis in GFP+ Vβ2 T cells expressing the empty vector (empty vector) or a PLZF transgene (Tg-PLZF; n = 4, donor Nos. 4, 6, and 9). (G) Expression of PLZF in Jurkat cells infected by a lentiviral empty vector (empty vector) or containing a PLZF transgene (Tg-PLZF). Gray histograms, isotype control; black lines, anti-PLZF. (H) FAS-induced apoptosis in Jurkat cells expressing PLZF (Tg-PLZF) or not (empty vector; n = 4). (I) Staurosporine-induced apoptosis in Jurkat TAg cells infected by a lentiviral empty vector (empty vector) or containing a PLZF transgene (Tg-PLZF) PLZF (Tg-PLZF; n = 3). (J) Activity of the promoter P1 of caspase 3 measured by a reporter luciferase assay in Jurkat TAg cells expressing PLZF (Tg-PLZF) or the empty vector. Data of 2 independent experiments with quintuplicates, mean ± SD. (K) Expression of actin, PLZF, caspase 3 (CASP3), cleaved caspase 3 (CASP3 cleaved), and Ku70 (as loading control) in wild-type Jurkat TAg cells (WT) or expressing PLZF (Tg-PLZF) or the empty vector by Western blotting. On the right, quantification of caspase 3 and cleaved caspase 3 expressions in Jurkat TAg cells expressing PLZF compared with the empty vector and normalized on actin expression. One representative experiment of 2. Paired, 2-tailed t tests in panels B, C, E, F, H, and I with data mean ± SD and n = number of independent experiments with duplicate (*P < .05, **P < .01, ***P < .001).
The transcription factor PLZF controls the proapoptotic phenotype of iNKT cells. (A) Expression of PLZF in expanded GFP+iNKT targeted by a control shRNA (sh-control) or specific for PLZF (sh-PLZF) by flow cytometry. Gray histograms, isotype control; black lines, anti-PLZF. One representative experiment of 4. (B) Staurosporine-induced apoptosis of GFP+iNKT cells targeted with a control shRNA (sh-control) or 2 shRNA specific for PLZF (sh-PLZF-1-, n = 6; sh-PLZF-2-, n = 3). (C) Same as in panel B except that GFP+iNKT cells were analyzed for FAS-induced apoptosis (left panel, n = 5). In the right panel, cells were repeatedly treated with anti-FAS antibodies every 24 hours and the proportions of GFP+ cells in the cultures were determined. Data represent the ratio of the percentage of GFP+ cells in the culture to the percentage of GFP+ cells at time 0. One representative experiment of 2 with 2 donors (Nos. 4 and 9). (D) Expression of PLZF in expanded Vβ2 T cells infected by a lentiviral empty vector (empty vector) or containing a PLZF transgene (Tg-PLZF). Gray histograms, isotype control; black lines, anti-PLZF. One representative experiment of 4. (E) Staurosporine-induced apoptosis in GFP+ Vβ2 T cells expressing the empty vector (empty vector) or a PLZF transgene (Tg-PLZF; n = 3, donors Nos. 4, 6, and 9). (F) Same as panel C. FAS-induced apoptosis in GFP+ Vβ2 T cells expressing the empty vector (empty vector) or a PLZF transgene (Tg-PLZF; n = 4, donor Nos. 4, 6, and 9). (G) Expression of PLZF in Jurkat cells infected by a lentiviral empty vector (empty vector) or containing a PLZF transgene (Tg-PLZF). Gray histograms, isotype control; black lines, anti-PLZF. (H) FAS-induced apoptosis in Jurkat cells expressing PLZF (Tg-PLZF) or not (empty vector; n = 4). (I) Staurosporine-induced apoptosis in Jurkat TAg cells infected by a lentiviral empty vector (empty vector) or containing a PLZF transgene (Tg-PLZF) PLZF (Tg-PLZF; n = 3). (J) Activity of the promoter P1 of caspase 3 measured by a reporter luciferase assay in Jurkat TAg cells expressing PLZF (Tg-PLZF) or the empty vector. Data of 2 independent experiments with quintuplicates, mean ± SD. (K) Expression of actin, PLZF, caspase 3 (CASP3), cleaved caspase 3 (CASP3 cleaved), and Ku70 (as loading control) in wild-type Jurkat TAg cells (WT) or expressing PLZF (Tg-PLZF) or the empty vector by Western blotting. On the right, quantification of caspase 3 and cleaved caspase 3 expressions in Jurkat TAg cells expressing PLZF compared with the empty vector and normalized on actin expression. One representative experiment of 2. Paired, 2-tailed t tests in panels B, C, E, F, H, and I with data mean ± SD and n = number of independent experiments with duplicate (*P < .05, **P < .01, ***P < .001).
MAIT cells exhibit a proapoptotic feature similar to iNKT cells
MAIT cells represent a second invariant T-cell population which accumulate in the intestinal lamina propria and blood in humans at a relatively high frequency (accounting for 1% to 8% of total T cells).6 MAIT cells share with iNKT cells numerous features distinguishing them from conventional T cells including high expression of PLZF.6 We therefore sought to determine whether MAIT cells behave as iNKT cells in terms of apoptosis sensitivity. MAIT cells corresponding to CD161highVα7.2+CD3+ were found to be 10-fold less prevalent in the blood of XIAP-deficient patients compared with healthy donors (> 1%), although they appeared slightly diminished but not significantly (P = .41) in the blood of SAP-deficient patients (Figure 7A-B). Next, apoptosis of fresh MAIT cells from PBMCs from 4 healthy donors was analyzed in response to anti-CD3 stimulation. MAIT cells exhibited a stronger apoptosis compared with that of Vβ2 T cells (Figure 7C). High-rate apoptosis of MAIT cells in response to CD3, FAS, or staurosporine stimulation was also observed in cell cultures of iNKT cell expansions, in which slowly growing MAIT cells are present (Figure 7D). The level of induced apoptosis in MAIT cells in the different conditions tested was similar to that observed in iNKT cells. Similar to iNKT cells, the proportion of fresh MAIT cells expressing activated caspases (> 10%) was elevated compared with Vβ2 T cells (< 2.5%; Figure 7E to compare with Vβ2 T cells in Figure 5B). Remarkably, fresh MAIT from 2 XIAP-deficient patients exhibited, similar to iNKT cells, an increased apoptosis after stimulation by staurosporine relative to cells of a healthy donor (Figure 7F). Finally, down-regulation of PLZF using a shRNA in MAIT cells significantly decreased their apoptosis in response to staurosporine (Figure 7G). Taken together, these observations indicate that similar to iNKT cells, MAIT cells are particularly sensitive to apoptotic stimuli, their survival being reciprocally dependent on XIAP and PLZF.
MAIT cells, similar to iNKT cells, are more prone to apoptosis than conventional T cells. (A) Flow cytometry analysis of MAIT cells (CD3+TCRVα7.2+CD161+). MAIT cells from PBMCs of 2 healthy donors (Ctr.) and 2 XIAP patients (XIAP) were detected by staining with anti-TCRVα7.2 and anti-CD161 antibodies and analyzed after gating on CD3+ cells. (B) Comparison of numbers of MAIT cells (TCRVα7.2+CD161+) in CD3+ lymphocytes from PBMCs of control healthy donors (Ctr.; n = 15), SAP-deficient patients (SAP; n = 5) and XIAP-deficient patients (XIAP; n = 16). Bars correspond to mean values of each group of cell numbers, unpaired t test (***P < .001). (C) Apoptosis of ex vivo MAIT and Vβ2 T cells from PBMCs of 4 healthy donors (Nos. 4, 6, 9, and 12) after stimulation with anti-CD3 antibody or staurosporine for 8 hours. (D) Apoptosis of cultured MAIT cells stimulated with anti-CD3 or anti-FAS antibodies. Data correspond to 1 experiment in duplicate (error bars correspond to SD) with 2 donors (Nos. 4 and 7). (E) Active caspases were detected by flow cytometry using FLICA in ex vivo MAIT cells from 2 healthy donors (Nos. 4 and 6) after gating on CD3+TCRVα7.2+CD161+. (F) Apoptosis of ex vivo MAIT and iNKT cells from PBMCs of 2 XIAP-deficient patients (P.1 and P.2 in Figure 1B) and from 1 healthy donor (No. 9) stimulated with staurosporine for 8 hours. Data correspond to 1 experiment. (G) Staurosporine induced apoptosis of ex vivo MAIT cells targeted by a control shRNA (sh-control) or a shRNA specific for PLZF (sh-PLZF-1-). Four independent experiments (n = 4) with 2 different donors (Nos. 21 and 22). Mean ± SD; paired, 2-tailed t test (*P < .05).
MAIT cells, similar to iNKT cells, are more prone to apoptosis than conventional T cells. (A) Flow cytometry analysis of MAIT cells (CD3+TCRVα7.2+CD161+). MAIT cells from PBMCs of 2 healthy donors (Ctr.) and 2 XIAP patients (XIAP) were detected by staining with anti-TCRVα7.2 and anti-CD161 antibodies and analyzed after gating on CD3+ cells. (B) Comparison of numbers of MAIT cells (TCRVα7.2+CD161+) in CD3+ lymphocytes from PBMCs of control healthy donors (Ctr.; n = 15), SAP-deficient patients (SAP; n = 5) and XIAP-deficient patients (XIAP; n = 16). Bars correspond to mean values of each group of cell numbers, unpaired t test (***P < .001). (C) Apoptosis of ex vivo MAIT and Vβ2 T cells from PBMCs of 4 healthy donors (Nos. 4, 6, 9, and 12) after stimulation with anti-CD3 antibody or staurosporine for 8 hours. (D) Apoptosis of cultured MAIT cells stimulated with anti-CD3 or anti-FAS antibodies. Data correspond to 1 experiment in duplicate (error bars correspond to SD) with 2 donors (Nos. 4 and 7). (E) Active caspases were detected by flow cytometry using FLICA in ex vivo MAIT cells from 2 healthy donors (Nos. 4 and 6) after gating on CD3+TCRVα7.2+CD161+. (F) Apoptosis of ex vivo MAIT and iNKT cells from PBMCs of 2 XIAP-deficient patients (P.1 and P.2 in Figure 1B) and from 1 healthy donor (No. 9) stimulated with staurosporine for 8 hours. Data correspond to 1 experiment. (G) Staurosporine induced apoptosis of ex vivo MAIT cells targeted by a control shRNA (sh-control) or a shRNA specific for PLZF (sh-PLZF-1-). Four independent experiments (n = 4) with 2 different donors (Nos. 21 and 22). Mean ± SD; paired, 2-tailed t test (*P < .05).
Discussion
In this study, we provided evidence that iNKT cells and MAIT cells are highly susceptible to apoptosis, a feature not shared with conventional T cells. Susceptibility to apoptosis affected both intrinsic and extrinsic pathways and was irrespective of the activation status indicative of a cell intrinsic proapoptotic phenotype. In agreement with these observations, expression of caspases 7 and 3 and their activated forms was found to be up-regulated in iNKT and MAIT cells before any stimulation. As caspases 3 and 7 are distal executioner caspases32 activated by many apoptosis pathways, their enhanced expression and activation probably accounts for the proapoptotic nature of invariant T cells. The exquisite XIAP dependency of iNKT and MAIT cells for survival is probably explained by the ability of XIAP to selectively block the activity of caspases-3 and 7.20 Removing XIAP from conventional T cells had a lesser impact on their survival in comparison with iNKT cells, consistent with the fact that conventional T cells expressed lower amounts of these caspases. We also noticed that the expression of XIAP is higher in expanded iNKT cells than in Vβ2 T cells. This could suggest a concordant regulation of caspases and XIAP with increased XIAP expression counteracting up to a certain magnitude consequences of the high caspase expression in iNKT cells.
The proapoptotic phenotype of iNKT cells and MAIT cells was found to be dependent of the transcription factor PLZF which is selectively highly expressed in these cells (in comparison to other T-cell subpopulations). Strengthening this observation, overexpression of PLZF in conventional T cells leads to their acquisition of a proapoptotic phenotype. PLZF is a key transcription factor required for the effector differentiation of iNKT cells in mice.27,28 In PLZF-deficient mice, the development of iNKT cells is partially impaired and iNKT cells failed to acquire innate-like features including memory phenotype and rapid secretion of large amounts of IL-4 and IFN-γ. The proapoptotic phenotype reported here thus represents a novel iNKT cell-associated feature also controlled by PLZF. In our experiments, the inhibition of PLZF expression only partially reduced the proapoptotic phenotype of iNKT cells probably because the down-regulation of PLZF was not complete. In addition, decreasing PLZF expression in mature iNKT cells from the periphery as we did, might not completely compromised their proapoptotic phenotype, which may in part also be acquired early in the development in the thymus. The proapoptotic phenotype could also under the control of other transcription factors in combination with PLZF, such as Hobit, which was recently shown to be involved in the terminal differentiation of iNKT cells.33
We also found that the proapoptotic phenotype of iNKT cells is shared by MAIT cells, the second invariant T-cell lineage in humans.7 Both populations share developmental, functional, and phenotypical features and are considered as innate-like T lymphocytes. iNKT cells are also autoreactive cells by the endogenous nature of the glycosphingolipids they recognize. Based on their unique features, it is tempting to speculate that the proapoptotic nature of iNKT and MAIT cells may serve to rapidly and tightly control their magnitude of activation and expansion to avoid deleterious effects. Interestingly, patients with a XIAP deficiency frequently develop severe colitis.15 A decreased number of MAIT cells as observed in XIAP-deficient patients might thus contribute to this phenotype, given their capacity to be activated by bacteria and their enrichment in the intestinal lamina propria.5
In the cohort of XIAP-deficient patients studied here, 2 patients have normal iNKT cell counts and have not been yet infected with EBV. A similar observation was previously reported in a group of XIAP-deficient patients in whom the HLH disease was not predominantly associated with EBV infection.34 These observations suggest that the depletion of iNKT cells in XIAP-deficiency could be secondary to EBV infection. EBV-infected cells could trigger activation-induced apoptosis of iNKT cells, an event that is exacerbated in the absence of XIAP. Activation may be direct through the recognition of agonist self-glycosphingolipids presented by EBV-infected cells, in a similar way as it was recently reported for HBV-infected cells.35 Inflammatory cytokines that accumulate in the context of the HLH may also trigger apoptosis of iNKT cells as suggested by the recent observation of decreased iNKT cell counts in patients with familial lymphohistiocytosis.36
Our demonstration of a proapoptotic feature of iNKT cells provides a reliable explanation for the iNKT cell defects reported in several pathologic contexts in humans including autoimmunity, allergic/inflammatory diseases, cancers, and infectious diseases that may trigger rapid exhaustion of these cells.1,9 Under such conditions, iNKT cell defects may probably be a consequence of the pathologic context which may preferentially contribute to exacerbated apoptosis of iNKT cells. Our study also showed that PLZF coordinates the effector functions of iNKT cells by limiting their cell survival with the acquisition of a proapoptotic phenotype, which may serve as a safeguard to their rapid activation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients, their families, and the healthy donors for cooperation. The authors also thank the National Institutes of Health Tetramer Core Facility for providing reagents.
This work was supported by grants from Inserm, ANR (ANR-08-MIEN-012-01; France) and the XLP Research Trust (United Kingdom), and a grant from the European Research Council (ERC-2009-AdG_20090506 n°FP7-249816). S.L. is a senior scientist of CNRS (France). S.G. is a fellowship recipient of the Ministère de la Recherche et de l'Ecole Polytechnique (France) and the Fondation ARC pour la Recherche sur le Cancer (ARC; France). C.A. is supported by the ARC, and E.M. by the ANR (France) and the Ligue Contre le Cancer (France).
Authorship
Contribution: S.G., S.S., and E.M. performed experiments, analyzed the data, and participated in writing the paper; C.L. and C.A. performed experiments; A.F., C.P., and O.L analyzed the data and participated in writing the paper; and S.L. coordinated the study, designed research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sylvain Latour, Unité Inserm 768, Hôpital Necker Enfants-Malades, Paris, France; e-mail:sylvain.latour@inserm.fr.