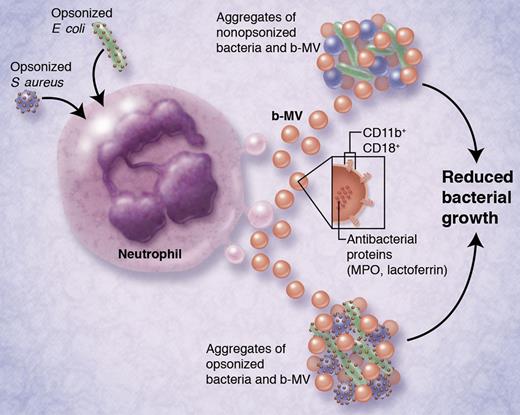
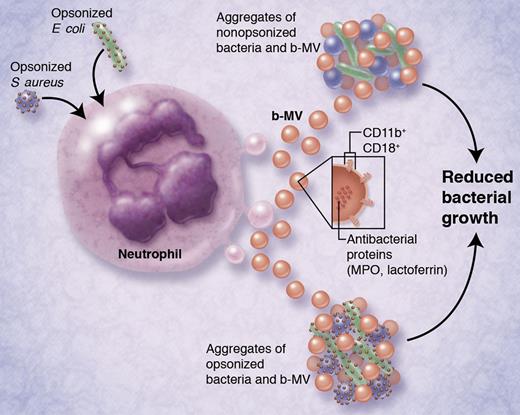
Neutrophils deploy extracellular microvesicles that can arrest the growth of bacteria.
Neutrophils are among the first innate immune cells to encounter invasive bacteria in host infection. The classical mechanism by which neutrophils kill bacteria is through phagocytosis and digestion of pathogenic microbes. Microbes are then eradicated by the release of reactive oxygen species and cytotoxic granule proteins inside the resulting phagosome. For many years this was considered the principal mechanism by which neutrophils controlled the growth of pathogenic bacteria.
Recent reports suggest that neutrophils have a wider repertoire of weapons against bacteria than previously appreciated. Neutrophils have been demonstrated to arrest the growth of pathogenic bacteria by the production of extracellular traps (NETs) resulting from the release of nuclear DNA together with antimicrobial proteins.1,2 The discovery of NETs has led to the notion that neutrophils are able to exert control over extracellular bacterial growth in addition to intracellular killing of bacteria. This has led to a paradigm shift in the role of neutrophils maintaining immunity.
In this issue of Blood, Timár and colleagues provide fascinating evidence for another immunologic mechanism in neutrophils that is directed at controlling the growth of bacteria.3 These are extracellular microvesicles, also known as microparticles or ectosomes, released by neutrophils in response to opsonized Staphylococcus aureus or Escherichia coli. Neutrophils are known to generate microvesicles spontaneously and in response to triggers such as f-Met-Leu-Phe, tumor necrosis factor, lipopolysaccharides, opsonized yeast and bacteria, and others.4,5 Previous reports have shown that neutrophil microvesicles affect platelets, endothelial cells, and macrophages.6-8 The report by Timár et al shows for the first time that neutrophil microvesicles also possess antibacterial activity. But their antibacterial activity depends on what triggers their formation.
In the paper by Timár and colleagues, microvesicles from human peripheral blood neutrophils were segregated into 3 different categories based on the activation status of the neutrophils from which they were derived: s-MV (for spontaneous), p-MV (for phorbol ester–stimulated), and b-MV (for bacterially induced microvesicles). Analysis of the protein content, size, and morphology of MV revealed that these were present in 2 sizes (∼ 100 and ∼ 500 nm, most of which were the larger size) and contain different types of proteins depending on the trigger used to evoke their production.
Each of the MV subpopulations possessed different antibacterial effects. While s-MV had no discernible antibacterial properties, p-MV were moderately effective, and b-MV were most potent at preventing bacterial growth. Intriguingly, the bacteriostatic effects of b-MV were evident regardless of whether bacteria were opsonized or not, even though opsonization of bacteria was required to activate b-MV production from neutrophils in the first place. Proteomic analysis of the MV subpopulations revealed that antibacterial proteins (myeloperoxidase, lactoferrin) were enriched in b-MV, which may be related to their differential effects on bacterial growth. Surface markers for b-MV included the integrins CD11b and CD18, which were necessary for their antibacterial properties (see figure).
Activation of neutrophils by opsonized bacteria (S aureus or E coli, at left) causes the release of bacterially induced microvesicles (b-MV; ∼ 500 nm in diameter). After their release, b-MV can aggregate both nonopsonized and opsonized bacteria, leading to reduced bacterial growth. Released b-MV contain antibacterial proteins myeloperoxidase (MPO) and lactoferrin, and express integrins CD11b and CD18 on their surfaces. Integrins are required for b-MV–mediated bacterial aggregation. This is proposed to be a novel bacteriostatic mechanism for host defense against pathogenic bacteria. Indeed, aggregates of b-MV and bacteria can be detected in the sera of bacteremic patients. Professional illustration by Alice Y. Chen.
Activation of neutrophils by opsonized bacteria (S aureus or E coli, at left) causes the release of bacterially induced microvesicles (b-MV; ∼ 500 nm in diameter). After their release, b-MV can aggregate both nonopsonized and opsonized bacteria, leading to reduced bacterial growth. Released b-MV contain antibacterial proteins myeloperoxidase (MPO) and lactoferrin, and express integrins CD11b and CD18 on their surfaces. Integrins are required for b-MV–mediated bacterial aggregation. This is proposed to be a novel bacteriostatic mechanism for host defense against pathogenic bacteria. Indeed, aggregates of b-MV and bacteria can be detected in the sera of bacteremic patients. Professional illustration by Alice Y. Chen.
A mechanism for the antibacterial properties of b-MV was proposed in which b-MV attach to bacteria via integrins, leading to the formation of aggregates of b-MV and bacteria. Aggregation of bacteria with b-MV did not have a bactericidal effect, but instead prevented their growth, and was therefore bacteriostatic. This antibacterial effect was reduced by treatment of b-MV with water or saponin, and was dependent on Ca2+, energy (glucose), and actin cytoskeletal remodeling, among other processes. The bacteriostatic effect of b-MV was not altered by inhibition of NADPH oxidase, suggesting that superoxide is not required for bacterial aggregation.
The investigators went further to demonstrate that neutrophil-derived MV (CD11b+CD177+) were routinely detected in the serum of healthy donors, and increased 5- to 6-fold in patients with documented S aureus bacteremia. Large CD11b+ aggregates of bacteria and MV were found in blood serum from bacteremic patients, which were rarely detected in healthy individuals.
The effects of MV on bacteria are distinct from those of NETs, which is supported by several comparisons. The formation of NETs is a lengthier process (2-4 hours) while maximum b-MV formation occurred within 20 minutes. The production of NETs is dependent on reactive oxygen species, whereas the formation and activity of b-MV were independent of respiratory burst. NETs do not require energy or other cellular structures, while b-MV were dependent on an intact vesicular structure, cytoskeletal remodeling, and an adequate supply of glucose.
In summary, these findings put forward a novel and important immunologic role for extracellular neutrophil MV in trapping and arresting bacteria. An important aspect of these findings is that neutrophil MV are freely mobile in systemic circulation, with the capacity to attach to bacteria in blood or tissues—even those that may have escaped opsonization. Further studies are anticipated that will investigate the mechanisms underlying b-MV release from neutrophils, which remain unknown.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■