Key Points
Administration of donor-specific regulatory T cells prevents chronic rejection of BM and skin allografts in the mouse.
Injected regulatory T cells induce the emergence of host regulatory T cells with similar specificity thus ensuring persistence of tolerance.
Abstract
Despite the use of immunosuppressive drugs, chronic allograft rejection remains a major hurdle in transplantation medicine. Induction of specific immunologic tolerance to antigens expressed by the graft would avoid its eventual functional loss and the severe side effects of paralyzing the immune system. We previously showed that donor-specific regulatory T-lymphocytes prevent rejection of fully allogeneic bone marrow (BM) grafts in mice. Thus generated hematopoietic chimeras then accepted skin and heart allografts of the same donor. We noticed that injected regulatory T-cells (Tregs) disappeared with time and investigated the mechanisms involved in the nevertheless long-term persistence of allograft tolerance. Using Tregs that can be depleted in vivo with diphtheria toxin, we show that injected cells are required for induction but not for maintenance of tolerance to BM allografts. We observed progressive deletion of donor-specific T-lymphocytes, accounting at least in part for maintenance of tolerance. Toxin-induced depletion of administered as well as host Tregs did not affect hematopoietic chimerism but it led to rapid loss of skin allografts. Therefore, our data show that newly generated host Tregs can prevent chronic allograft rejection. Long-lasting tolerance to allografts is thus achieved.
Introduction
Transplantation of allogeneic tissue and organs is severely hindered by the vigorous reaction of the host’s immune system to the foreign graft. Unless inhibited by immunosuppressive drugs, this alloreactivity leads to rapid rejection. However, these medicaments do not prevent chronic allograft rejection. Moreover, the induced paralysis of the immune system explains the increased incidence of cancer and opportunistic infections. The lifelong use of drugs is also associated with direct toxic side effects. Means to prevent chronic allograft rejection should therefore be developed.1,2
Despite the random nature of the somatic rearrangements that determine the antigen-specificity of developing T lymphocytes, these cells normally do not attack self-tissues. Several mechanisms are involved in this self-tolerance.3,4 For example, developing autospecific T cells die upon recognition of their peptide/major histocompatibility complex (MHC) ligand expressed by thymic dendritic cells. Indeed, the T-cell repertoire developing in mice in which thymic dendritic cells do not express MHC molecules is strongly autoreactive in vitro and in vivo.5-8 Some autospecific T cells nevertheless escape to the periphery.9,10 These cells are kept under control by regulatory T-cell (Treg)-populations. The most extensively studied Treg express the co-receptor CD4 and Foxp3. Patients and mice carrying mutations in the gene encoding this forkhead/winged helix transcription factor rapidly develop a lethal autoimmune syndrome, best illustrating the central role of Treg in the control of autospecific lymphocytes.11-13 Self-tolerance is therefore maintained by at least 2 important mechanisms: deletion and regulation. Induction of genuine tolerance to donor antigens, presumably also needs these 2 mechanisms.
Seminal work by Billingham et al14 showed that congenital hematopoietic chimerism strongly favors acceptance of skin grafts of the same donor. Deletion of autospecific T cells presumably played a major role in this phenomenon. However, unless chimerism induced Treg, which has never been shown, it appears unlikely that it will lead to full tolerance to the skin grafts. Indeed, despite the substantial delay in acute rejection, chronic rejection is not prevented by hematopoietic chimerism (see review by Pasquet et al15 ). We and others, therefore, assessed the capacity of Treg to induce long-lasting transplantation tolerance in the mouse. Donor-specific Treg were administered to sublethally irradiated host mice at the time of bone marrow (BM) transplantation. Thus, the hosts accepted the fully allogeneic BM grafts.16,17 When these hematopoietic chimeras were grafted with skin or heart from the same donor, transplants survived for at least 100 days and chronic rejection was fully prevented. The combination of the induction of hematopoietic chimerism and the administration of Treg of appropriate specificity, therefore, appeared to ensure full tolerance to the grafts.18 Separately, these 2 approaches also strongly favor acceptance of allografts,19,20 but best results were obtained by associating them.
Whereas, in absence of rejection, hematopoietic chimerism is maintained by self-renewing stem cells, little is known about the life expectancy of administered Treg. When injected into mutant animals in which production of Treg is impaired, Treg can persist over prolonged periods.21 In our earlier work we showed that injected Treg survived substantially longer in the presence of hematopoietic cells for which they were specific than in their absence.18 However, 9 weeks after injection, their representation in the blood had dropped substantially and it was unclear if these cells would be home to tissues or entirely disappear. Therefore, it was important to assess if persistence of administered Treg is required for long-term maintenance of tolerance to the graft. Here we address this issue by first quantifying the persistence of injected Treg in different tissues. We then actively eliminated these cells in vivo and monitored survival of BM and skin allografts. Given we observed that injected Treg become dispensable for persistent tolerance to the grafts, we also investigated mechanisms involved in the long-term prevention of chronic rejection.
Methods
Mice
Mice were purchased from the Centre de Recherche et d’Elevage Janvier (Le Genest St. Isle, France). C57BL/6 (B6) Thy1.1 and DEREG mice22 were bred in our animal facility. All experiments involving animals were performed in compliance with relevant laws (authorization #31 09 555 45), and institutional guidelines were approved by the ethics committee (MP/01/40/11/06).
Antibodies
Anti (α)H-2Kb-FITC or -biotin (AF6-88.5); αH-2Kd-PE (SF1-1.1), αH-2Kk-PE(36-7-5); αCD4-Pacific Blue (RM4-5); αCD8-Pacific Blue (53.6.7); αCD3-Pacific Orange (500A2); αCD25-APC (PC61); αVβ3, Vβ4, Vβ5, Vβ6, and Vβ14-biotin (BD Biosciences, Heidelberg, Germany); αNK-FITC (DX5); αCD4-PECy7 (GK1.5); αThy1.1-APC (HiS51) and αB220-APC (RA3-6B2); αCD25-PE (PC61.5); purified F4/80 (BM8) and αFoxp3 mAb (FJK-16s); Streptavidin-eFluor710 (eBioscience, San Diego, CA). Hybridoma supernatants of αFcγRII/III (2.4G2), αCD8 (53.6.7), αMHC class II (M5/114.15.2), and αThy1.2 (AT83).
Purification and in vitro culture of CD4+CD25+ Treg
CD4+CD25+ splenic T cells were purified and cultured with γ-irradiated splenocytes as previously described.16 Cell purity was checked by flow cytometry on a FACSCalibur (BD Bioscience, San Jose, CA) and was routinely ≥95%.
BM and skin transplantation
Skins and T-cell depleted BM were transplanted as previously described.18 Skins were considered rejected if ≥70% of the surface was necrotic.
In vivo depletion of Treg
Chimeras were intraperitoneally injected with 1.5 μg Diphtheria Toxin (Sigma-Aldrich, St. Quentin Fallavier, France) diluted in PBS on 2 consecutive days.
Flow cytometry
Cell suspensions were stained using saturating concentrations of antibody. Acquisition was performed on an LSRII or a FORTESSA cytometer (Becton Dickinson, Franklin Lakes, NJ) and data were analyzed using FlowJo software (Tree Star, Ashland OR).
Histological analysis
Skin biopsies were fixed in 10% buffered formalin and embedded in paraffin. Sections were stained as indicated. F4/80 and αFoxp3 antibody binding was revealed using αrat Ig-biotin (Dako, Glostrup, Denmark) and streptavidine-HRP (Vector Laboratories, Berlingame, CA).
Statistical analysis
Statistical significance was determined by using Student t test with Bonferroni correction.
Results
Administered Treg wane away with time
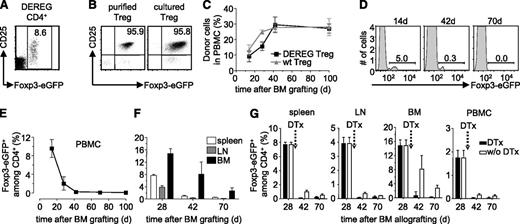
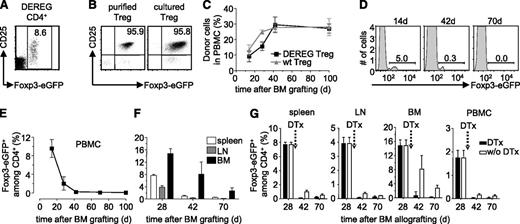
We previously showed that donor-specific Treg durably prevented rejection of semi and fully allogeneic BM allografts.16 To assess if administered Treg need to persist to maintain tolerance to the donor, we set up an experimental system in which Treg can be deleted in vivo. DEREG mice carry a transgenic bacterial artificial chromosome construct in which the Foxp3-promoter drives expression of the diphtheria toxin receptor and enhanced green fluorescent protein (eGFP).22 Practically all CD4+CD25high Treg in C57BL/6 (B6) DEREG mice express eGFP (Figure 1A). We isolated CD4+CD25high cells from spleens of transgenic animals (Figure 1B, left panel) and cultured them with (donor x host)F1 (ie, [CBA × B6]F1) mice-derived–irradiated splenocytes as stimulators in presence of high levels of interleukin-2, required for the proliferation of Treg. Semi-allogeneic F1 splenocytes were used because they present alloantigens via the direct and indirect pathways. This allows for the expansion of Treg specific for indirectly presented alloantigens required for prevention of chronic rejection of skin and heart allografts.18 After a 2-week culture period, Foxp3-expression, as assessed by eGFP fluorescence, was maintained (Figure 1B, right panel). We then used these donor-specific Treg to protect a BM allograft. Recipient B6 mice were sublethally irradiated (5 Gy γ), reconstituted with T cell-depleted CBA BM, and simultaneously injected with the in vitro cultured DEREG Treg. Thus, the fully allogeneic BM graft was protected from rejection by the recipient’s immune system for more than 100 days (Figure 1C). Wild-type (WT) and DEREG Treg were equally efficient in inducing tolerance to the CBA BM allograft (ie, similar levels of hematopoietic chimerism were observed). In protected mice administered DEREG Treg were clearly visible among blood CD4+ T lymphocytes (Figure 1D). However, DEREG Treg waned away with time and after 70 days they were no longer visible in peripheral blood mononuclear cells (PBMC) of the chimeras (Figure 1D-E). At this time, also in spleen, lymph nodes, and BM, administered Treg had almost completely disappeared (Figure 1F).
Administered Treg wane away with time. (A) DEREG splenocytes were analyzed by flow cytometry. CD25 vs Foxp3-eGFP expression on electronically gated CD4+ cells. (B) Expression of CD25 vs Foxp3-eGFP by purified DEREG Treg was analyzed before (left) and after (right) in vitro culture with donor-type irradiated splenocytes as stimulators. Dot plots are representative of 4 experiments. Numbers indicate percentages within indicated electronic gates. Sublethally irradiated B6 WT mice were grafted with CBA BM under cover of 2.106 DEREG Treg in vitro cultured with (CBA × B6)F1 antigen-presenting cells. (C) Hematopoietic chimerism in PBMC and percentage of Foxp3-eGFP+ cells among CD4+ T lymphocytes in (D-E) blood and (F) spleen, lymph node (LN), and BM was assessed at the indicated time points. Indicated are mean values ± SD (n ≥ 3, 4 independent experiments). (G) Four weeks after BM grafting, chimeric mice were left untreated (white bars) or received (black bars) 2 consecutive injections of diphtheria toxin (DTx). Percentage of Foxp3-eGFP+ cells among CD4+ T lymphocytes was analyzed in spleen, lymph node (LN), BM, and PBMC. Indicated are mean values ± SD (n = 5, 2 independent experiments).
Administered Treg wane away with time. (A) DEREG splenocytes were analyzed by flow cytometry. CD25 vs Foxp3-eGFP expression on electronically gated CD4+ cells. (B) Expression of CD25 vs Foxp3-eGFP by purified DEREG Treg was analyzed before (left) and after (right) in vitro culture with donor-type irradiated splenocytes as stimulators. Dot plots are representative of 4 experiments. Numbers indicate percentages within indicated electronic gates. Sublethally irradiated B6 WT mice were grafted with CBA BM under cover of 2.106 DEREG Treg in vitro cultured with (CBA × B6)F1 antigen-presenting cells. (C) Hematopoietic chimerism in PBMC and percentage of Foxp3-eGFP+ cells among CD4+ T lymphocytes in (D-E) blood and (F) spleen, lymph node (LN), and BM was assessed at the indicated time points. Indicated are mean values ± SD (n ≥ 3, 4 independent experiments). (G) Four weeks after BM grafting, chimeric mice were left untreated (white bars) or received (black bars) 2 consecutive injections of diphtheria toxin (DTx). Percentage of Foxp3-eGFP+ cells among CD4+ T lymphocytes was analyzed in spleen, lymph node (LN), BM, and PBMC. Indicated are mean values ± SD (n = 5, 2 independent experiments).
Diphtheria toxin-mediated in vivo depletion of Treg
These data suggested that persistence of injected donor-specific Treg is not required for maintenance of tolerance to the BM allograft. However, at 70 days after injection, a very small number of DEREG Treg still remained detectable, especially in BM (Figure 1F). Therefore, we cannot formally conclude that administered Treg no longer play a role in protection of the graft. To directly address this question, next we depleted administered DEREG Treg by injection of diphtheria toxin. Preconditioned WT B6 mice were grafted with CBA BM and simultaneously injected with in vitro cultured donor-specific WT or DEREG Treg. Diphtheria toxin was injected 4 weeks after BM transplantation. Two and 6 weeks after toxin injection, administered DEREG (ie, Foxp3-eGFP expressing) Treg were no longer detected in the spleen, BM, lymph nodes, and PBMC (Figure 1G). These results were confirmed using an experimental setup in which administered DEREG Treg could be traced using the allelic marker Thy1.2. Again, upon administration of diphtheria toxin, we observed a very efficient depletion of administered Treg (supplemental Figure 1). Therefore, the injection of diphtheria toxin very efficiently depleted administered DEREG Treg.
Administered Treg are required for induction, but not maintenance, of tolerance to MHC mismatched BM allografts
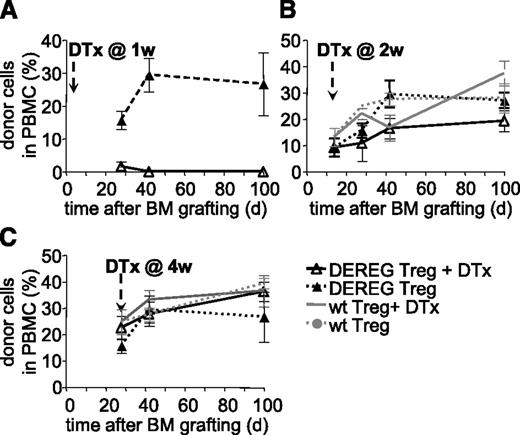
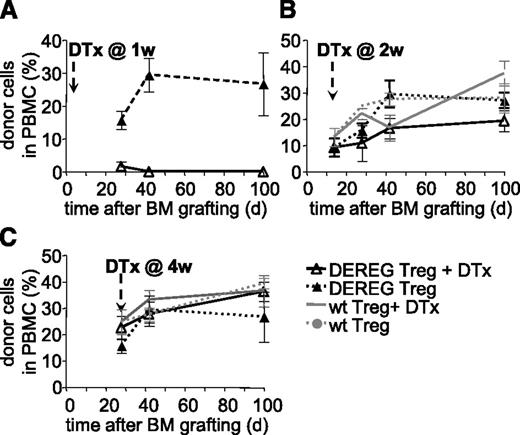
We next assessed if persistence of administered Treg is required for maintenance of tolerance. WT B6 mice were preconditioned, grafted with CBA BM, and simultaneously injected with donor-specific DEREG Treg. Mice injected with diphtheria toxin at 1 week after BM grafting did not develop hematopoietic chimerism (Figure 2A). This effect was due neither to a nonspecific effect of the toxin (mice injected with WT Treg and treated with toxin developed chimerism, not shown) nor to an intrinsic defect of DEREG Treg (mice injected with DEREG Treg but not treated with toxin developed chimerism) (Figure 2A). Therefore, administered Treg are required during the initial phases of tolerance induction. These data also confirmed that injection of diphtheria toxin efficiently depleted injected DEREG Treg.
Administered Treg are dispensable for persistence of tolerance to BM allografts. Sublethally irradiated B6 WT mice were grafted with CBA BM coinjected with 2.106 DEREG or WT Treg in vitro cultured with (CBA × B6)F1 antigen-presenting cells. Treg depletion by diphtheria toxin (DTx) injection was performed 1 (A), 2 (B), or 4 (C) weeks after transplantation. Allograft survival was followed up to 100 days. Shown is the percentage of donor cells among PBMC, as assessed by flow cytometry, mean values ± SD (n ≥ 5, 4 independent experiments).
Administered Treg are dispensable for persistence of tolerance to BM allografts. Sublethally irradiated B6 WT mice were grafted with CBA BM coinjected with 2.106 DEREG or WT Treg in vitro cultured with (CBA × B6)F1 antigen-presenting cells. Treg depletion by diphtheria toxin (DTx) injection was performed 1 (A), 2 (B), or 4 (C) weeks after transplantation. Allograft survival was followed up to 100 days. Shown is the percentage of donor cells among PBMC, as assessed by flow cytometry, mean values ± SD (n ≥ 5, 4 independent experiments).
Next we performed similar experiments but injected diphtheria toxin at 2 or 4 weeks after BM grafting and Treg injection. In both cases, hematopoietic chimerism developed normally and persisted for at least 100 days (Figure 2B-C). No significant difference in chimerism was observed between the groups injected with WT or DEREG Treg, treated or not with diphtheria toxin. Therefore, it appears that 2 weeks after transplantation, administered Treg are no longer required for maintenance of tolerance to the BM allograft.
After depletion of administered Treg, tolerance to BM allografts appears maintained by deletion of donor-specific T cells, not by host Treg
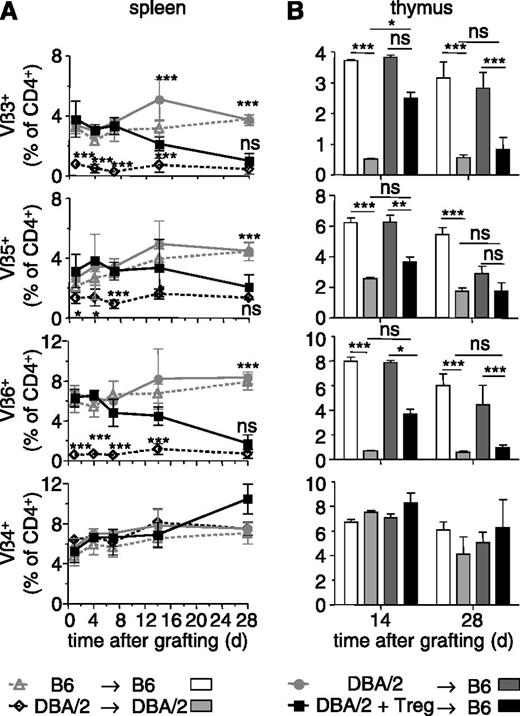
We then assessed which mechanisms were involved in the tolerance to BM allografts that persisted despite depletion of administered Treg. First, we wished to investigate what happens to donor-specific T lymphocytes. To track such cells, we chose to use DBA/2 donors. These mice present a large number of mammary tumor virus-encoded superantigens by their I-Ed MHC class II molecules. T cells expressing several T cell receptor (TCR) Vβ segments, among which Vβ3, Vβ5, Vβ6, but not Vβ4 and Vβ14, recognize these superantigens.23 Preconditioned B6 (Thy1.2) mice were grafted with T cell-depleted DBA/2 BM and injected with donor-specific B6 Thy1.1 Treg. The TCR Vβ repertoire of host (ie, Thy1.2+ H-2Kb+) T cells was analyzed in spleen and thymus of these chimeras. In sublethally irradiated B6 mice that had received a DBA/2 BM in absence of Treg, and therefore that rapidly rejected the allograft, the TCR Vβ repertoire in the spleen did not change much during the 28-day follow-up period and was similar to that observed in B6 → B6 control chimeras (Figure 3A). In contrast, in B6 mice that had received a DBA/2 BM allograft and donor-specific Treg, and that accepted the allograft and became chimeric, superantigen-specific TCR Vβ3, 5, and 6-expressing CD4+ T cells gradually disappeared and reached levels found in DBA/2 → DBA/2 control chimeras by 28 days (Figure 3A). Similar results were obtained for the thymus (Figure 3B). The representation of CD4+ T cells expressing Vβ4 (Figure 3A-B) and Vβ14 (not shown), which do not recognize donor-encoded superantigens, was similar in spleen and thymus of all 4 types of chimeras studied. Peripheral and central deletion of donor-specific CD4+ T cells, therefore, was gradually established in the chimeras and most surely contributed to the maintenance of tolerance after depletion of administered Treg.
BM allografts induce deletion of donor-specific T cells. Sublethally irradiated B6 mice were grafted with DBA/2 BM coinjected (or not) with 2.106 Thy1.1+ Treg in vitro cultured in presence of DBA/2 antigen-presenting cells. Control syngeneic chimeras were realized by reconstituting sublethally irradiated B6 and DBA/2 mice with syngeneic BM. TCR-Vβ3, Vβ5, Vβ6-expressing CD4+ T cells are specific for superantigens presented in DBA/2 (but not B6) mice. The percentage of Vβ+ cells among host CD4+ T cells was assessed by flow cytometry in the spleen (A) and thymus (B) at the indicated time points after reconstitution. Vβ4-expressing T cells do not recognize superantigens in DBA/2 mice. Indicated are mean values ± SD (n ≥ 4, 4 independent experiments). *P < .05, **P < .01, ***P < .001. ns, not significant, Student t test.
BM allografts induce deletion of donor-specific T cells. Sublethally irradiated B6 mice were grafted with DBA/2 BM coinjected (or not) with 2.106 Thy1.1+ Treg in vitro cultured in presence of DBA/2 antigen-presenting cells. Control syngeneic chimeras were realized by reconstituting sublethally irradiated B6 and DBA/2 mice with syngeneic BM. TCR-Vβ3, Vβ5, Vβ6-expressing CD4+ T cells are specific for superantigens presented in DBA/2 (but not B6) mice. The percentage of Vβ+ cells among host CD4+ T cells was assessed by flow cytometry in the spleen (A) and thymus (B) at the indicated time points after reconstitution. Vβ4-expressing T cells do not recognize superantigens in DBA/2 mice. Indicated are mean values ± SD (n ≥ 4, 4 independent experiments). *P < .05, **P < .01, ***P < .001. ns, not significant, Student t test.
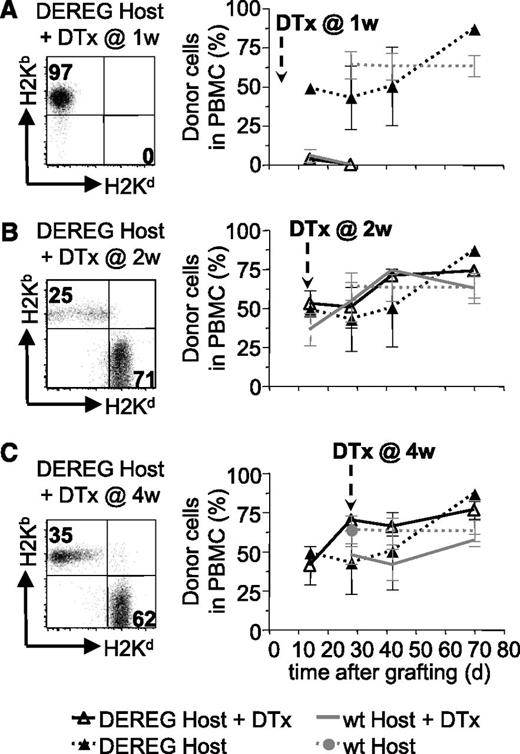
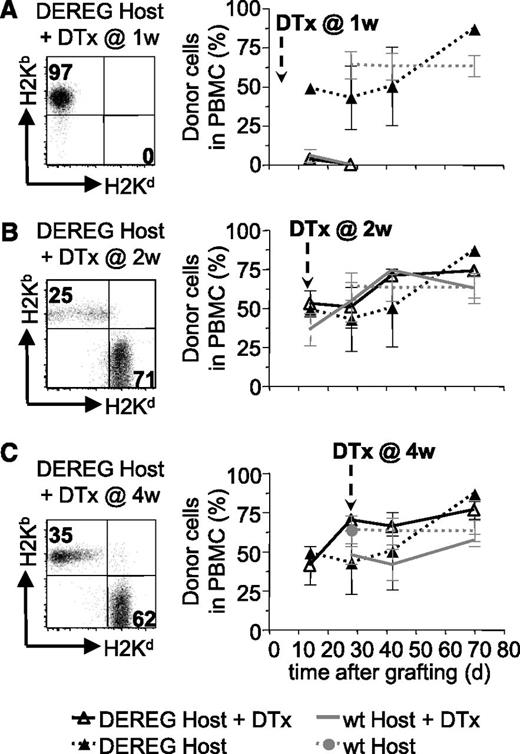
We also investigated if host Treg played a role in the maintenance of tolerance to the BM allograft after depletion of administered Treg. To this end, we preconditioned B6 DEREG hosts and grafted them with DBA/2 BM coadministered with donor-specific DEREG Treg. Injection of diphtheria toxin into these mice would deplete administered, as well as host, Treg. Rapid reconstitution of newly developing Treg avoids autoimmune pathology.22 As expected, depletion of administered plus host Treg (but not host Treg only, supplemental Figure 2) at 1 week after reconstitution led to rejection of the BM allograft (Figure 4A). Injection of diphtheria toxin at 2 or 4 weeks did not lead to rejection of the allografts (Figure 4B-C). These results strongly suggest that host Treg are not involved in the maintenance of tolerance to the BM allografts after depletion of administered Treg.
Host Treg are not required for persistence of BM tolerance. Sublethally irradiated DEREG or WT mice were grafted with DBA/2 BM under cover of 2.106 DEREG Treg in vitro cultured in presence of (B6 × DBA/2)F1 antigen-presenting cells. Treg depletion, by diphtheria toxin (DTx) injection, was performed 1 (A), 2 (B), or 4 (C) weeks after transplantation. BM allograft survival was assessed by flow cytometry (illustrated in left panels) up to 10 weeks after grafting by analyzing the percentage of allogeneic cells in PBMC (quantitative assessment in right panels). Indicated are mean values ± SD (n ≥ 4, 4 independent experiments).
Host Treg are not required for persistence of BM tolerance. Sublethally irradiated DEREG or WT mice were grafted with DBA/2 BM under cover of 2.106 DEREG Treg in vitro cultured in presence of (B6 × DBA/2)F1 antigen-presenting cells. Treg depletion, by diphtheria toxin (DTx) injection, was performed 1 (A), 2 (B), or 4 (C) weeks after transplantation. BM allograft survival was assessed by flow cytometry (illustrated in left panels) up to 10 weeks after grafting by analyzing the percentage of allogeneic cells in PBMC (quantitative assessment in right panels). Indicated are mean values ± SD (n ≥ 4, 4 independent experiments).
Persistence of administered Treg is not required for tolerance to skin allografts
We next investigated if persistence of injected Treg was required for maintenance of tolerance to fully allogeneic skin grafts. WT B6 hosts were preconditioned and reconstituted with DBA/2 BM. DEREG Treg in vitro expanded using (donor × host)F1 (ie, [DBA/2 × B6]F1) antigen-presenting cells, and therefore specific for directly and indirectly presented alloantigens, were injected with the BM allograft. Thus generated hematopoietic chimeras were grafted with skin from the same donor strain 6 weeks after BM reconstitution. Grafts were monitored for macroscopic signs of rejection and, if no rejection was observed at the end of the 100-day observation period, they were taken for histologic analysis of chronic rejection.
In a first experiment, we depleted administered DEREG Treg at 10 weeks after BM reconstitution (ie, at 4 weeks after skin grafting). During the 100-day observation period, we did not observe any macroscopic signs of rejection of the skins (Figure 5A-B, upper panels). Histologic analysis of the grafts taken at 100 days after skin grafting did not reveal any signs of (chronic) rejection either (Figure 5C, upper panels). Most importantly, we did not see infiltration by mononuclear cells (eg, macrophages) or by eosinophils, previously observed in chronic rejection of skin allografts.18,24 Therefore, it appears that once the skin allograft is in place, administered Treg are dispensable for the maintenance of tolerance.
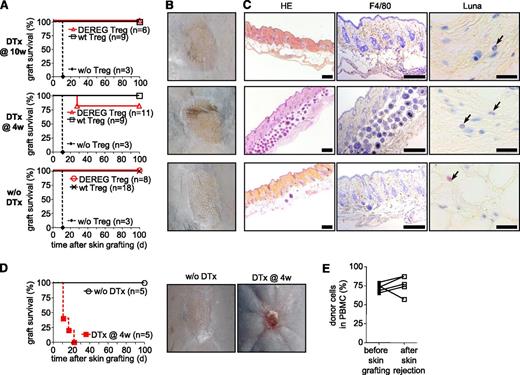
Upon depletion of administered Treg, host Treg are required for acceptance of skin allografts. (A-C) Sublethally irradiated WT B6 mice were grafted with DBA/2 BM under cover of 2.106 DEREG or WT Treg in vitro cultured in presence of (B6 × DBA/2)F1 antigen-presenting cells. Mice were left untreated or received diphtheria toxin (DTx) 4 or 10 weeks after reconstitution, as indicated. Skin allografts were placed 6 weeks after BM grafting. Control mice were not injected with Treg at the time of BM transplantation. (A) Skin allograft survival was monitored daily by assessment of macroscopic signs of rejection. Graft-survival curves for indicated conditions (4 independent experiments). Representative macroscopic (B) and histologic (C) features of skins 100 days after transplantation. (HE, hematoxylin and eosin; F4/80, immunohistocheministry with antibody to the macrophage marker F4/80; Luna, Luna’s eosinophil stain) Scales represent 200 µm for HE and F4/80 staining and 20 µm for Luna staining. (D-E) Sublethally irradiated DEREG mice were grafted with DBA/2 BM under cover of DEREG Treg in vitro cultured in presence of (B6 × DBA/2)F1 stimulator cells. Injected Treg were depleted or not by diphtheria toxin (DTx) injection 4 weeks after BM grafting. DBA/2 Skin transplantation was performed 2 weeks later. Skin allograft survival was monitored daily. (D, left panel) Skin allograft survival during the 100-day observation period. (D, right panels) In toxin-treated mice, rejection was macroscopically clearly visible (2 independent experiments). (E) Chimerism was analyzed in PBMC 2 days before skin transplantation and immediately after rejection in toxin-treated mice.
Upon depletion of administered Treg, host Treg are required for acceptance of skin allografts. (A-C) Sublethally irradiated WT B6 mice were grafted with DBA/2 BM under cover of 2.106 DEREG or WT Treg in vitro cultured in presence of (B6 × DBA/2)F1 antigen-presenting cells. Mice were left untreated or received diphtheria toxin (DTx) 4 or 10 weeks after reconstitution, as indicated. Skin allografts were placed 6 weeks after BM grafting. Control mice were not injected with Treg at the time of BM transplantation. (A) Skin allograft survival was monitored daily by assessment of macroscopic signs of rejection. Graft-survival curves for indicated conditions (4 independent experiments). Representative macroscopic (B) and histologic (C) features of skins 100 days after transplantation. (HE, hematoxylin and eosin; F4/80, immunohistocheministry with antibody to the macrophage marker F4/80; Luna, Luna’s eosinophil stain) Scales represent 200 µm for HE and F4/80 staining and 20 µm for Luna staining. (D-E) Sublethally irradiated DEREG mice were grafted with DBA/2 BM under cover of DEREG Treg in vitro cultured in presence of (B6 × DBA/2)F1 stimulator cells. Injected Treg were depleted or not by diphtheria toxin (DTx) injection 4 weeks after BM grafting. DBA/2 Skin transplantation was performed 2 weeks later. Skin allograft survival was monitored daily. (D, left panel) Skin allograft survival during the 100-day observation period. (D, right panels) In toxin-treated mice, rejection was macroscopically clearly visible (2 independent experiments). (E) Chimerism was analyzed in PBMC 2 days before skin transplantation and immediately after rejection in toxin-treated mice.
We next investigated if administered Treg can be depleted before skin grafting. Diphtheria toxin was injected 4 weeks after BM grafting. Skin from the same donor strain was transplanted 2 weeks later. During the 100-day observation period, no macroscopic signs of rejection were observed in 9 of 11 thus treated mice (Figure 5A-B, middle panels) and histologic analysis of the grafts did not reveal any signs of chronic rejection (Figure 5C, middle panels).
Host Treg are required for maintenance of skin allograft tolerance
Central and peripheral deletion, as shown in Figure 3, may be involved in the continued protection from chronic rejection of the skin grafts after depletion of injected Treg. We wished to investigate the possibility that host Treg also played a role. B6 DEREG mice were preconditioned and grafted with fully allogeneic DBA/2 BM under cover of DEREG Treg specific for directly and indirectly presented alloantigens. Four weeks later, administered and host Foxp3+ Treg were depleted by injection of the diphtheria toxin. Treg depletion did not change the hematopoietic chimerism (Figure 4C). Two weeks later, DBA/2 skins were transplanted on these chimeras. Grafts were monitored for signs of rejection during a 100-day observation period. Depletion of administered and host Treg in DEREG hosts led to rapid rejection, which was clearly, macroscopically visible (Figure 5D right panel). All grafts on DEREG hosts (5 of 5 performed) were rejected by 23 days (Figure 5D, left panel). Importantly, in all these mice the BM allograft remained in place (Figure 5E), and the loss of tolerance was therefore specific for the skin grafts. These data show that host Treg were involved in the continued prevention of skin rejection after depletion of injected Treg. They also demonstrate that DEREG host Treg are effectively depleted by injection of diphtheria toxin in our experimental model and thus confirm that host Treg are not required for maintenance of the allogeneic BM graft (Figure 4). Combined, our data demonstrate that administered Treg had transmitted their role in the protection of fully allogeneic skin grafts to host Treg that became sufficient for the prevention of chronic rejection.
Host Treg acquire graft-protective capacities similar to that of administered Treg
We previously showed that Treg specific for directly presented donor antigens (ie, presented by donor MHC molecules), protected skin, and heart allografts from acute but not chronic rejection. By contrast, Treg specific for indirectly presented donor antigens (ie, presented by host MHC molecules) fully prevented chronic rejection.18 We wished to investigate if the capacity to protect from acute vs chronic rejection is transmitted from administered to host Treg. When Treg were specific for directly and indirectly presented donor antigens, chronic rejection of the skin allografts was prevented, even after depletion of administered Treg (Figure 5). We then performed similar experiments but used Treg specific for directly presented alloantigens only. These cells protected skin grafts from acute rejection (supplemental Figure 3A) but failed to prevent chronic rejection, thus confirming our previously reported results (supplemental Figure 3B-C). When administered DEREG Treg specific for directly presented donor antigens were depleted at 4 weeks post-BM grafting, subsequently transplanted skin grafts did not undergo acute rejection but clear signs of chronic rejection were observed (supplemental Figure 3A-C). It therefore appears that host Treg that protected skin grafts from rejection had acquired the capacity to protect from acute and/or chronic rejection similar to that of administered Treg. In both cases some sparse host Treg could be found inside the skin grafts, suggesting that they may act locally (supplemental Figure 4).
Discussion
Previously published data from our laboratory and those of others demonstrated the potential of Treg to induce tolerance to BM, skin, and heart allografts in mice.17-19,25 Since injected Treg vanish with time, it was important to study if tolerance would persist in their absence. Here we show that injected Treg are essential for the induction of tolerance to BM allografts. By contrast, once tolerance is established, its maintenance no longer requires injected Treg. Mice that had accepted the BM allograft were also tolerant to subsequent skin grafts from the same donor strain. Injected Treg were not required for acceptance of the skin grafts. However, when injected Treg were depleted, host Treg became critical for skin graft acceptance. Therefore, it appears that the Treg-mediated immunotherapy against skin graft rejection leads to development of host Treg-mediated tolerance. This mechanism will ensure long-term tolerance to allografts.
Administration of in vitro expanded Treg specific for directly presented donor antigens leads to permanent protection of BM allografts in mice.16 Treg-mediated immunotherapy may therefore be envisaged for clinical protocols in which administration of donor stem cells or BM is used to promote tolerance to kidney allografts, for example. Treg administration may allow for induction of stable hematopoietic chimerism, not detected in the clinical protocols, and thus further improve results.15,26,27 Importantly, our data demonstrate that once the administered Treg had induced tolerance, its maintenance no longer required the presence of these cells. The long-term persistence of donor chimerism also indicates that progenitor cells are not rejected. Deletion of donor-specific T cells upon interaction with donor hematopoietic cells, as also shown by others before, probably plays an important role in this persistent, self-sustained, tolerance.28 Depletion of injected and host Treg at 2 weeks did not lead to rejection of the BM allograft, despite the presence of some donor-specific T cells. Therefore, it appears that the latter cells must be anergic29 or kept under control by regulatory cells not expressing Foxp3. Whatever the explanation, host Foxp3-expressing Treg are not involved.
In our experimental approach, skin grafts are placed after hosts accepted BM grafts. The hematopoietic chimerism alone is insufficient for full tolerance to the skin grafts, which is consistent with the literature.15 Here we show that in chimeras the administered Treg are not required for acceptance of skin grafts. However, after depletion of injected Treg, host Treg became critically involved in the prevention of skin allograft rejection. Despite the fact that they rejected the skin grafts, recipients in which administered and host Treg were depleted did not reject the BM allograft. T cells specific for tissue-specific antigens, which apparently are not deleted (or rendered anergic) by hematopoietic cells, therefore, presumably rejected the skin grafts. These data confirm that hematopoietic chimerism is insufficient for maintenance of tolerance to allogeneic skin in this model, and that Treg are required. More importantly, our data demonstrate that injected Treg had transferred their tolerogenic role to host Treg.
Transmission of tolerogenic activity from one T-cell population to another one is known as “infectious tolerance” (see reviews by Kendal and Waldmann30 and Cobbold et al31). Allograft tolerance can be induced by blockade of CD4 and CD8 coreceptors, for example.32,33 Treg from tolerant mice transferred tolerance to a secondary host.34 When the transferred donor T cells were subsequently depleted, tolerance persisted, and it is thought that host Foxp3+ Treg are involved.35 Using a very different experimental system, we directly show here that administered Treg had transferred their tolerogenic activity to host Treg. In this report, “host” Treg are defined as those cells that can be depleted with diphtheria toxin. Even at later time points, donor BM-derived CD4+ T cells represented only a small minority of the total pool of CD4+ T cells in our experimental model (supplemental Figure 5), potentially explaining their manifest incapacity to protect the skin allografts.
Treg specific for directly presented donor-antigens protected skin grafts from acute but not from chronic rejection. This observation is consistent with a role for donor-specific antibody (the generation of which requires indirect recognition of donor antigens) in chronic organ-graft rejection.36 When administered Treg were depleted, the host Treg involved in protection of the skin grafts from acute rejection still failed to prevent chronic rejection. Injection of Treg with indirect donor-specificity protected from chronic rejection. When these Treg were depleted, the host Treg involved in protection of the skin grafts still prevented chronic rejection. These observations strongly suggested that initially administered and emerging host Treg had similar specificities. This conclusion is consistent with data showing that hemagglutinin-specific Treg can induce Treg with the same specificity from responder T cells in vivo.37 We postulate that interaction of administered Treg with host cells presenting donor antigens generates a tolerogenic microenvironment in which locally activated conventional T cells differentiate into Treg. This may occur under influence of TGF-β or depletion of essential amino acids, both known to induce Foxp3-expression.37-40 Formally, we cannot exclude the possibility that host Treg of appropriate specificity may expand or develop in the host thymus under influence of the mixed hematopoietic chimerism. However, hematopoietic chimerism alone fails to prevent chronic rejection, and the latter possibility appears rather unlikely. These issues merit further investigation.
To our knowledge, this is the very first demonstration that Treg can transfer their tolerogenic capacity to other Treg in hematopoietic chimeras in absence of other donor tissue. It strongly suggests the involvement of hematopoietic cells, probably dendritic cells, acting in secondary lymphoid organs. In any case, solid donor tissue is not required.
Finally, in previous reports it was shown that CD4+CD25+Foxp3+ Treg could mediate infectious transplantation tolerance.34,35 This conclusion was based on adoptive transfer experiments, and the potential implication of other regulatory populations was not assessed. By in vivo depleting only Foxp3+ Treg, we abolished development of tolerance to skin grafts. Potential other regulatory populations were clearly insufficient for prevention of skin allograft rejection in our experimental model.
In conclusion, experimental Treg-mediated immunotherapy against acute and chronic rejection of skin allografts does not require persistence of administered Treg. A newly emerging host Treg population fully prevents chronic rejection. This mechanism allows for long-term maintenance of tolerance. Administration of in vitro manipulated Treg for the treatment of graft rejection or graft-versus-host disease for example, should turn out to be an attainable goal,41 and our results indicate that thus induced tolerance should be self-perpetuating.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Sylvie Guerder, Jean-Charles Guéry, and Olivier Joffre for critical reading of the manuscript, and Drs Fatima-Ezzahra L'Faqihi-Olive and Valérie Duplan-Eche (Inserm U1043 flow cytometry facility), the personnel of the Inserm US006 Animalerie Exploration Fonctionnelle/histology facility (Dr Talal Al Saati, Florence Capilla, and Alain Marrot) and the personnel of the Inserm US006 Animalerie Exploration Fonctionnelle/Centre Régional d'Exploration Fonctionnelle et Ressources Expérimentales animal facility, in particular, Maryline Calise, for expert technical assistance.
This work was supported by institutional funds, the Agence Nationale pour la Recherche (ANR-08-BLAN-0187), the Région Midi-Pyrénées (08004389), and the Agence de la Biomédecine (2007).
Authorship
Contribution: L.P., P.R., and J.P.M.v.M. designed experiments; L.P. and J.-Y.D. performed experiments and analyzed results; T.S. contributed transgenic mice; L.P., J.-Y.D., P.R. and J.P.M.v.M. analyzed the data; and L.P., P.R., and J.P.M.v.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joost P. M. van Meerwijk, Inserm U1043, BP 3028, 31024 Toulouse Cedex 3, France; e-mail: joost.van-meerwijk@inserm.fr.