Key Points
The large extracellular domains of the tyrosine phosphatases CD45 and CD148 prevent them from inhibiting T-cell receptor triggering.
These domains are required for optimal segregation from the engaged T-cell receptor, supporting the kinetic-segregation model of triggering.
Abstract
T-cell receptor (TCR) triggering results in a cascade of intracellular tyrosine phosphorylation events that ultimately leads to T-cell activation. It is dependent on changes in the relative activities of membrane-associated tyrosine kinases and phosphatases near the engaged TCR. CD45 and CD148 are transmembrane tyrosine phosphatases with large ectodomains that have activatory and inhibitory effects on TCR triggering. This study investigates whether and how the ectodomains of CD45 and CD148 modulate their inhibitory effect on TCR signaling. Expression in T cells of forms of these phosphatases with truncated ectodomains inhibited TCR triggering. In contrast, when these phosphatases were expressed with large ectodomains, they had no inhibitory effect. Imaging studies revealed that truncation of the ectodomains enhanced colocalization of these phosphatases with ligated TCR at the immunological synapse. Our results suggest that the large ectodomains of CD45 and CD148 modulate their inhibitory effect by enabling their passive, size-based segregation from ligated TCR, supporting the kinetic-segregation model of TCR triggering.
Introduction
T cells are stimulated through the T-cell receptor (TCR) when it binds cognate peptide presented by a major histocompatibility complex molecule (pMHC) on another cell. As a consequence of ligation, immunoreceptor tyrosine-based activation motifs (ITAMs) in the cytoplasmic domains of the TCR/CD3 complex are phosphorylated by lymphocyte-specific protein tyrosine kinase (Lck). These phosphorylated ITAMs recruit ζ-chain-associated protein tyrosine kinase 70 (Zap-70) to the membrane, and Zap-70 phosphorylates substrates such as linker of activated T cells (LAT).1 Despite the extensive research in this field, the mechanism by which the binding of TCR to pMHC leads to phosphorylation of TCR/CD3 ITAMs is still contested, and several models have been proposed.2 One common feature of some of these models is that TCR triggering is initiated by changes in the relative concentrations of membrane tyrosine kinases and phosphatases in the vicinity of ligated TCR.2 The principal membrane tyrosine phosphatases involved in regulating TCR-induced tyrosine phosphorylation are CD45 and CD148.3 The importance of this dynamic equilibrium between kinase and phosphatase activity in TCR triggering was highlighted in studies that use phosphatase inhibitors such as pervanadate.4-6 Treatment of T cells with these inhibitors alone, in the absence of any TCR ligand, was sufficient to induce full activation of TCR signaling pathways, ranging from early events such as phosphorylation of TCR ITAMs, Zap-70, and LAT to late events such as interleukin 2 (IL-2) production.4-6
Several mechanisms have been proposed for perturbation of relative kinase/phosphatase concentrations on TCR engagement.2 One mechanism is colocalization of the CD8 or CD4 coreceptors, which are associated with Lck, with TCR/pMHC complex when coreceptors bind to the pMHC. However, coreceptor binding to pMHC is not essential for, and appears to follow, initial TCR triggering, suggesting that other mechanisms must be involved.7 A second proposed mechanism is the association of engaged TCR with lipid rafts enriched in Lck.8 A third mechanism, proposed by the kinetic-segregation (K-S) model of TCR triggering, is that there is passive (signaling-independent) segregation of CD45 and CD148 from engaged TCR driven by their large ectodomains.9-11 The K-S model postulates that TCR/pMHC interactions take place in small, close-contact zones in which there is a close juxtapositioning of adjacent membranes from the T cell and the pMHC-presenting cell. As a consequence, molecules with large ectodomains, such as CD45 and CD148, will be excluded from the vicinity of the engaged TCR. This will result in an increase in the kinase/phosphatase ratio surrounding the engaged TCR, which will endure as long as the TCR remains bound to the pMHC, leading to increased phosphorylation of TCR ITAMs and other substrates and the propagation of TCR signaling. In support of the K-S model, imaging studies have shown that both CD45 and CD148 are segregated from sites of TCR engagement and triggering.12-15
The K-S model postulates that the ectodomains of CD45 and CD148 have a critical role in TCR triggering because of their large size. Low-resolution electron microscopy studies16,17 have estimated the CD45 ectodomain size as ranging from ∼28 to ∼50 nm, depending on the splice isoform3 (Discussion). Although the structure of the CD148 ectodomain has not been determined, the fact that it has 8 to 10 highly N-glycosylated fibronectin domains that are ∼4 nm long (predicted length, 32-40 nm) and an ∼80-aa mucin-like N-terminal region (predicted length, ∼15 nm) suggests an overall length of ∼47 to 55 nm.10 A key prediction of the K-S model is that truncation of the CD148 and CD45 ectodomains will abrogate TCR triggering because of the lack of segregation of these molecules from areas of TCR/pMHC engagement. Previous studies attempting to investigate this have been inconclusive.13,18 Reconstitution of CD45-deficient cells with forms of CD45 with ectodomains of different lengths revealed that a large ectodomain was required to reconstitute TCR triggering.13,18 However, because CD45 has a primarily activatory effect on TCR triggering in this system, it was not possible to evaluate the role of the ectodomain in regulating its inhibitory function.19,20 Replacement of CD148 ecto- and trans-membrane domains with the equivalent regions from the raft-associated LAT molecule inhibited TCR triggering,13 but it was not possible to establish whether this was the result of truncation of the ectodomain or the targeting of CD148 to lipid rafts.
This study examines whether the size of the CD45 and CD148 ectodomains functions to regulate their inhibitory effects on TCR triggering by contributing to their segregation from engaged TCR. Using T cells already expressing endogenous CD45, we show that truncation of the large ectodomains of exogenous CD45 and CD148 enables them to inhibit TCR triggering and prevents their segregation from engaged TCR. Furthermore, we show through ectodomain substitution with another large ectodomain, and by antibody-induced forced segregation, that the observed abrogation of TCR triggering by these exogenous phosphatases is the result of the smaller size of their truncated ectodomains. Our results confirm that the large ectodomains of CD45 and CD148 regulate their inhibitory effects on TCR triggering and suggest that they do this by enhancing their segregation from the engaged TCR.
Materials and methods
DNA constructs
The cDNA for murine CD45 and CD148 were a gift from A. Weiss (Howard Hughes Medical Institute, Chevy Chase, MD). Truncated and chimeric constructs and mutants were generated by polymerase chain reaction–based mutagenesis and checked by double-stranded deoxyribonucleic acid sequencing (Source BioScience). All constructs consisted of the murine CD148 leader sequence followed by a FLAG tag and AvrII restriction site. The ectodomains of rat CD2 (amino acids 13-202), rat Thy1 (amino acids 20-130), or rat CD43 (amino acids 8-231) were used in the chimeric constructs, followed by a linker of 3 alanines with the transmembrane and cytosolic domain of murine CD45 (amino acids 565-1291), which was followed by a linker of an arginine and threonine and then the monovalent green fluorescent protein (GFP). A catalytically inactive mutant of the Thy1-CD45 chimeric construct was designed with a Cys→Ser mutation within the first phosphatase domain (C840S).21 The full-length murine CD148 (amino acids 29-1199) and a truncated form consisting of the 2-membrane proximal fibronectin type III domains (amino acids 620-1199) was inserted after the FLAG tag followed by a linker of an arginine and threonine and then the monovalent GFP. A catalytically inactive mutant of the truncated CD148 construct was designed with a Cys→Ser mutation within the phosphatase domain (C1140S).22 The various constructs were cloned into pBMN (Dr Garry Nolan's laboratory, Stanford University, CA).
Cell lines, transduction, antibodies, flow cytometry, and western blots
2B4 cells23 are a mouse CD4+ T-cell hybridoma line specific for moth cytochrome c(88-103) (ANERADLIAYLKQATK) peptide presented on the murine I-Ek MHC, and B3Z cells24 are a mouse CD8+ T-cell hybridoma line specific for ovalbumin(257-264) (SIINFEKL) presented on the murine H-2Kb MHC. Various constructs were transduced using an ecotropic packaging system developed by the Nolan laboratory. Matching cell surface expression levels (supplemental Figures 1, 4A, and 5, available on the Blood Web site) were obtained by FACS using an anti-FLAG antibody (Sigma). Quantum Simply Cellular anti-mouse IgG calibration beads (Bangs Laboratories, Inc) were used to measure the surface expression levels of transduced CD45 and CD148 and endogenous CD45. Transduced CD148 and CD45 were expressed at levels below 34 000 molecules per cell, whereas at least endogenous CD45 was present at levels in excess of 850 000 molecules per cell. Polyclonal populations of transduced cells were used for all experiments unless stated. Antibodies used for western blot included anti-pZap-70 (PY319) (Cell Signaling), anti-Zap-70 (Cell Signaling), anti-LAT (Upstate), anti-pLAT Y191 (Invitrogen), anti-β Actin (Sigma), anti-Rabbit IgG-HRP (Cell Signaling), and anti-Mouse IgG-HRP (Cell Signaling). Western blots were performed as previously described.25 Flow cytometry was performed on a FACS Excalibur (Becton Dickinson) and analyzed on FlowJo software (Treestar, Inc) gated on live cells. Cell sorting was performed on a MoFlo (Cytomation), with live cells stained with anti-FLAG.
T-cell activation
For antibody stimulation, T cells were incubated in plates coated with 1 μg/mL anti-mouse CD3ε antibody 145-2C11 (Biolegend), and/or 0.3 to 20 μg/mL anti-FLAG antibody (Sigma), and/or 0.02 to 20 μg/mL anti-rCD2 (OX34, Abcam) antibody for 14 to 24 hours. Alternatively, cells were stimulated with streptavidin-coated beads (Bangs Laboratories, Inc.) coated with biotinylated anti-mouse CD3ε 145-2C11 (Biolegend) at a 1:1 ratio with cells.
For peptide-MHC stimulation, 2B4 T cells were also stimulated with Chinese hamster ovary (CHO) cells expressing the cognate pMHC (I-Ek presenting the MCC peptide at varying concentrations), as previously described.26 B3Z cells were stimulated with CHO cells expressing a single-chain trimer consisting of the 2 chains from H-2Kb and the cognate peptide (SIINFEKL) previously shown to stimulate these cells.25 Supernatants were collected and subjected to IL-2 sandwich enzyme-linked immunosorbent assay with a capture rat anti-mouse IL-2 (JES6-1A12; Becton Dickinson) antibody and a biotinylated detection rat anti-mouse IL-2 (JES6-5H4; Becton Dickinson) antibody coupled to Extravidin (Sigma), as previously described.25
Supported lipid bilayers and TIRF microscopy
Anti-mouse CD3ε antibody (2C11) was monobiotinylated, as described elsewhere,27 and liposomes and bilayer preparation were performed as described previously.28 Briefly, 1,2-dioleoyl-sn-glycero-3-phosphocholine/NTA-DGS/biotinyl cap PE liposomes were deposited on cleaned coverslips in flow chambers (FCS2; Bioptechs) at a final density of 12.5 mol% NTA-1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] and 0.01 mol% biotinyl cap PE [1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(cap biotinyl)] (Avanti Polar Lipids). Coverslips were blocked with casein, and NTA groups charged with 100 μM NiSO4. After incubation with 5 μg/mL streptavidin, monobiotinylated 2C11 was coupled to streptavidin on bilayers by incubation for 20 minutes at 5 μg/mL. ICAM-1 containing a C-terminal 12-histidine tag was incorporated into bilayers at 200 molecules/μm2. Imaging was performed on a Nikon Ti microscope using a 100× TIRF objective, NA 1.49. Flow chambers were maintained at 37°C during imaging, using a Focht Chamber System heating unit. Hybridomas stably expressing GFP fusion constructs were injected into flow chambers and imaged for the first 60 seconds after contact with the bilayer. TCR distribution was followed by labeling cells with nonblocking anti-TCRβ (H57) antigen binding fragments conjugated with Alexa Fluor 568 (f/p ∼5). When cells were treated with PP2, they were incubated with 20 μM PP2 at 37°C for 10 minutes before analysis; the PP2 concentration was maintained during imaging.
Image analysis
All image processing was performed using ImageJ (National Institutes of Health) software. After background subtraction of GFP and AF568 channels, Pearson’s correlation coefficients were calculated for individual cells, using the interference reflection microscopy (IRM) channel to define cell regions of interest.
Statistics
Results
Forms of CD148 and CD45 with short ectodomains inhibit IL-2 secretion in a phosphatase-dependent manner
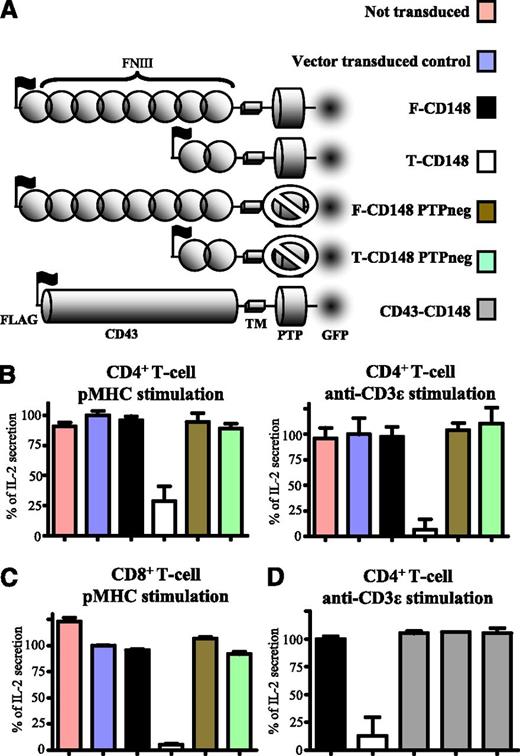
It has previously been shown that overexpression of CD148 in T cells can abrogate TCR signaling.3 To examine the role of the CD148 ectodomain, we compared the effects of CD148 constructs in which the ectodomain size is varied (Figure 1A). Either full-length CD148 or a truncated form of CD148 containing only the 2 most membrane-proximal FN domains were transduced into a mouse CD4+ T-cell hybridoma (Figure 1A). When stimulated with either cells presenting cognate pMHC or plate-immobilized anti-mouse CD3ε antibody, cells expressing the truncated form of CD148 showed reduced IL-2 secretion compared with untransduced cells or cells transduced with the vector control (Figure 1B). In contrast, expression of comparable levels of full-length CD148 had no effect on IL-2 expression (Figure 1B). Furthermore, expression of truncated CD148 with a mutation in the phosphatase domain (C1140S) that renders it catalytically inactive did not inhibit IL-2 secretion, indicating that this inhibition required tyrosine phosphatase activity. Truncated CD148 did not affect responses to stimuli that act downstream of proximal tyrosine phosphorylation (supplemental Figure 2). Constructs lacking the C-terminal GFP tag showed a similar trend, indicating that the GFP tag had no confounding effects (supplemental Figure 3). Similar responses were observed when these CD148 constructs were expressed in CD8+ T-cells (Figure 1C and supplemental Figure 4B). Inhibition of IL-2 secretion by truncated CD148 was not overcome by increasing the amount of stimulatory antibody (supplemental Figure 4B). These results suggest that the large ectodomain of CD148 prevents its tyrosine phosphatase domain from inhibiting TCR signaling.
The size of CD148 ectodomain modulates its inhibitory effect on T-cell activation. (A) Schematic depiction of the CD148 constructs used in this study. The ectodomains comprised either the full-length form of CD148 or the truncated form of CD148 or the ectodomain from CD43 (CD43-CD148). Catalytically inactive (PTPneg) forms of full-length and truncated CD148 had a cysteine to serine substitution (C1140S) in the phosphatase domain. All constructs contained an N-terminal FLAG tag to stain and match surface expression levels by FACS and a C-terminal GFP tag for imaging. (B) Control (untransduced or vector-transduced) 2B4 T cells or 2B4 T cells expressing comparable levels of the indicated CD148 construct (supplemental Figure 1) were stimulated with either CHO cells expressing I-Ek, presenting the cognate MCC peptide (left) or plate-immobilized anti-mouse CD3ε (right) and IL-2 secretion analyzed after 14 to 18 hours. (C) Control or transduced B3Z T cells expressing comparable levels of the indicated CD148 construct (supplemental Figure 4A) were stimulated with CHO cells expressing cognate pMHC as a single-chain trimer and IL-2 secretion analyzed after 14 to 18 hours. (D) 2B4 T-cells transduced with the indicated CD148 constructs were stimulated with plate-immobilized anti-mouse CD3ε and IL-2 secretion examined after 14 to 18 hours. Three different CD43-CD148 transduced clones were tested. Error bars represent the SD of the mean from at least 3 replicates. For (B) and (C), IL-2 secretion data were normalized with 0% and 100%, representing unstimulated and stimulated vector-transduced controls, respectively. In (D), IL-2 secretion was normalized to full-length CD148 transduced cells.
The size of CD148 ectodomain modulates its inhibitory effect on T-cell activation. (A) Schematic depiction of the CD148 constructs used in this study. The ectodomains comprised either the full-length form of CD148 or the truncated form of CD148 or the ectodomain from CD43 (CD43-CD148). Catalytically inactive (PTPneg) forms of full-length and truncated CD148 had a cysteine to serine substitution (C1140S) in the phosphatase domain. All constructs contained an N-terminal FLAG tag to stain and match surface expression levels by FACS and a C-terminal GFP tag for imaging. (B) Control (untransduced or vector-transduced) 2B4 T cells or 2B4 T cells expressing comparable levels of the indicated CD148 construct (supplemental Figure 1) were stimulated with either CHO cells expressing I-Ek, presenting the cognate MCC peptide (left) or plate-immobilized anti-mouse CD3ε (right) and IL-2 secretion analyzed after 14 to 18 hours. (C) Control or transduced B3Z T cells expressing comparable levels of the indicated CD148 construct (supplemental Figure 4A) were stimulated with CHO cells expressing cognate pMHC as a single-chain trimer and IL-2 secretion analyzed after 14 to 18 hours. (D) 2B4 T-cells transduced with the indicated CD148 constructs were stimulated with plate-immobilized anti-mouse CD3ε and IL-2 secretion examined after 14 to 18 hours. Three different CD43-CD148 transduced clones were tested. Error bars represent the SD of the mean from at least 3 replicates. For (B) and (C), IL-2 secretion data were normalized with 0% and 100%, representing unstimulated and stimulated vector-transduced controls, respectively. In (D), IL-2 secretion was normalized to full-length CD148 transduced cells.
To investigate whether truncation of CD148 enhanced the inhibitory effect because of a reduction in its size, we substituted the entire ectodomain of CD148 with the ectodomain of CD43 (Figure 1A). The CD43 ectodomain shares no homology with either CD45 or CD148 but is similarly large (∼45 nm) and highly glycosylated.29 T cells transduced with chimeric CD43- CD148 and full-length CD148 displayed similar levels of TCR ligand-induced IL-2 secretion, whereas truncated CD148 was strongly inhibitory (Figure 1D). This suggests that it is the large size of the CD148 ectodomain that prevents it from inhibiting T-cell activation.
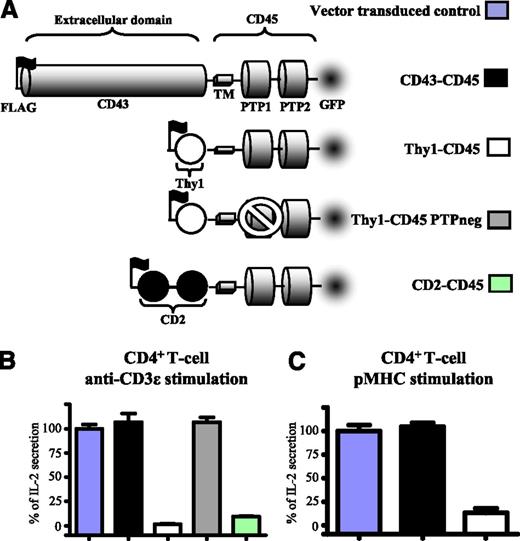
To investigate the role of the ectodomain of CD45 in TCR signaling, 3 chimeric forms of CD45 were transduced into CD4+ T-cells (Figure 2A). These comprised murine CD45 transmembrane and cytosolic domains and the ectodomain of Thy1, CD2, or CD43. These ectodomains vary dramatically in length: Thy-1 and CD2 ectodomains consist of 1 and 2 Ig-like domains, and so would be expected to extend up to ∼4 and ∼7 nm from the plasma membrane, respectively.30 As observed with CD148, expression of CD45 chimeras with small ectodomains inhibited IL-2 secretion by T-cells stimulated with either immobilized anti-CD3ε (Figure 2B) or cells presenting cognate pMHC (Figure 2C). This inhibition was not observed when the CD148 chimera had the large CD43 ectodomain or when phosphatase was catalytically inactive (Figure 2B). These results show that a large CD45 ectodomain functions to prevent it from inhibiting TCR signaling through its tyrosine phosphatase activity.
The size of CD45 ectodomain modulates its inhibitory effect on T-cell activation. (A) Schematic depiction of the CD45 constructs used in this study. They comprised the mouse CD45 transmembrane and cytosolic domains and the ectodomains of rat Thy1 (1 Ig-like domain), rat CD2 (2 Ig-like domains), or rat CD43. A catalytically inactive form of Thy1-CD45 (Thy1-CD45 PTPneg) had a cysteine-to-serine substitution (C840S) in the membrane-proximal phosphatase domain. All constructs contained an N-terminal FLAG tag to stain and match surface expression levels by FACS and a C-terminal GFP tag for imaging. 2B4 T-cells expressing comparable levels of the indicated CD45 construct (supplemental Figure 5) were stimulated with either (B) plate-immobilized anti-mouse CD3ε or (C) CHO cells expressing I-Ek presenting the cognate MCC peptide (left) and IL-2 secretion analyzed after 14 to 18 hours. Error bars represent the SD of the mean from at least 3 replicates. Data normalized to vector-transduced controls as in Figure 2.
The size of CD45 ectodomain modulates its inhibitory effect on T-cell activation. (A) Schematic depiction of the CD45 constructs used in this study. They comprised the mouse CD45 transmembrane and cytosolic domains and the ectodomains of rat Thy1 (1 Ig-like domain), rat CD2 (2 Ig-like domains), or rat CD43. A catalytically inactive form of Thy1-CD45 (Thy1-CD45 PTPneg) had a cysteine-to-serine substitution (C840S) in the membrane-proximal phosphatase domain. All constructs contained an N-terminal FLAG tag to stain and match surface expression levels by FACS and a C-terminal GFP tag for imaging. 2B4 T-cells expressing comparable levels of the indicated CD45 construct (supplemental Figure 5) were stimulated with either (B) plate-immobilized anti-mouse CD3ε or (C) CHO cells expressing I-Ek presenting the cognate MCC peptide (left) and IL-2 secretion analyzed after 14 to 18 hours. Error bars represent the SD of the mean from at least 3 replicates. Data normalized to vector-transduced controls as in Figure 2.
Forms of CD45 and CD148 with short ectodomains abrogate TCR triggering and proximal signaling
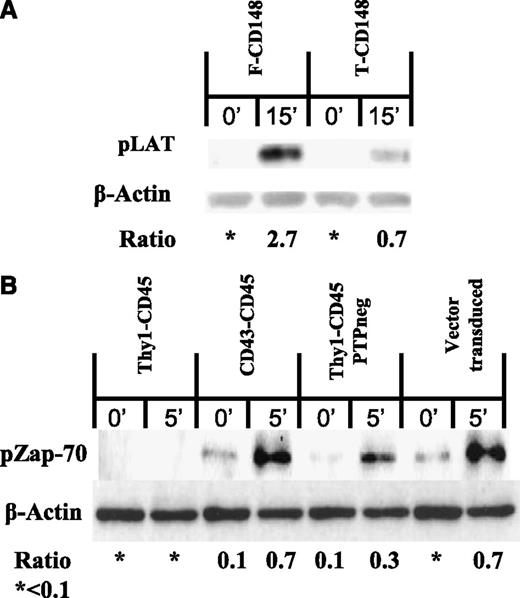
Because IL-2 secretion is a late readout of TCR triggering, we next examined early tyrosine phosphorylation events in the TCR triggering pathway; namely, phosphorylation of Zap-70 and LAT, both of which are recruited into the TCR signalosome.31 Truncation of the CD148 ectodomain resulted in decreased phosphorylation of LAT on TCR engagement by immobilized anti-CD3ε (Figure 3A). Similarly, expression of Thy-1-CD45 abrogated Zap-70 phosphorylation on TCR engagement (Figure 3B). This was not observed with the CD43-CD45 chimera or the catalytically inactive Thy-1-CD45 chimera (Figure 3B). Taken together, these results suggest that the truncation of CD148 and CD45 ectodomains inhibits TCR signaling by enabling them to dephosphorylate membrane-proximal molecules in the TCR triggering pathway.
Forms of CD148 and CD45 with short ectodomains abrogate TCR proximal signaling. (A) Immunoblot of phosphorylated LAT (pLAT) and β-actin in B3Z T-cells transduced with either full-length CD148 or truncated CD148 and stimulated with anti-mouse CD3ε coated beads for 15 minutes. (B) Immunoblot of phosphorylated Zap-70 (pZap-70) and β-actin in 2B4 T cells transduced with the indicated CD45 constructs (or vector as control) and stimulated with anti-mouse CD3ε coated beads for 5 minutes. All immunoblots are representative of 3 replicates. The densitometry ratio between either pLAT or pZAP-70 and β-actin was calculated in ImageJ (National Institutes of Health).
Forms of CD148 and CD45 with short ectodomains abrogate TCR proximal signaling. (A) Immunoblot of phosphorylated LAT (pLAT) and β-actin in B3Z T-cells transduced with either full-length CD148 or truncated CD148 and stimulated with anti-mouse CD3ε coated beads for 15 minutes. (B) Immunoblot of phosphorylated Zap-70 (pZap-70) and β-actin in 2B4 T cells transduced with the indicated CD45 constructs (or vector as control) and stimulated with anti-mouse CD3ε coated beads for 5 minutes. All immunoblots are representative of 3 replicates. The densitometry ratio between either pLAT or pZAP-70 and β-actin was calculated in ImageJ (National Institutes of Health).
Segregation of membrane phosphatases and TCR on an activating surface
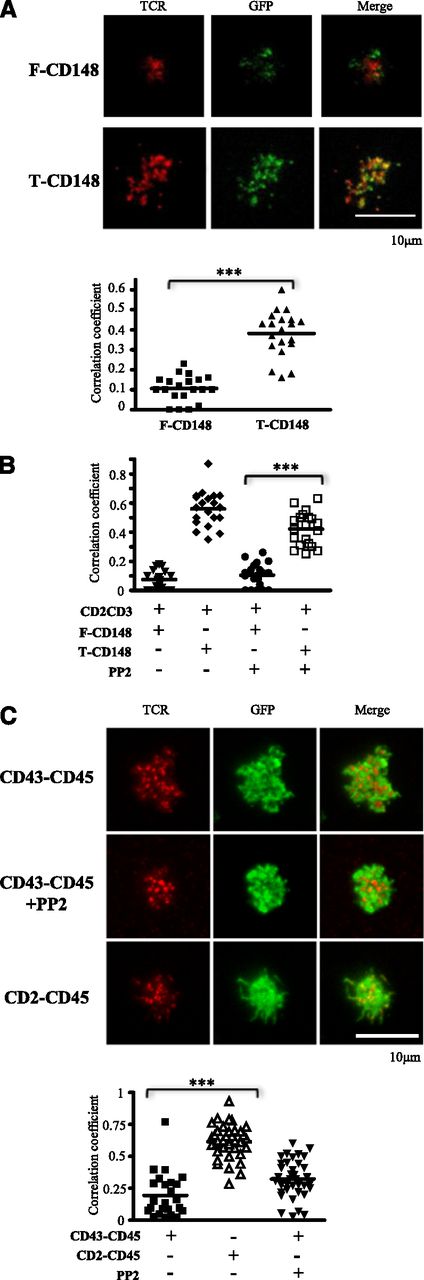
One possible mechanism by which truncation of CD45 and CD148 ectodomains inhibits TCR triggering is enabling these molecules to colocalize with engaged TCR. We therefore examined whether truncation of CD148 and CD45 ectodomains influenced their localization with respect to engaged TCR. B3Z T-cells expressing either full-length or truncated CD148-GFP were imaged on planar bilayers presenting anti-mouse CD3ε as a TCR ligand (Figure 4A). There was significantly greater colocalization of TCR microclusters with the truncated form of CD148 than with full-length CD148 (Figure 4A).
Truncation of CD148 and CD45 ectodomains enhances colocalization with TCR microclusters. (A) B3Z T cells expressing the indicated CD148-GFP chimera (green) were imaged by TIRF microscopy after initial contact with a planar bilayer that contained glycosylphosphatidylinositol-ICAM-1 and Cy5-labeled (red) monobiotinylated anti-mouse CD3ε. Representative images are shown (upper). Pearson correlation coefficients between the GFP and Cy5 fluorescence were analyzed in 20 cell contacts (lower). (B) B3Z T cells expressing CD2-TCRζ-mCherry and the indicated CD148-GFP chimera were preincubated with or without PP2 and then imaged in contact with a planar bilayer that contained glycosylphosphatidylinositol-ICAM-1 and anti-rat CD2. Pearson’s correlation coefficients between GFP and mCherry fluorescence were analyzed in 20 cell contacts. (C) 2B4 cells expressing the indicated CD45-GFP chimera (green) were preincubated with or without PP2 and imaged by TIRF microscopy after initial contact with a planar bilayer that contained ICAM-1 and monobiotinylated anti-mouse CD3ε. CD45 protein was imaged through its C-terminal GFP tag, and TCRβ was detected using a specific antibody (H57) Fab′ fragment labeled with AF568 (upper). Pearson’s correlation coefficients between the GFP and AF568 were analyzed on more than 25 cell contacts (lower). ***P < .01, using Student t test.
Truncation of CD148 and CD45 ectodomains enhances colocalization with TCR microclusters. (A) B3Z T cells expressing the indicated CD148-GFP chimera (green) were imaged by TIRF microscopy after initial contact with a planar bilayer that contained glycosylphosphatidylinositol-ICAM-1 and Cy5-labeled (red) monobiotinylated anti-mouse CD3ε. Representative images are shown (upper). Pearson correlation coefficients between the GFP and Cy5 fluorescence were analyzed in 20 cell contacts (lower). (B) B3Z T cells expressing CD2-TCRζ-mCherry and the indicated CD148-GFP chimera were preincubated with or without PP2 and then imaged in contact with a planar bilayer that contained glycosylphosphatidylinositol-ICAM-1 and anti-rat CD2. Pearson’s correlation coefficients between GFP and mCherry fluorescence were analyzed in 20 cell contacts. (C) 2B4 cells expressing the indicated CD45-GFP chimera (green) were preincubated with or without PP2 and imaged by TIRF microscopy after initial contact with a planar bilayer that contained ICAM-1 and monobiotinylated anti-mouse CD3ε. CD45 protein was imaged through its C-terminal GFP tag, and TCRβ was detected using a specific antibody (H57) Fab′ fragment labeled with AF568 (upper). Pearson’s correlation coefficients between the GFP and AF568 were analyzed on more than 25 cell contacts (lower). ***P < .01, using Student t test.
Although truncation could enhance colocalizing by preventing size-based exclusion, other explanations are possible. These include enhanced cis-association of truncated CD148 and the TCR, or a reduction in active segregation secondary to abrogation of TCR triggering. To investigate these alternatives, we examine colocalization between CD148 chimeras and a chimeric antigen receptor comprising the ectodomain of rat CD2 fused to TCRζ (CD2-TCRζ) and C-terminal mCherry.25,32 The ectodomain of this chimeric CD2-TCRζ construct will span a similar distance to that of native TCR.9 In these cells, truncation of the CD148 ectodomain enhanced its colocalization with CD2-TCRζ when stimulated on a planar bilayer presenting anti-rat CD2 (Figure 4B). Furthermore, this pattern of colocalization was also observed when the T cells were preincubated with PP2, an inhibitor of Src kinases that blocks TCR signaling (Figure 4B). These results suggest the enhanced colocalization observed after truncation of the CD148 ectodomain are not secondary to changes to cis-association with the TCR or TCR signaling.
As observed with CD148, the CD45 chimera with a shorter ectodomain (CD2-CD45) showed significantly greater colocalization with engaged TCR than the CD45 chimera with the large ectodomain (CD43-CD45) (Figure 4C and supplemental Figure 6A). Reduced colocalization of TCR and CD43-CD45 persisted in the presence of PP2, indicating that it is not secondary to TCR triggering (Figure 4C). To test whether there were differences in membrane spacing in relation to the bilayer, we quantified IRM images from CD43-CD45- and CD2-CD45-expressing cells (supplemental Figure 6B). We observe no significant difference in IRM signal at the synapse, suggesting comparable proximity of the cell surface with the bilayers when TCR is ligated (supplemental Figure 6C). Furthermore, we observe no significant difference in the TCR fluorescence within microclusters between CD43-CD45- and CD2-CD45-expressing cells (supplemental Figure 7), indicating normal TCR-ligand association. Taken together, these findings suggest that truncation of the CD45 and CD148 ectodomains enhances colocalization of these proteins with engaged TCR.
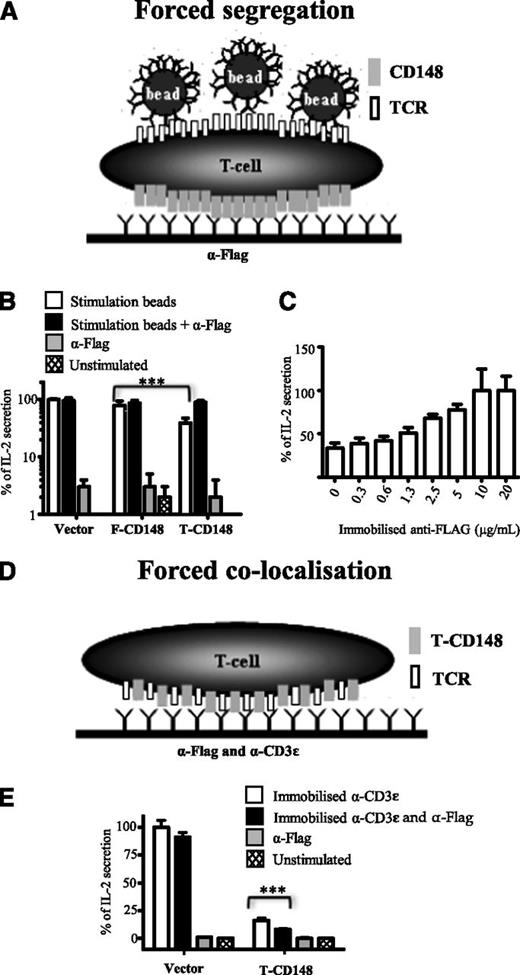
Forced segregation of truncated CD148 from engaged TCR restores TCR signaling
To further investigate the mechanism by which truncation of CD148 inhibited TCR triggering, we examined whether forced segregation of truncated CD148 from the engaged TCR reversed this effect (Figure 5). Segregation was induced by plating the T cells on surfaces coated with anti-FLAG antibody (all CD148 constructs have an N-terminal FLAG tag) and then stimulating them with anti-CD3ε-coated beads (Figure 5A). Segregation of truncated CD148 restored bead-stimulated IL-2 secretion to the same levels seen with vector-transduced T cells, whereas segregation of full-length CD148 had no effect (Figure 5B). Restoration of bead-stimulated IL-2 secretion was dependent on the concentration of immobilized anti-FLAG (Figure 5C).
Antibody-induced segregation of truncated CD148 rescues TCR signaling. (A) Schematic depiction of forced segregation experiment. B3Z T cells expressing FLAG-tagged CD48 are plated onto surfaces presenting immobilized anti-FLAG antibody before stimulation with anti-mouse CD3ε beads. By accumulating FLAG-tagged CD148 on the basal side of the cell, this forces segregation from apical areas where the beads engage TCR. (B) B3Z cells expressing the indicated form of CD148 and plated onto anti-FLAG or control surfaces were exposed to anti-mouse CD3ε (stimulation) or control beads IL-2 secretion determined after 16 hours after stimulation. Data are normalized with IL-2 secretion from unstimulated and stimulated vector transduced cells set to 0% and 100%, respectively. ***P < .01, using paired Student t test. (C) B3Z cells expressing the truncated form of CD148 were plated onto surfaces coated with the indicated concentration of immobilized anti-FLAG, stimulation with anti-mouse CD3ε beads, and IL-2 secretion determined after 16 hours. Data normalized to IL-2 secretion observed with highest levels of anti-FLAG (100%) and IL-2 secretion from unstimulated cells was set to 0%. (D) Schematic depiction of forced colocalization experiment. B3Z cells expressing FLAG-tagged truncated CD148 was plated onto surfaces containing both immobilized anti-FLAG and anti-mouse CD3ε. (E) Control B3Z cells or B3Z cells expressing the truncated form of CD148 were plated onto surfaces coated with the indicated combination of anti-FLAG and anti-mouse CD3ε antibodies and IL-2 secretion determined after 16 hours of stimulation. Data are normalized with IL-2 secretion from unstimulated and stimulated vector-transduced cells set to 0% and 100%, respectively. Error bars represent the SD of the mean from at least 3 replicates.
Antibody-induced segregation of truncated CD148 rescues TCR signaling. (A) Schematic depiction of forced segregation experiment. B3Z T cells expressing FLAG-tagged CD48 are plated onto surfaces presenting immobilized anti-FLAG antibody before stimulation with anti-mouse CD3ε beads. By accumulating FLAG-tagged CD148 on the basal side of the cell, this forces segregation from apical areas where the beads engage TCR. (B) B3Z cells expressing the indicated form of CD148 and plated onto anti-FLAG or control surfaces were exposed to anti-mouse CD3ε (stimulation) or control beads IL-2 secretion determined after 16 hours after stimulation. Data are normalized with IL-2 secretion from unstimulated and stimulated vector transduced cells set to 0% and 100%, respectively. ***P < .01, using paired Student t test. (C) B3Z cells expressing the truncated form of CD148 were plated onto surfaces coated with the indicated concentration of immobilized anti-FLAG, stimulation with anti-mouse CD3ε beads, and IL-2 secretion determined after 16 hours. Data normalized to IL-2 secretion observed with highest levels of anti-FLAG (100%) and IL-2 secretion from unstimulated cells was set to 0%. (D) Schematic depiction of forced colocalization experiment. B3Z cells expressing FLAG-tagged truncated CD148 was plated onto surfaces containing both immobilized anti-FLAG and anti-mouse CD3ε. (E) Control B3Z cells or B3Z cells expressing the truncated form of CD148 were plated onto surfaces coated with the indicated combination of anti-FLAG and anti-mouse CD3ε antibodies and IL-2 secretion determined after 16 hours of stimulation. Data are normalized with IL-2 secretion from unstimulated and stimulated vector-transduced cells set to 0% and 100%, respectively. Error bars represent the SD of the mean from at least 3 replicates.
One possible confounding explanation for these results is that the anti-FLAG antibodies inhibit CD148 phosphatase activity by inducing homodimerization, which has been reported to inhibit other receptor protein tyrosine phosphatases, including CD45.33-35 To rule this out, we examined the effect of placing the anti-FLAG antibody on the same surface as the anti-CD3ε antibody used to stimulate the cells (Figure 5D). Under these circumstances, the anti-FLAG antibody did not reverse the inhibitory effect of the truncated CD148 on anti-CD3ε-induced IL-2 production (Figure 5E). Indeed, the inhibition was slightly enhanced (Figure 5E), presumably because of forced colocalization of truncated CD148 and the engaged TCR. Taken together, these results demonstrate that the forced segregation of truncated CD148 reversed its inhibitory effect on TCR triggering, supporting our hypothesis that truncation of the CD148 inhibits TCR triggering by allowing colocalization.
Discussion
CD45 and CD148 have both activatory and inhibitory roles on TCR signal transduction.19,20,36,37 The activatory role is to dephosphorylate an inhibitory site at the C-terminal end of Src kinases such as Lck, which is needed to enable Lck to be fully activated on receptor engagement. The inhibitory role includes dephosphorylation of an activatory site in Lck, preventing its full activation, and dephosphorylation of substrates of Lck and ZAP-70. Because the activatory role of these molecules is fulfilled by low levels of CD45,19,20 by performing our experiments on T cells with endogenous expression levels of CD45 we were able to focus on inhibitory effects of exogenous CD148 and CD45.
Our results demonstrate that the ectodomains of CD148 and CD45 regulate their inhibitory effects on TCR triggering. When their ectodomains were truncated, exogenous CD45 and CD148 inhibited TCR triggering. When replaced with an equally large ectodomain domain from CD43, the inhibitory effect was reversed. Similarly, when truncated CD148 was forcibly segregated from the region of TCR engagement, this inhibitory effect was reversed. Finally, TIRF microscopy revealed that truncation of the ectodomain enhanced colocalization of CD148 and CD45 with the engaged TCR. Taken together, these findings suggest that an important function of the large CD148 and CD45 ectodomains is to mediate passive (signaling-independent) segregation of these phosphatases from the site of TCR engagement, thereby preventing them from inhibiting TCR triggering.
These findings strongly support the K-S model of TCR triggering.9-11 This model postulates that a key step in TCR triggering is the segregation of membrane tyrosine phosphatases such as CD45 and CD148 from the site of TCR engagement, and that this segregation is a passive process driven by the large size of their ectodomains. It has also been proposed that the K-S mechanism is important for signal transduction by many other small receptors where signaling involves phosphorylation of cytoplasmic tyrosine residues by extrinsic membrane-associated Src tyrosine kinases.11 Indeed, there is evidence to support a role for the K-S mechanism in the case of natural killer receptors,38,39 CD28,40 and Dectin-1.41 A role in B-cell receptor triggering has also been proposed.42 Given that CD45 and CD148 are ubiquitously expressed,3 it is likely that their large ectodomains may play a similar regulatory role in many cells. Recently, James and Vale reported that although a CD45 ectodomain was required for segregation from engaged TCR, some segregation was observed even when the CD45 ectodomain was truncated.43 One possible explanation for this is that lateral “crowding” can drive segregation if, for example, mobile TCR/pMHC complexes cluster in high densities at the interface. Such an effect may also account for the fact that the truncated form of CD148 inhibited TCR triggering induced by immobilized anti-CD3 antibodies more strongly than it inhibited triggering induced by mobile pMHC presented on cells (Figures 1B and supplemental Figure 3). Because they are immobilized, the TCR/antibody complexes would presumably be less able to form high-density clusters.
Our findings are consistent with but extend previous studies on the role of the CD45 and CD148 ectodomains on regulating TCR signaling. In our previous investigation of the role of the CD45 ectodomain, we reconstituted CD45-deficient Jurkat cells with various CD45 constructs.18 In these cells, TCR signaling is blocked because Lck is phosphorylated on its inhibitory C-terminal site. Introduction of wild-type exogenous CD45 restored signaling by dephosphorylating this site. In contrast, chimeric forms of CD45 with short ectodomains did not restore TCR signaling, whereas forms with the large CD43 ectodomain did restore signaling.18 Although this was consistent with the results presented here, because the activatory role of CD45 was critical in this previous study, it was not possible to exclude the possibility that a large ectodomain was required for its activatory function. Indeed, some findings suggested that a large, highly glycosylated ectodomain may somewhat enhance its activatory function, possibly by influencing its association with lipid rafts.18 In our current study, the T cells used already expressed sufficient endogenous CD45 to fulfill this activatory role. Thus, exogenous CD45 had either no effect or was inhibitory, enabling us to establish that a large, bulky ectodomain negatively regulates CD45’s inhibitory effect.
Lin and Weiss examined the role of the CD148 ectodomain in T-cell activation.13 They replaced the transmembrane and ectodomain of CD148 with the transmembrane and ectodomain of LAT. This substantially enhanced the inhibitory effect of exogenous CD148. However, because the LAT substitution not only truncated the ectodomain but, through its transmembrane domain, potentially targeted CD148 to lipid rafts, it was not possible to conclude that the ectodomain regulated its inhibitory effect. In contrast, in the current study we show that only truncating the CD148 ectodomain, while leaving the transmembrane domain intact, enhances its inhibitory effect. Furthermore, we show that a different, but equally large, ectodomain from CD43 was able to prevent CD148 from inhibiting TCR triggering. This demonstrates that the large ectodomain of CD148 regulates it ability to inhibit TCR triggering.
Although our findings suggest that the CD45 and CD148 ectodomains have a passive mechanical role in mediating segregation from areas of close contact, there is evidence that they have other roles. The CD45 ectodomain has been reported to regulate homodimerization of CD45,44 and it has been proposed that CD45 homodimerization inhibits its catalytic activity.34 Another proposed role for the CD45 and CD148 ectodomains is interaction with ligands. A number of lectin-like molecules have been reported to bind CD45, including Galectins and CD22.3 However, this may reflect the abundance of CD45 and its high level of glycosylation, and the functional significance of these interactions are not clear. Recently, it has been reported that the ectodomain of the cell surface molecule Syndecan-2 binds to the CD148 ectodomain, and this does not appear to require glycosylation.45 These postulated functional roles, homodimerization and ligand engagement, are unlikely to explain the effects we observed when we truncated the CD148 and CD45 ectodomains, as they were reversed by substituting the native ectodomain with the CD43 ectodomain, which has no similarity other than being equally large and highly glycosylated.
One unusual feature of CD45 that is conserved between species is that the membrane distal portion of the CD45 ectodomains varies in length as a result of alternative splicing of 3 exons encoding the A, B, and C protein segments.3,46 Although CD45 is expressed in all T cells, the isoform that is expressed varies during T-cell development and on T-cell activation.47,48 Thymocytes express a form of CD45 lacking all 3 exons (CD45RO), whereas peripheral T cells have a mixture of isoforms. On T-cell activation, there is a tendency to express isoforms with fewer exons. As the isoforms would vary significantly in size from ∼28 nm for the smallest CD45RO isoform to ∼50 nm for the largest CD45ABC isoform,16,17 it is possible that this variation regulates its exclusion from close-contact areas by altering the size of the ectodomain. Another possible role for variable splicing of these exons is to alter the activity of CD45 by influencing homodimerization.44
Interestingly, the expression of CD148 varies significantly during T-cell development, and this differs somewhat between mice and humans.36 CD148 is expressed at higher levels in naive human T cells and is virtually absent in naive mouse T cells, but expression is substantially upregulated after T-cell activation.36 One possible explanation for this upregulation on T-cell activation is that it matches the increase in cell surface Lck that is also observed on T-cell activation,49 to maintain the balance between tyrosine kinase and phosphatase activity at the cell surface. This could be necessary to prevent spontaneous TCR triggering or triggering induced by very low affinity pMHC. The large ectodomain would ensure that CD148 was appropriately excluded from areas of close contact so that appropriate signaling after agonist pMHC engagement was not inhibited.
In conclusion, our results demonstrate that the large ectodomains of receptor tyrosine phosphatases CD45 and CD148 regulate their inhibitory effect on TCR triggering and suggest that they do this by mediating their passive segregation from areas of close cell–cell contact. Although the role of the cytoskeleton or lipid domains in the segregation of the long form of the phosphatase cannot be ruled out, it is difficult to envisage a mechanism by which the cytoskeleton or lipid are able to segregate only long forms of a phosphatase in a manner independent of signaling. These results strongly support the kinetic segregation model of TCR triggering, although they do not rule out contributions from other mechanisms, such as ligand-induced aggregation or conformational change. It is notable that a number of other receptor tyrosine phosphatases have large ectodomains whose function is poorly understood.50 Our results raise the question as to whether their size contributes to their exclusion from or localization within cell/cell or cell/matrix contact areas.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ross Benkhebab for performing some experiments, A. Weiss for providing CD45 and CD148 cDNA, N. Rust for assistance with cell sorting, and N. Barclay, S. Davis and members of the P.A. van der Merwe laboratory for valuable discussion and advice.
This work was supported by the UK Medical Research Council, EP Abraham Research Fund, Cancer Research Institute Postdoctoral Fellowship (K.C.) and a National Institutes of Health grant (R37AI043542) to M.L.D.
Authorship
Contribution: The majority of this work was completed by S.-P.C. and K.C., with the following exceptions: H.Z. performed the TIRF imaging on CD148; M.B. performed flow cytometry and western blotting experiments; A.B.B. performed some CD148 experiments; and M.L.D. and P.A.v.d.M. contributed to the direction, approach, data interpretation, and writing of this paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: P. Anton van der Merwe, Sir William Dunn School of Pathology, University of Oxford, South Parks Rd, Oxford, OX1 3RE, United Kingdom; e-mail: anton.vandermerwe@path.ox.ac.uk.
References
Author notes
S.-P.C. and K.C. contributed equally to this study.