In 1988, the gene responsible for the autosomal recessive disease ataxia- telangiectasia (A-T) was localized to 11q22.3-23.1. It was eventually cloned in 1995. Many independent laboratories have since demonstrated that in replicating cells, ataxia telangiectasia mutated (ATM) is predominantly a nuclear protein that is involved in the early recognition and response to double-stranded DNA breaks. ATM is a high-molecular-weight PI3K-family kinase. ATM also plays many important cytoplasmic roles where it phosphorylates hundreds of protein substrates that activate and coordinate cell-signaling pathways involved in cell-cycle checkpoints, nuclear localization, gene transcription and expression, the response to oxidative stress, apoptosis, nonsense-mediated decay, and others. Appreciating these roles helps to provide new insights into the diverse clinical phenotypes exhibited by A-T patients—children and adults alike—which include neurodegeneration, high cancer risk, adverse reactions to radiation and chemotherapy, pulmonary failure, immunodeficiency, glucose transporter aberrations, insulin-resistant diabetogenic responses, and distinct chromosomal and chromatin changes. An exciting recent development is the ATM-dependent pathology encountered in mitochondria, leading to inefficient respiration and energy metabolism and the excessive generation of free radicals that themselves create life-threatening DNA lesions that must be repaired within minutes to minimize individual cell losses.
Introduction
Ataxia-telangiectasia (A-T) is an autosomal recessive disorder characterized by a broad spectrum of disease phenotypes that can be viewed from many perspectives, much like the Indian parable of the blind men probing different parts of an elephant while not seeing the entirety of the animal, because each relies on only 1 methodology—his touch. The A-T phenotype is similarly complex and includes progressive neuronal degeneration, ocular telangiectasias, variable immunodeficiency, and cancer susceptibility,1,,,,,,,-9 whereas the overall functions of the ataxia telangiectasia mutated (ATM) protein suggest a much broader pathology. Indeed, the extended phenotype can include growth retardation, premature aging, insulin resistance, manifestations of mitochondrial dysfunction, inadequate responses to oxidative stress, and adverse reactions to the DNA-damaging agents commonly used to treat cancer. Cells derived from A-T patients exhibit cytoskeletal abnormalities,4 requirements for serum growth factors and clastogenic factors,5,10,11 chromosomal instabilities,12,-14 chromatin changes,15 hypersensitivity to ionizing radiation,16,17 and aberrant checkpoint controls.18
The gene responsible for A-T was first localized to chromosome 11q22.3-23.1 by Gatti et al19 using mathematical analysis of cosegregation (linkage) data.19 During the next 7 years, the region was “fine mapped” by an international consortium,20,,,,-25 and then identified as the ATM gene by Savitsky et al.26 ATM is a high-molecular-weight (350 kDa) protein kinase, and it is a member of the large phosphoinositidyl 3-kinase-related protein kinase (PIKK) family; other family members include the DNA-dependent protein kinase catalytic subunit (DNA-PKcs), A-T and Rad3-related protein (ATR), mammalian target of rapamycin (mTOR)/FKBP-rapamycin-associated protein (mTOR/FRAP), and ATX/SMG.18,27,28 Much of the structural similarity of these proteins lies in their domains for (1) kinase activity; (2) FRAP-ATM-TRRAP (FAT); and (3) C-terminal FAT (FATC) and binding sites for p53, BLM, and other ATM-binding proteins.29
In steady-state cells, ATM appears to exist as an inactive dimer/tetramer that can be rapidly activated and recruited to sites of double-strand breaks (DSBs) by the Mre11-Rad50-NBS1 (MRN complex) proteins via its interaction with the C-terminal domain of NBS1. Once bound and retained by the MRN complex at a DSB, ATM undergoes autophosphorylation at a critical Ser1981 residue, forming active monomers/dimers that are important for their role(s) in homologous recombinational repair.8,29,-31 Several reports suggest that the sustained activation of ATM in response to damage is also modulated by a number of accessory proteins that must be retained in a complex surrounding the DSB, including MDC1,32 53BP1,33 BRCA1,34 the protein phosphatases PP2A and WIP1,35,-37 and TIP60, the histone acetyl-transferase that acetylates ATM on lysine residue (K3016) during its autophosphorylation at Ser1981.38 Once activated, ATM controls cell-cycle–checkpoint signaling, perhaps to maintain a clear temporal separation between DNA repair and cell division, which probably should not be happening at the same time. ATM coordinates: (1) the G1-S cell-cycle checkpoint by phosphorylating the tumor suppressor protein p53 at Ser15; (2) the G2/M checkpoint by phosphorylating the protein kinase Chk2 at Thr68; and (3) the intra-S cycle checkpoint by phosphorylating SMC1 at Ser957 and Ser966.8,18,39
The role of ATM in maintaining genomic stability by coordinating the DNA-damage response and regulating cell-cycle damage checkpoints is consistent with it being most abundantly expressed in the nucleus. Indeed, most clinical measurements of ATM are performed on nuclear preparations from peripheral blood lymphocytes or lymphoblastoid cell lines. However, pools of ATM also exist in the cytoplasm of many varying cell types. Early studies reported that ATM was found almost exclusively in the cytoplasm of differentiated (postmitotic) neurons, Purkinje cells, and neuronlike SH-SY59 cells.40,-42 However, fractions of ATM are also found in association with cytoplasmic organelles and with proteins involved in vesicle trafficking.43 ATM has also been shown to localize to microsomal compartments that include peroxisomes.44,-46 Together, these findings have led some investigators to suggest that ATM has separate functions in cytoplasmic-signaling pathways whose disruption might contribute to the clinical pleiotropy of A-T.
In this review, we explore cytoplasmic ATM signaling and highlight the emergence of the next generation of research on ATM functions and its potential impact on the pathogenesis and treatment of A-T. We focus on the role of cytoplasmic ATM in (1) oxidative stress-induced responses; (2) TORC signaling; (3) the pentose phosphate pathway (PPP); (4) mitochondrial function; and (5) insulin signaling and cardiovascular disease.
ATM responses to oxidative stress
The DNA of eukaryotic cells is continuously exposed to reactive oxygen species (ROS) generated either by exposure to physical sources (UV light and ionizing radiation), or via the physiological functions of numerous intracellular enzymes, including NADPH oxidases, xanthine oxidase, cyclooxygenases, cytochrome P450, lipoxygenases, and those of immune cells.47,48 However, the vast majority of intracellular ROS is produced by the respiratory chain in the mitochondria.49 ROS are an important class of toxicants that are capable of causing damage to lipids and proteins and are able to generate a plethora of DNA lesions, including oxidized DNA bases, apurinic/apyrimidinic (AP) sites, and single- and double-strand breaks.50,51 When the damage caused by ROS exceeds the cells’ capacity for repair, a general self-perpetuating state of oxidative stress is produced. Conversely, reducing the oxidation of ATM should (1) improve the efficiency of DNA repair by increasing ATM kinase activity; and (2) reduce cancer risk.
Early studies showed that A-T fibroblasts were more susceptible than normal cells to oxidative damage caused by the prooxidant, hydrogen peroxide (H2O2).52 In addition, A-T cells were hypersensitive to ROS produced by activated neutrophils and by the xanthine oxidase.53 Together, these observations prompted Rotman and Shiloh54,55 to suggest that A-T is in essence a disorder of oxidative stress and that ATM might well act as a sensor for redox homeostasis. Since that time, ATM-deficient cells have consistently exhibited overwhelming sensitivity to agents that generate ROS and cause oxidative DNA damage, such as IR, t-butyl hydroperoxide, chromium IV, and nitric oxide (NO).56,57 Moreover, the basal expression levels of several different oxidative-damage responsive pathways involving p53, p21, Gadd45, NFκB, hemeoxygenase (HO-1), and manganese superoxide dismutase (MnSOD), have been shown as constitutively elevated in A-T cells and in certain tissues from Atm−/− mice.58,,-61 A-T patients also appear to exhibit significantly reduced levels of several antioxidants such as vitamin A and E. Cultured ATM-deficient fibroblasts and lymphoblasts show reduced synthesis of important antioxidants such as glutathione.62,63 Levels of lipid peroxidation and oxidized DNA bases, such as 8-hydroxy-2-deoxyguanosine (8-OHdG), have also been elevated in A-T cells.64 Furthermore, ATM modulates the self-renewal of hematopoietic stem cells by regulating cellular ROS levels.65 Precisely how ATM coordinates the regulation of cellular redox homeostasis is not clearly understood, but the results of several recent studies described later show that ATM is directly activated by certain oxidative damaging agents, regulates the production of glutathione, and participates in several important oxidative stress-signaling pathways.
Direct activation by ATM oxidation
Although it has long been appreciated that cytoplasmic ATM participates in various oxidative stress-signaling pathways, its direct activation by oxidants/prooxidants had not been previously demonstrated until Guo et al66 showed that ATM is activated by exposure to H2O2, as evidenced by ATM autophosphorylation and downstream trans-phosphorylation of p53 (Ser15) and Chk2 (Thr68). The H2O2-dependent activation of ATM also proceeds in the absence of physical DNA-damage, because the MRN complex was not required for the activation of ATM in the presence of H2O2, and several key protein markers of direct DNA damage, such as H2AX and KAP1 (Kruppel-associated box [KRAB]-associated protein 1), were not phosphorylated.66
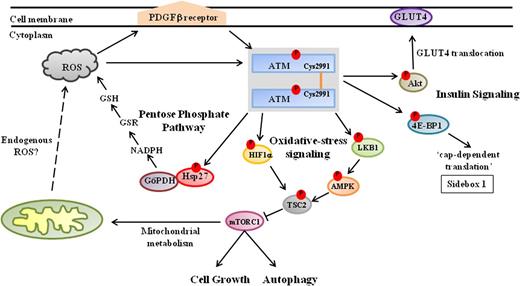
In the absence of DNA damage, ATM usually exists as a noncovalently associated dimer that dissociates into active monomers upon DNA damage. When Guo et al66 treated either purified ATM in vitro or otherwise normal human cells with H2O2, they were able to recover ATM predominantly in the form of active covalent dimers, which appeared to involve intermolecular disulfide bond formation in the C-terminus of ATM. On further experimentation, these authors determined that a cysteine residue, Cys2991, located in the FATC domain of ATM, is required for intermolecular disulfide bond formation and is essential for activation of ATM by H2O2. When a Cys2991 to Leu2991 (C2991L) ATM mutant was expressed in A-T lymphoblasts, it could not be activated by H2O2 treatment (Figure 1).66,,,,,,,-74 Similar to their findings with the C2991L mutant, Guo et al66 reported that although the spontaneously occurring ATM mutant, R3047X, could be fully activated by damaged DNA, presented together with the MRN complex in vitro, it could not be activated by H2O2; this is interesting, because the R3047X mutation is missing the last 10 amino acids of the FATC domain. On the basis of these results, the authors proposed a model explaining, at least in part, how ATM might be dually activated for a role(s) in either oxidative stress-responsive (signaling) pathways, or in response to DNA damage. After exposure to ROS-generating agents (IR, H2O2, and the like), oxidative activation of ATM for its role(s) in oxidative stress pathways would predominate in an ROS-dependent fashion. However, the prevailing reducing environments in cells could then be expected to decrease the oxidative activation of ATM, thereby leading instead to the sustained activation of ATM by DNA damage and the MRN complex for its roles in DNA-damage signaling and repair.66,76 In this context, it would be interesting to determine whether the H2O2-modified ATM dimers described by Guo et al66 participate in certain of those oxidative stress-response pathways (see later) already known to be operating in the cytoplasm, and whether ATM can be similarly modified and activated in response to endogenously generated ROS. These interactions will also have to be reexamined in Mre11-, RAD50-, and nibrin-deficient cells.
ATM kinase signals to diverse metabolic pathways in the cytoplasm. During in vitro experiments, oxidants/prooxidants induce the phosphorylation and activation of ATM dimers that appear to be linked via intermolecular disulfide bonds formed at a conserved C2991 residue in the C- terminus.66 It remains to be fully clarified whether these ATM dimers are similarly activated in response to endogenously generated ROS—acting directly on ATM, or else via the platelet-derived growth factor receptor β67 —and are responsible for regulating (1) expression of mTORC1; (2) synthesis of reduced glutathione (GSH) via the PPP; and (3) insulin-induced protein synthesis in response to oxidative stress. Nevertheless, the activation of ATM in response to oxidative stress leads to the inhibition of mTORC1 activity via the LKB1/AMPK/TSC2 signaling cascade. Activated ATM phosphorylates LKB1 (Thr366), which in turn phosphorylates AMPK (Thr172). AMPK subsequently phosphorylates TSC2 (Thr1271, Ser1387), which inhibits the expression of mTORC1, thereby promoting autophagy.68,-70 Furthermore, ATM regulates TSC2 activity in response to hypoxia by directly phosphorylating HIF1α (Ser696),71 and hence blocks mTORC1 activity. In addition, activated ATM induces complex formation between glucose-6-phosphate dehydrogenase (G6PD) and Hsp27, which increases the production of NADPH via the PPP, resulting in elevated intracellular levels of the antioxidant glutathione.72 Cytoplasmic ATM also regulates protein synthesis in response to insulin by phosphorylating 4E-BP1 (Ser111) and enhancing mRNA translation.73 Moreover, ATM phosphorylates Akt (Ser473) in response to insulin, which stimulates the translocation of the glucose transporter 4 (GLUT4) complex into the cell membrane via an as yet undetermined mechanism.74,75
ATM kinase signals to diverse metabolic pathways in the cytoplasm. During in vitro experiments, oxidants/prooxidants induce the phosphorylation and activation of ATM dimers that appear to be linked via intermolecular disulfide bonds formed at a conserved C2991 residue in the C- terminus.66 It remains to be fully clarified whether these ATM dimers are similarly activated in response to endogenously generated ROS—acting directly on ATM, or else via the platelet-derived growth factor receptor β67 —and are responsible for regulating (1) expression of mTORC1; (2) synthesis of reduced glutathione (GSH) via the PPP; and (3) insulin-induced protein synthesis in response to oxidative stress. Nevertheless, the activation of ATM in response to oxidative stress leads to the inhibition of mTORC1 activity via the LKB1/AMPK/TSC2 signaling cascade. Activated ATM phosphorylates LKB1 (Thr366), which in turn phosphorylates AMPK (Thr172). AMPK subsequently phosphorylates TSC2 (Thr1271, Ser1387), which inhibits the expression of mTORC1, thereby promoting autophagy.68,-70 Furthermore, ATM regulates TSC2 activity in response to hypoxia by directly phosphorylating HIF1α (Ser696),71 and hence blocks mTORC1 activity. In addition, activated ATM induces complex formation between glucose-6-phosphate dehydrogenase (G6PD) and Hsp27, which increases the production of NADPH via the PPP, resulting in elevated intracellular levels of the antioxidant glutathione.72 Cytoplasmic ATM also regulates protein synthesis in response to insulin by phosphorylating 4E-BP1 (Ser111) and enhancing mRNA translation.73 Moreover, ATM phosphorylates Akt (Ser473) in response to insulin, which stimulates the translocation of the glucose transporter 4 (GLUT4) complex into the cell membrane via an as yet undetermined mechanism.74,75
Activation of ATM by membrane receptors in response to oxidative stress
More recently, important roles for the activation of ATM by membrane receptors have been described for primarily postmitotic neuronal cells experiencing oxidative stress. Kim et al67 reported that membrane-bound tyrosine kinases, including the platelet-derived growth factor receptor β (PDGFRB), are required to activate ATM under conditions of oxidative stress. PDGFRB expression is important for protecting neurons from glutamatergic excitotoxicity.77 In P19-derived neuronlike cells, ATM underwent rapid autophosphorylation of Ser1981 in response to H2O2 treatment. The activation of ATM was strongly suppressed by AG1433, a specific inhibitor of PDGFRB signaling.67 Notably, the activation of ATM in response to X-ray irradiation was not suppressed by AG1433, suggesting that this activation of ATM by the PDGFRB receptor does not proceed via the DNA-damage response. Moreover, excessive excitation of neurons by the glutamate receptor agonist, kainic acid, stimulated phosphorylation of ATM in the cytoplasm of P19 cells. In addition, Kim et al67 showed that expression of PDGFRB is induced by the transcription factor ZFHX3 (ATBF1); in turn, the expression of ZFHX3 appears to be regulated by ATM, which activates the cyclic AMP response element-binding protein (CREB) to bind to a CRE consensus-binding site located near the ZFHX3 promoter, at least in retinoic acid (RA)-induced differentiated P19 cells. Kainic acid treatment also caused the formation of vacuolar structures in the neurons indicative of autophagy, leading Kim et al67 to suggest that membrane receptors might protect neurons in response to excessive excitation by signaling to cytoplasmic ATM-dependent autophagic processes to initiate the removal of dysfunctional organelles.
ATM and mTORC1 signaling
The mTOR is a 290 kDa serine-threonine kinase and, like ATM, is a member of the PIKK family. mTOR is important for regulating cell growth in response to a variety of environmental stresses, including nutrient starvation, hypoxia, DNA damage, and osmotic stress.78 The mTOR kinase is often found in 2 functionally distinct complexes, the so-called mTOR complex 1 (mTORC1) and mTORC2. mTORC1 activity inhibits the process of autophagy, which is usually responsible for recycling damaged organelles and the retrieval of cellular nutrients. Moreover, tight regulation of mTORC1 activity also appears physiologically important, because its deregulation has been associated with the onset of different disease phenotypes, including cancer and diabetes,78 which are among the most debilitating clinical phenotypes associated with A-T. Interestingly, ATM appears to downregulate mTORC1 activity in response to oxidative stress in an indirect fashion by activating the tuberous sclerosis 2 (TSC2) tumor suppressor (Figure 1). 79
TSC2/tuberin normally participates in energy sensing and growth-factor signaling, and was identified as a negative regulator of the mTOR kinase in the mTORC1 complex (Figure 1).68 TSC2/tuberin is activated by the AMP-activated protein kinase (AMPK), which phosphorylates TSC2/tuberin at several sites, including Thr1271 and Ser1387, thereby leading to the repression of mTORC1 activity under conditions of energy stress. AMPK is in turn regulated through the serine/threonine liver kinase B1 (LKB1), which phosphorylates AMPK at Thr172.69 Importantly, ATM was shown to phosphorylate LKB1 (Thr366 in human; Thr363 in rodents) in response to damage.70
The mechanism for repression of mTORC1 in response to oxidation stress had not been previously demonstrated until Alexander et al79 showed that mTORC1 activity was repressed in ATM-proficient lymphoblasts in response to H2O2 exposure, whereas little if any repression of mTORC1 occurred in ATM-deficient cells, a phenomenon that was successfully reproduced in Atm+/+ vs Atm−/− mouse embryonic fibroblasts. These same investigators also showed that TSC2/tuberin activity, being modulated by ATM via its activation of the LKB1/AMPK signaling cascade, was required for repression of mTORC1 in response to oxidative stress; mTORC1 activity was strongly repressed in Tsc2+/+ but not Tsc2−/− fibroblasts on exposure to H2O2. Collectively, these results led the authors to suggest that a signaling pathway exists between ATM and TSC2 to inhibit mTORC1 in response to oxidative damage caused by H2O2.79
ATM also appears to be involved in inhibiting mTORC1 activity under hypoxic conditions, wherein inhibition of mTORC1 activity usually requires the dissociation of the inhibitory 14-3-3α proteins from the TSC2 complex (not shown in Figure 1). The REDD1 (regulated in development and DNA-damage response 1) protein acts as a negative regulator of mTORC1 by binding to 14-3-3α, thereby provoking TSC2/14-3-3 dissociation and mTORC1 inhibition.71,80 The HIF-1α transcription factor is responsible for regulating expression of the REDD1 gene in response to hypoxia. Under hypoxic conditions, HIF-1α becomes stabilized during dimerization with HIF-β in the cytoplasm, before the HIF-1α/HIF−β heterodimer translocates to the nucleus to regulate the expression of hypoxia-inducible genes, including the REDD1 gene.81 Recently, Bencokova et al82 reported that ATM is activated under hypoxic conditions in a manner that is independent of both the DNA-damage–inducible MRN complex and mitochondrial signaling. Soon after, Cam et al71 showed that hypoxia-activated ATM stabilizes HIF-1α by phosphorylating Ser696 (Figure 1). In addition, these authors demonstrated that the translocation of HIF-1α into the nucleus of ATM-deficient cells was greatly attenuated; in turn, the induction of REDD1 was also strongly repressed in ATM−/− fibroblasts under hypoxic conditions. Thus, ATM appears to inhibit mTORC1 activity during hypoxia by regulating cellular levels of HIF-1α.71
ATM and the PPP
Consentino et al72 provide evidence to suggest that the reduced rates of glutathione biosynthesis exhibited by A-T cells might stem from ATM’s role in regulating the synthesis of the multifunctional pyridine nucleotide, nicotinamide adenine dinucleotide phosphate (NADPH), during the operations of the catabolic PPP. Reduced glutathione (GSH) is an important cellular antioxidant, and it is converted to its oxidized form glutathione disulfide (GSSG) on reaction with ROS. The regeneration of GSH is catalyzed by glutathione reductase (GSR), which requires the reducing cofactor NADPH (Figure 1).83 Intracellular levels of NADPH are maintained by the PPP, which is also responsible for generating metabolic intermediates required for the synthesis of aromatic amino acids, vitamin B6, and nucleic acids.
The PPP can operate at the same time as glycolysis, under both aerobic and anaerobic conditions. The pathway begins when glucose-6-phosphate (G6P) is oxidized to 6-phosphogluconate, which in turn is oxidized to ribose 5-phosphate and carbon dioxide; NADPH is produced during these 2 oxidation steps. Under aerobic conditions, the PPP yields 2 molecules of NADPH for each glucose molecule metabolized to pyruvate.83 Using Xenopus extracts, Consentino et al72 showed that ATM is a posttranscriptional activator of the PPP. ATM activation is required for stimulating complex formation between the heat shock protein, Hsp27, and the rate-limiting PPP enzyme glucose-6-phosphate dehydrogenase (G6PDH). The Hsp27-G6PDH complex appears to increase G6PDH activity, thereby increasing the production of NADPH.72 Although the precise mechanism(s) by which the affinity of Hsp27 for G6PDH is increased by ATM remains to be established, Consentino et al72 have shown that Hsp27 phosphorylation is ATM dependent. Thus, cytoplasmic fractions of ATM might be regulating glutathione biosynthesis, and hence the overall antioxidant capacity of cells, by modulating Hsp27-dependent activities in the PPP.
ATM and mitochondria
The human mitochondrial genome is a circular double-stranded DNA molecule of ∼16 600 base pairs. The mitochondrial DNA (mtDNA) encodes 37 genes: 13 genes coding for the protein subunits of the electron transport chain/oxidative phosphorylation system (ETC/OXPHOS system), whereas another 24 genes specify 2 ribosomal RNAs (rRNAs) and 22 transfer RNAs (tRNAs) that are required to synthesize the 13 ETC/OXPHOS subunits.84 Mitochondria are only semiautonomous, however, and the bulk of the proteins of the ETC/OXPHOS system, as well as those required for mtDNA replication, transcription, repair, and mitochondrial metabolism, are encoded by over 1000 nuclear genes (nDNA) and must be imported into the mitochondria after their production in the cytoplasm.85
The ETC/OXPHOS system is composed of 5 complexes: complex I (NADH ubiquinone oxidoreductases), complex II (succinate ubiquinone oxidoreductases), complex III (ubiquinone-cytochrome c reductase), complex IV (cytochrome c oxidase), and complex V (ATP synthase), all located in the inner mitochondrial membrane.84,85 Although the flux of protons at these sites powers the ATP synthase (complex V) and subsequently leads to the generation of ATP in the mitochondrial matrix,85 this process seems inherently inefficient and can lead to the endogenous production of damaging ROS species. Thus, for example, it is estimated that 1% to 5% of electrons flowing through the ETC system are inadvertently donated to molecular oxygen to form superoxide (O2−), which can eventually lead to the formation of prooxidants and several damaging ROS species, including H2O2, hydroxyl radicals (•OH), and peroxynitrite anions (ONOO−).86 Mitochondrial dysfunction leading to the production of excessive ROS is thought to underlie the aging process and to contribute to the disease phenotypes associated with cancer, diabetes, and many progressive neurodegenerative disorders, such as Alzheimer disease, Parkinson disease, Huntington disease, and amyotrophic lateral sclerosis.87,88
The results of earlier studies hinted that ATM-deficient cells are also associated with varying abnormal mitochondrial functions. The mitochondria-specific superoxide dismutase MnSOD was elevated in the cerebrum and cerebellum of ATM-deficient mice.89 In addition, Stern et al90 reported that mitochondrial respiration was deregulated in ATM-deficient mice, was elevated in the cerebellum, and was reduced in the cerebrum.90 We investigated mitochondrial functions in lymphoblasts established from A-T patients.45 Although we found that confluent cultures of normal and A-T lymphoblasts had similar numbers of mitochondria, we also found that A-T cells exhibited (1) an abnormal structural organization of mitochondria; (2) larger populations of mitochondria with decreased membrane potential (ΔΨ); and (3) an increase in the basal expression levels of several nuclear DNA-encoded oxidative damage responsive genes whose proteins are targeted to the mitochondria, including polymerase γ, mitochondrial topoisomerase I, peroxiredoxin 3, and MnSOD. Consistent with these results, we observed that overall mitochondrial respiratory activity was diminished in A-T lymphoblasts but could be rescued by treating the cells with the antioxidant, α lipoic acid (ALA), or by the expression of full-length ATM.45 These results showed that ATM is required for the regulation of mitochondrial function. Since that time, several investigators have reported on varying mitochondrial numbers and mitochondrial dysfunction in ATM-deficient human and animal cells.
Similar to our results obtained with lymphoblasts established from A-T patients,45 Eaton et al91 showed that confluent cultures of normal and A-T human fibroblasts had similar numbers of mitochondria. However, when these investigators studied early-passage (60% confluent) cultures, they found that A-T fibroblasts had ∼50% fewer mitochondria than normal, a phenomenon that could be reproduced when normal fibroblasts were treated with the ATM inhibitor KU-55933. Furthermore, these investigators observed that the levels of certain subunits of the ribonucleotide reductase complex were misregulated in ATM-deficient fibroblasts. More importantly, the low levels of ribonucleotide-reductase activity correlated significantly with the reduced numbers of mitochondria evident in the early-passage ATM-deficient fibroblasts. Collectively, these results suggest that ATM-induced regulation of the ribonucleotide reductase complex is essential for mitochondrial biogenesis.91
In contrast to earlier reports,45,91 Valentin-Vega et al46 studied mitochondria in thymocytes derived from Atm−/− mice and found that early passage Atm−/− thymocytes accumulated an abnormally high mitochondrial mass, which was accompanied by elevated mitochondrial respiration rates. However, Valentin-Vega et al46 argue that the abnormally high mitochondrial mass they observed was not due to an increase in mitochondrial biogenesis but rather to defects in mitophagy (a type of mitochondrial autophagy). Consistent with this notion, Atm−/− thymocytes were proficient at performing conventional autophagy but displayed reduced levels of the DJ-1 protein, which these authors suggested is involved in the recycling of abnormal mitochondria.46 Levels of complex I and ATP were also significantly decreased in the ATM-deficient thymocytes46 ; in turn, these findings were consistent with those of previous studies showing that the activities of complex I and complex IV (cytochrome c oxidase) in mitochondria of ATM-deficient mice were greatly diminished in skeletal muscle and liver extracts, respectively.92,93 It is interesting that Valentin-Vega et al46 showed that the abnormally high mitochondrial mass, elevated mitochondrial respiration rates, and mitochondrial complex I deficiency in Atm−/− thymocytes could all be reversed when these cells were made heterozygous for the Beclin-1 gene, which encodes the Beclin-1 protein regulator of autophagy. It should be recognized, however, that the mechanisms by which Beclin-1 regulates mitochondrial biogenesis, as well as complexes of the mitochondrial ETC/OXPHOS system and respiration rates, require further elucidation.
Taken together, an emerging operational model implicates ATM as a hierarchical protein not only for DNA processing and repair but as well for maintaining mitochondrial homeostasis, in ways that may be cell and/or tissue dependent. It will further be necessary to delineate the direct vs indirect effects of ATM on mitochondrial function. Furthermore, because repair of mitochondrial DNA damage remains largely unexplored, there is little, if any, experimental evidence in the literature to date describing a role for ATM in directly and/or indirectly coordinating the repair of mitochondrial DNA damage. Along this line, it is noteworthy that several ATM-dependent substrates with roles in the DNA damage response, including, Mre11, CREB, p53, BRCA1, and ATM-related kinases (eg, mTOR), have been shown to translocate to the mitochondria after activation and appear to be required for proper mitochondrial function.94,,,,,-100
ATM, insulin signaling, and cardiovascular disease
Among the clinical hallmarks associated with A-T is an increased risk of developing insulin resistance and type 2 diabetes.101,102 Although poorly documented in the medical literature, A-T homozygotes appear to be at an increased risk of developing vascular disease, and this may apply to heterozygotes as well but to a lesser degree. This may be associated with mitochondrial genome instability and dysfunction or perhaps reflects a much more basic defect of impaired ATM function.
ATM appears to make a number of important contributions to cell growth and proliferation by regulating insulin-mediated signaling and glucose homeostasis, a pathway that may ultimately affect mate selection by controlling the size of sexually selected weapons, such as antlers in deer and horns of rhinoceros beetles.103 Yang and Kastan73 published data suggesting that ATM participates in insulin-induced protein synthesis. These authors reported that insulin activates ATM kinase activity in vivo, and confirmed that ATM phosphorylates 4E-BP1 on Ser111 in vitro (Sidebox 1).73,104 Moreover, ATM-dependent phosphorylation of 4E-BP1 was apparent in normal human and mouse embryonic fibroblasts treated with insulin.73 It would appear, therefore, that an insulin-resistance phenotype of ATM-deficient cells could, in part at least, be explained by the disruption to insulin-dependent regulation of protein synthesis.
“Cap-dependent translation” pathway
The “cap-dependent translation” pathway involves modulating the recruitment and binding of mRNA to the 40S ribosomal subunit. Successful recruitment of mRNA to the 40S ribosomal subunit is dependent upon the availability of the eIF4E subunit, which is responsible for recognizing and binding the 7-methylguanosine cap structure located at the 5′ terminus of mRNA. In resting cells, the eIF4E subunit is usually sequestered by 1 of its binding partners, 4E-BP1, to form the eIF4E-4E-BP1 complex. The formation of the eIF4E-4E-BP1 complex prevents the eIF4E subunit from binding to the scaffolding protein eIF4G; the eIF4E-eIF4G complex is required for recruitment of mRNA to the 40S ribosomal subunit and formation of the protein-translation initiation complex, eIF4F. In the presence of insulin and other growth factors, however, the 4E-BP1 subunit is phosphorylated leading to the subsequent release of eIF4E, formation of the eIF4E-eIF4G complex, and the initiation of translation of mRNA.104 ATM kinase activity is required for the phosphorylation of 4E-BP1 (on Ser111) in response to insulin, at least in vitro.73 It seems plausible, therefore, that the insulin-resistance phenotype exhibited by ATM-deficient cells could stem in part at least from the constitutive formation of the eIF4E-4E-BP1 complex, which would be expected to inhibit the initiation of translation of mRNA in response to insulin in these cells.
Disruption of insulin-controlled glucose transport is thought to be a major contributing factor to the development of insulin resistance and type 2 diabetes. Mice with defects in the cell-surface glucose transporter 4 (GLUT4) complex developed insulin resistance and glucose intolerance. ATM appears to regulate insulin-mediated glucose uptake by phosphorylating the serine-threonine kinase Akt, which is known to be involved in many cellular processes, including proliferation, apoptosis, cell-cycle control, and glucose metabolism. In addition, Akt is directly phosphorylated at Ser473 and Thr308 in response to insulin.74,105 When ATM kinase activity was blocked in mouse skeletal muscle cells, Akt phosphorylation was greatly diminished, and this event appeared to be associated with a significant reduction in insulin-mediated glucose uptake.75 Furthermore, although Atm−/− muscle cells transfected with wild-type ATM showed normal translocation of the GLUT4 complex to the cell surface in response to insulin, translocation of GLUT4 was blunted in cells stably expressing a kinase dead-ATM (Figure 1).75 These results suggest, at least in skeletal muscle cells, that ATM is involved in insulin-dependent signaling and glucose homeostasis by sustaining the increased phosphorylation of Akt and enhancing the translocation of the cell surface GLUT4 complex. ATM-dependent control of glucose transport by GLUT4 most likely plays an even more critical role in brain and cerebellar development and can now be evaluated by PET (Positive Emission Tomography) studies comparing glucose transport receptor functions by 2-FDG and 4-FDG tracers in Atm-deficient animal models.106,107
ATM also appears to regulate insulin functions and glucose homeostasis indirectly through a p53-dependent pathway. ATM phosphorylates the human p53 tumor suppressor Ser15 (Ser18 on murine p53), thereby regulating its transcriptional activity. Armata et al108 studied insulin sensitivity in mice with a p53 Ser18 to Ala18 mutation. The loss of p53 Ser18 led to increased metabolic stress and to the development of glucose intolerance and insulin resistance. More importantly perhaps, the insulin resistance exhibited by late-passage primary fibroblasts could be ameliorated by N-Acetyl cysteine (NAC) treatment. The addition of NAC in the diet also rendered the p53 Ser18-deficient mice glucose tolerant.108 Together these data imply that ATM phosphorylation of p53, followed by p53-dependent regulation of cellular ROS levels, is an important determining factor in the physiological regulation of glucose homeostasis.
Besides being prone to developing insulin resistance, A-T patients may also be at an increased risk of developing cardiovascular disease. Early epidemiology data suggested that even ATM heterozygosity might elevate the risk of death from cardiovascular disease.109 Schneider et al105 also showed that ATM haploinsufficiency accelerated diet-induced atherosclerotic lesions in apoE null mice, along with other features associated with the metabolic syndrome, including insulin resistance and glucose intolerance. In addition, these authors found that ATM deficiency led to increases in the activity of the Jun N-terminal kinase (JNK) in multiple tissues of apoE null mice, which correlated with an increase in insulin resistance and accelerated atherosclerosis.105 Furthermore, when Atm+/+ apoE−/− mice were treated with low doses of the ATM-activating drug, chloroquine, JNK activity was suppressed and was accompanied by decreases in diet-induced atherosclerosis, leading these authors to suggest that ATM-dependent suppression of JNK mediates susceptibility to atherosclerosis. Mercer et al93 showed that Atm+/−apoE−/− mice developed accelerated atherosclerosis and multiple features of the metabolic syndrome, including hypertension, hypercholesterolemia, obesity, steatohepatitis, and glucose intolerance. Moreover, in Atm+/−apoE−/− mice, atherosclerosis was attenuated by transplantation with Atm+/+ bone marrow, whereas the metabolic syndrome was not. Intriguingly, Atm+/− apoE−/− mouse tissues, particularly liver, appeared to show signs of mitochondrial DNA damage and intrinsic mitochondrial dysfunction, including reduced mitochondrial oxidative phosphorylation. The reduction in oxidative phosphorylation in Atm+/−apoE−/− mice was subsequently linked to the deletion of the so-called “4977 bp common mitochondrial-DNA deletion,” which spans 5 tRNA genes, as well as several genes encoding respiratory chain polypeptides.93,110 Thus, the onset of accelerated atherosclerosis, at least in mice, appears to involve mitochondrial dysfunction initiated by ATM-deficiency and perhaps mitochondrial genome instability.
A recent genome-wide association study of 1024 metformin-treated patients with Type 2 diabetes localized a glycemic response to metformin to the genomic region around the ATM gene, based on associations with 14 SNPs.111 This observation was replicated in 2 follow-up cohorts totaling 2896 subjects, which further localized the strongest genome-wide association study associations to SNPs in the 3′UTR region of ATM and the adjacent C11org65 gene just downstream. Metformin has been used as a first-line therapeutic for Type 2 diabetes for more than 50 years and is known to activate AMPK by inhibiting the mitochondrial respiratory chain, with a subsequent increase in cellular AMP.112,113 When ATM kinase activity was inhibited by KU-55933, in a rat hepatoma cell line, AMPKpThr172 activity was significantly reduced. This is not unexpected considering that liver kinase LKBpThr266 is phosphorylated in an ATM-dependent manner and, as mentioned previously, activated LKB then phosphorylates AMPK at Thr172 (Figure 1). Nonetheless, there is significant variability in the glycemic response to metformin. The authors hope that this work will lead to the design of new metformin derivatives for patients with diabetes.
Epilogue
Recognizing the cytoplasmic roles of ATM has far-reaching translational implications for A-T and other related disorders. For example, acknowledging ATM as a rate-limiting mitochondrial protein begs the question as to whether mitochondrial dysfunction is an important pathogenetic factor in other neurodegenerative disorders besides A-T.87 The work in our laboratory suggests that many “radiosensitive” disorders have mitochondrial respiratory changes similar to those associated with A-T.45,114,-116
Recognizing A-T as the prototype for another overarching syndrome of X-ray sensitivity, cancer susceptibility, immunodeficiency, neurologic aberrations, and DSBs (XCIND syndrome) expands the impact of identifying new roles for ATM and allows confirmation of predicted pathways and interactions between proteins.117 Already we recognize phenocopies for the MRN complex proteins in the diseases associated with mutations in Rad50118 and NBS, as well as the synergistic roles and overlapping phenotypes of A-T and Mre11 deficiency.119 Infants presenting with severe combined immunodeficiency (SCID) variants, who are both severely immunodeficient and severely radiosensitive, carry mutations primarily in genes of the nonhomologous end joining pathway and not, thus far, of the homologous recombination pathway or ubiquitinylation ligase cascade.114,116,120 On the other hand, T-cell development defects continue to implicate class switch recombination,119 whereas lymphoma pathology appears to involve primarily B-cell developmental defects and early DSB response mechanisms.14,119,121,122 However, there are also many exceptions to these generalizations.
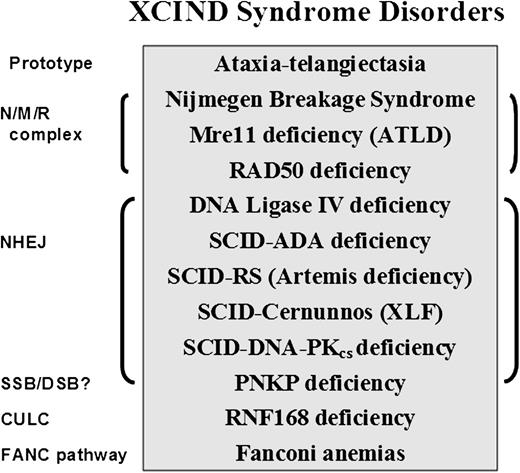
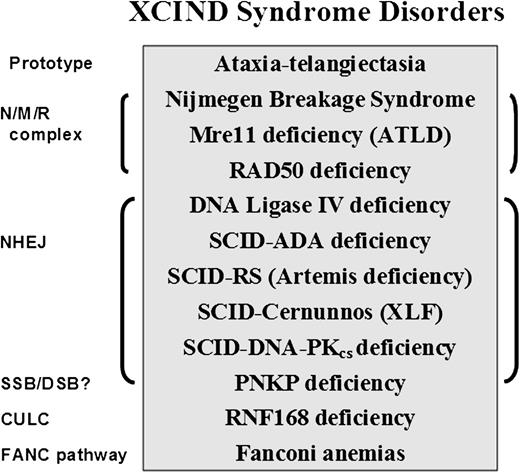
The XCIND syndrome disorders (Figure 2)114,116,117,119,120,123,,,-127 primarily include cancers of the lymphoid system, despite the many early deaths from overwhelming infections.1,2 If being considered for stem cell transplantation or radiation therapy for cancer, XCIND patients are also at risk of receiving life-threatening doses of radiation and/or chemotherapy.114 The first and last inclusion criteria (X-ray sensitivity and DSB repair) separate the XCIND syndrome from another syndrome that might include single-strand break disorders, such as xeroderma pigmentosum, Cockayne syndrome, and Seckle syndrome, which are not typically sensitive to ionizing radiation and generally activate separate and distinct DNA-repair pathways.128 Thus, a patient suspected of having XCIND syndrome would not be expected to manifest skin sensitivity to sunlight and the anticipated malignancies would most likely involve the lymphoid system rather than the skin or kidney cancers that are more characteristic of single-strand break disorders. Such insights may prove useful in designing new cancer treatments that are based on the principles of synthetic lethality.129
XCIND syndrome disorders and associated DNA-repair pathways. The prototype for the XCIND syndrome is A-T, involving X-ray sensitivity, Cancer susceptibility, Immunodeficiency, Neurologic involvement, and DSB repair.114 The NMR complex is necessary for recruitment of ATM to a DSB and activation of its kinase activity. Patients lacking NBS (nibrin), Mre11 (aka ATLD), and Rad50 proteins are usually children with features of XCIND syndrome, although the types of neurologic involvement differ for the 3 disorders.119,123 Nonhomologous end-joining (NHEJ) repair comprises the major DSB repair pathway in man. Patients lacking functional proteins in this pathway often present in infancy as SCID, although DNA ligase IV deficiency has been described as well in children with primary dwarfism and in adults.120,124 PNKP protein has both polynucleotide kinase and phosphatase functions, moving the phosphate groups from 5′ to 3′, thereby optimizing conditions for ligation in end-joining. PNKP may also play a role in single-strand break repair. Affected infants display intractable seizures, microcephaly, and developmental delay.125 Absence of RNF168 protein results in poor retention of 53BP1 and BRCA1 at sites of DSB, as well as at other predicted interactions within the chromatin ubiquitin ligase cascade (CULC).117 Two patients with RNF168 have been described, both with adult-onset immunodeficiency, neurologic involvement, and growth retardation116,117,126 ; severe microcephaly was noted in 1, with normal intelligence.116 The inclusion of Fanconi anemia (itself a syndrome) within this partial list of XCIND syndrome-associated disorders is based arguably on the manifestations of radiosensitivity,127 and many of the patients have also been associated with untoward responses to chemotherapy.114 The disorders listed herein are inherited as autosomal recessives.
XCIND syndrome disorders and associated DNA-repair pathways. The prototype for the XCIND syndrome is A-T, involving X-ray sensitivity, Cancer susceptibility, Immunodeficiency, Neurologic involvement, and DSB repair.114 The NMR complex is necessary for recruitment of ATM to a DSB and activation of its kinase activity. Patients lacking NBS (nibrin), Mre11 (aka ATLD), and Rad50 proteins are usually children with features of XCIND syndrome, although the types of neurologic involvement differ for the 3 disorders.119,123 Nonhomologous end-joining (NHEJ) repair comprises the major DSB repair pathway in man. Patients lacking functional proteins in this pathway often present in infancy as SCID, although DNA ligase IV deficiency has been described as well in children with primary dwarfism and in adults.120,124 PNKP protein has both polynucleotide kinase and phosphatase functions, moving the phosphate groups from 5′ to 3′, thereby optimizing conditions for ligation in end-joining. PNKP may also play a role in single-strand break repair. Affected infants display intractable seizures, microcephaly, and developmental delay.125 Absence of RNF168 protein results in poor retention of 53BP1 and BRCA1 at sites of DSB, as well as at other predicted interactions within the chromatin ubiquitin ligase cascade (CULC).117 Two patients with RNF168 have been described, both with adult-onset immunodeficiency, neurologic involvement, and growth retardation116,117,126 ; severe microcephaly was noted in 1, with normal intelligence.116 The inclusion of Fanconi anemia (itself a syndrome) within this partial list of XCIND syndrome-associated disorders is based arguably on the manifestations of radiosensitivity,127 and many of the patients have also been associated with untoward responses to chemotherapy.114 The disorders listed herein are inherited as autosomal recessives.
Microcephaly is frequently encountered among patients with XCIND disorders (eg, A-T Fresno, NBS, Rad50, Cernunnos/XLF-SCID, DNA ligase IV deficiency, RNF168 deficiency, and PNKP [Polynucleotide Kinase/Phosphatase] deficiency) and is an easily recognized clinical finding, even before birth (see Figure 2 legend for additional references). Thus, microcephaly represents a potential early biomarker of aberrant DSB repair, hereditary cancer susceptibility, immunodeficiency, and radiosensitivity, which, when used to increase the clinical suspicion of an underlying XCIND syndrome, would also justify laboratory testing of DNA repair and mitochondrial functions. In conclusion, the evolving phenotype of “A-T” is highlighting additional translational associations to as yet unrealized ATM-related functions.
Acknowledgments
The authors thank Pamela and George Smith for the encouragement and long-term support of A-T research and the A-T Medical Research Foundation.
This work was partially funded by USPHS grants NS052528 and AI067769, and California Institutes for Regenerative Medicine (CIRM) RT2-01920.
Authorship
Contribution: M.A. and R.A.G. contributed equally to the preparation and writing of this review and have no conflict of interest with any of the stated contents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard A. Gatti, David Geffen School of Medicine at UCLA, Los Angeles Department of Pathology & Laboratory Medicine, 675 Charles Young Dr South, MRL 4-736, Los Angeles, CA 90095, e-mail: rgatti@mednet.ucla.edu.
References
Author notes
M.A. and R.A.G. contributed equally to this study.