Key Points
High-dose intensive factor VIII treatment increases the risk for inhibitor development in patients with severe hemophilia A.
In patients with severe hemophilia A, factor VIII prophylaxis decreases inhibitor risk, especially in patients with low-risk F8 mutations.
The objective of this study was to examine the association of the intensity of treatment, ranging from high-dose intensive factor VIII (FVIII) treatment to prophylactic treatment, with the inhibitor incidence among previously untreated patients with severe hemophilia A. This cohort study aimed to include consecutive patients with a FVIII activity < 0.01 IU/mL, born between 2000 and 2010, and observed during their first 75 FVIII exposure days. Intensive FVIII treatment of hemorrhages or surgery at the start of treatment was associated with an increased inhibitor risk (adjusted hazard ratio [aHR], 2.0; 95% confidence interval [CI], 1.3-3.0). High-dose FVIII treatment was associated with a higher inhibitor risk than low-dose FVIII treatment (aHR, 2.3; 95% CI, 1.0-4.8). Prophylaxis was only associated with a decreased overall inhibitor incidence after 20 exposure days of FVIII. The association with prophylaxis was more pronounced in patients with low-risk F8 genotypes than in patients with high-risk F8 genotypes (aHR, 0.61, 95% CI, 0.19-2.0 and aHR, 0.85, 95% CI, 0.51-1.4, respectively). In conclusion, our findings suggest that in previously untreated patients with severe hemophilia A, high-dosed intensive FVIII treatment increases inhibitor risk and prophylactic FVIII treatment decreases inhibitor risk, especially in patients with low-risk F8 mutations.
Introduction
Patients with severe hemophilia A (factor VIII [FVIII] activity < 0.01 IU/mL) have a bleeding diathesis characterized by spontaneous joint and muscle bleeding. The current standard of care of children with severe hemophilia A is primary prophylaxis: regular FVIII infusions aimed at prevention of joint damage that are started from the first joint bleeding onward or earlier.1,2
Most patients with hemophilia do not mount a clinically measurable immune response toward FVIII. In ∼30% of patients, however, such FVIII antibodies develop, rendering FVIII treatment ineffective and impairing the functional status of patients.3,4
The capacity to develop inhibitors varies from one individual to another and depends on the interaction of multiple genetic and nongenetic risk factors. The causative F8 genotype is an important genetic risk factor.5,6 Other reported genetic risk factors are a family history of inhibitor development,7 ethnicity,7 HLA genotype8,9 and polymorphisms in immune regulatory genes.10,,,-14
Nongenetic risk factors for the occurrence of inhibitory antibodies are largely related to FVIII treatment. It has been suggested that this may be partly explained by the immunologic danger theory.15 According to the immunologic danger theory, inhibitor development may be influenced by the extent of tissue damage at the time of FVIII infusions. This model proposes that antigen-presenting cells need to be activated by alarm signals to elicit an effective antibody response.15 Because the FVIII protein itself does not contain “alarm signals,”16 immune tolerance should occur on exposure to infused FVIII, unless FVIII is accompanied by alarm signals that trigger the maturation of dendritic cells.17 During FVIII treatment of major bleeding or surgery, a patient is exposed to high doses of FVIII in combination with tissue damage and inflammation. According to the danger theory, substances released from damaged tissue could activate antigen-presenting cells, which might subsequently present FVIII antigen with up-regulated costimulatory signals to T lymphocytes. These cells then enhance antibody formation.15 Indeed, repeated high-dose FVIII treatment given for major bleeding and surgery has been associated with an increased risk for inhibitor development.18,,-21
Low-dose prophylactic FVIII infusions, however, are given in the absence of tissue damage. Antigen-presenting cells present the FVIII antigen to T lymphocytes in the absence of costimulatory signals. This may cause the induction of immune tolerance by the generation of regulatory T cells from naïve T cells or cause anergy of previously primed T cells.17 Observations that prophylaxis is associated with a reduced risk for inhibitor development support this hypothesis.18,22,23 However, it is currently unclear whether the timing of the introduction of prophylaxis and the dose and the frequency of prophylactic FVIII infusions affect the risk for inhibitor development.
We set up a large, international cohort study to examine the association of the intensity of treatment with inhibitor incidence among previously untreated patients with severe hemophilia A. We studied high-dose intensive FVIII treatment of bleeds or surgery and prophylactic FVIII treatment, including the dose, frequency, and time of onset of prophylaxis.
Patients and methods
Patients
We aimed to include consecutive previously untreated patients with severe hemophilia A (FVIII activity <0.01 IU/mL) born between January 1, 2000, and January 1, 2010, diagnosed in one of the 29 participating hemophilia treatment centers. Patients who were referred because of the presence of an inhibitor were excluded. Approval was obtained from every center’s institutional review board. Written informed consent was obtained from the parents or guardians of all participants in accordance with the Declaration of Helsinki.
Data collection
Detailed data on potential treatment-related determinants were uniformly collected in all centers: all administrations of FVIII up to 75 exposure days or inhibitor development, including dates of infusion, doses and brands of FVIII products, reasons for treatment, types of bleeding, and surgery. Patients were monitored until the study end point, which was either the development of a clinically relevant inhibitor or a cumulative number of 75 exposure days.
The determination of clinically relevant inhibitor development was based on all performed inhibitor tests and recovery measurements (in case of borderline positive inhibitor test results) in patients who ever had a positive inhibitor measurement.
In the majority of centers (92%), patients were routinely screened for inhibitor development after every 1 to 5 exposure days during the first 20 exposure days and at least every 3 months thereafter. All centers closely monitored patients for clinical signs of inhibitor development and performed inhibitor and recovery testing at any clinical suspicion of inhibitor development.
All data collected were repeatedly checked for completeness and inconsistencies using prespecified protocols. Data-monitor visits were performed at least annually at each center, including ascertainment of 100% of included and excluded patients, hemophilia diagnosis, and F8 genotype, as well as ascertainment of a minimal 10% of source data on FVIII treatments.
Relatively few data were missing (supplemental Methods). For the current analyses, data collected until May 1, 2011, were used.
Outcomes
The primary outcome of the study was clinically relevant inhibitor development, defined as at least 2 positive inhibitor titers combined with a decreased in vivo FVIII recovery up to the 75th exposure day.18 A positive inhibitor titer was defined according to the cutoff level of the inhibitor assay used in each center’s laboratory. The FVIII recovery was considered to be decreased when it was less than 66% of the expected FVIII activity level 15 minutes after infusion of FVIII. The expected level of FVIII activity was calculated according to Lee et al.24
The secondary outcome was high titer inhibitor development, defined as the occurrence of a clinically relevant inhibitor with a peak titer of at least 5 Bethesda Units per mL (BU/mL).25
Determinants
Intensive FVIII treatment.
Intensive FVIII treatment was evaluated by peak treatment moments, surgical procedures, duration between exposure days, and dose of FVIII product.
Peak treatment moments and surgical procedures.
“Peak treatment moments” were defined as episodes of treatment with FVIII for bleeding or surgery on at least 3, 5, or 10 consecutive days. We studied “surgical procedures” for which replacement therapy lasting at least 3 consecutive days was given.18 The corresponding time-dependent variables were defined as the “after peak treatment moment” or “after surgical procedure.”
Duration between exposure days.
“Duration between exposure days” was the measure for frequency of exposures; it was defined as the period between each exposure day and 5 exposure days before this exposure day.
Dose of FVIII product.
To study the association between inhibitor occurrence and the “dose of FVIII product,” we calculated at each time point the mean FVIII dose per kilogram bodyweight of the previous 5 exposure days.
Because prophylaxis influences these factors to a large extent, the analyses of duration between exposure days and the dose of FVIII product were performed in patients who were treated on demand only.
Prophylaxis.
Ascertaining the moment when prophylaxis was truly started is not always clear-cut, because of venous access problems or lack of cooperation of the child. Therefore, we defined prophylaxis in 2 ways, analogous with the analysis of randomized controlled trials: the “per-protocol analysis” and “intention-to-treat analysis.”
Regular prophylaxis.
First, the “per-protocol analysis” was used to study the effect of regular prophylaxis on inhibitor development. Prophylactic FVIII infusions were all infusions of FVIII given to prevent bleeding. The start of regular prophylaxis was defined as the moment on which at least 3 consecutive prophylactic FVIII infusions had been given within a period of at least 15 days. Its effect was compared with on-demand treatment, which was defined as FVIII treatment without any prophylactic FVIII exposures. From the moment that at least 1 prophylactic infusion was given, until the criteria of regular prophylaxis were met, patients were categorized into a residual group, which was not included in the comparison.
This analysis was designed to assess the maximal effect of prophylactic FVIII infusions.
Intention to start prophylaxis.
Second, in the “intention-to-treat analysis,” we assessed the effect of the intention to start prophylaxis on inhibitor development, regardless of any delays or failures to start regular prophylaxis. This analysis was designed to estimate the effect of a prophylactic treatment regimen in clinical practice, including any surgery for venous access needed to accomplish prophylaxis. With this analysis, we aimed to avoid potential overestimation of the effect of prophylaxis. Because the intention to start prophylaxis was not registered as such, we defined it as the moment when either at least 1 prophylactic infusion was given or at the time of any surgical procedures to facilitate venous access (implantation of a central venous access device or creation of an arteriovenous fistula).
We assumed that after prophylaxis was started, its effect continued during the period up to the 75th exposure.
Dose and frequency of prophylaxis.
To investigate the effect of the dose and frequency of FVIII infusions in prophylaxis, regular prophylaxis was categorized into 4 arbitrarily defined categories of prophylaxis: (1) once a week, less than 30 IU/kg/infusion; (2) once a week, more than 30 IU/kg/infusion; (3) more than once a week, less than 30 IU/kg/infusion; and (4) more than once a week, more than 30 IU/kg/infusion. On-demand treatment was the reference.
Start of prophylaxis
To assess the effect of starting prophylaxis early, we defined “start of prophylaxis ≤15ED” as the start of regular prophylaxis before a cumulative number of 15 or fewer exposure days of on-demand treatment. Fifteen exposure days was chosen because this was the median number of exposure days at which patients started prophylaxis. Prophylaxis exposure days before a cumulative number of 15 or fewer exposure days were compared with on-demand treatment days. In these analyses, all prophylaxis exposure days of patients who started prophylaxis after their first 15 exposure days were censored.
Varying prophylaxis effect.
The cumulative inhibitor incidence curves for patients receiving prophylaxis and patients treated on demand showed that the difference between these patients only occurred later during treatment. For this reason, we also estimated the values of the HR allowing it to vary during 75 exposure days. The period of 1 to 75 exposure days was categorized into arbitrarily defined periods (1-10, 11-20, 21-30, 31-40, and 40-75 days).
Effect of prophylaxis in patients with high-risk and low-risk genotypes
Prophylaxis may have a different effect in patients, dependent on their F8 genotype. To investigate this, we assessed the relationship between prophylaxis and inhibitor development according to the presence of a high-risk or low-risk F8 genotype. High-risk F8 gene mutations were large gene deletions, nonsense mutations, and intron 1 and 22 inversions; low-risk F8 gene mutations were small deletions and insertions, missense mutations, and splice site mutations.
Sensitivity analyses of prophylaxis
Several sensitivity analyses on the effect of prophylaxis were performed. The methods and results are described in supplemental material.
Data analyses
We used survival analysis methods with the cumulative number of exposure days as the time variable instead of calendar time (pooled logistic regression). This method accounts for varying risks according to the cumulative number of exposure days, and its interpretation is similar to that of a Cox regression, with exposure days as time-variable and time-dependent covariates.26 Patients who had not yet reached the study end point were included in the analyses and were censored at the last exposure day. In the analyses with “high titer inhibitor development” as the outcome, censoring occurred at the last exposure day in noninhibitor patients and at the last exposure day at inhibitor development in patients with low titer inhibitors. HRs are interpreted as relative risks throughout the study.
Crude as well as adjusted hazard ratios (aHRs) are presented. The aHRs for time-fixed determinants (treatment-related factors at the first exposure) and associations assessed in patients treated on demand only (FVIII dose and duration between exposure days) were calculated using multivariable pooled logistic regression models. We used marginal structural models with an inverse probability of treatment weighting to adjust the associations between time-varying determinants and inhibitor development for confounders.27 We adjusted for possible determinants that could have confounded the specific associations studied, independent of their statistical significance in univariate analyses.
S.G. and J.G.v.d.B. analyzed the data. All authors have access to the primary data.
Role of the funding source
The RODIN Study was supported by unrestricted research grants from Bayer Healthcare and Baxter Bioscience. The companies did not have a role in the study design, data collection, data analysis, or the writing of this manuscript. As agreed in the contracts, the companies received a copy of this manuscript 2 weeks before submission.
Results
Patient characteristics
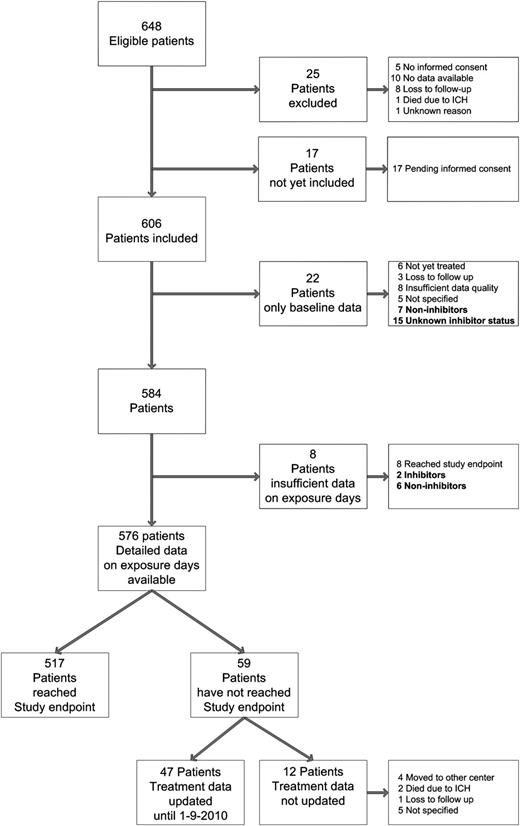
Figure 1 presents an overview of the study population. In this study, 576 patients were included on whom detailed exposure data were available. The baseline and treatment characteristics are presented in supplemental Table 1 and Table 1.
Overview of included patients. A total of 648 patients were eligible for this study. Of these, 17 patients were eligible but were not yet included in the study, and 25 patients were excluded by the centers' investigators for various reasons. Baseline data were available in 606 patients (93.5%). Of these 606 patients, 6 were not treated with FVIII products during the study period, 15 had an unknown inhibitor status because of unavailable data, 59 were treated on less than 75 exposure days (median, 18 days; IQR, 7-36 days; range, 1-69 days), 179 had development of clinically relevant inhibitors, and 347 patients were treated with FVIII on 75 exposure days without the development of inhibitors.
Overview of included patients. A total of 648 patients were eligible for this study. Of these, 17 patients were eligible but were not yet included in the study, and 25 patients were excluded by the centers' investigators for various reasons. Baseline data were available in 606 patients (93.5%). Of these 606 patients, 6 were not treated with FVIII products during the study period, 15 had an unknown inhibitor status because of unavailable data, 59 were treated on less than 75 exposure days (median, 18 days; IQR, 7-36 days; range, 1-69 days), 179 had development of clinically relevant inhibitors, and 347 patients were treated with FVIII on 75 exposure days without the development of inhibitors.
Treatment characteristics of included patients with detailed data (N = 576)
| Characteristics . | No. (%) or median (IQR) . | Range . |
|---|---|---|
| Age at first exposure (mo.) | 9.8 (5.3-13.4) | 0-55.8 |
| Age at 75th exposure day (mo.)* | 26.0 (19.5-34.5) | 2.9-115.0 |
| Duration between 1st and 75th exposure day (mo.)* | 15.6 (10.2-23.8) | 2.6-84.8 |
| Intensity of treatment | ||
| Treatment characteristics at first treatment | ||
| Reason of first exposure to FVIII | ||
| Hemorrhage, including head trauma | 488 (84.7) | NA |
| Prophylaxis | 68 (11.8) | NA |
| Surgery | 20 (3.5) | NA |
| Peak treatment moment at first exposure to FVIII | ||
| Peak treatment moment at least 3 days | 149 (25.9) | NA |
| Peak treatment moment at least 5 days | 98 (17) | NA |
| Peak treatment moment at least 10 days | 40 (6.9) | NA |
| Major surgery at first exposure to FVIII | 13 (2.3) | NA |
| Treatment characteristics at subsequent exposure days | ||
| Peak treatment moment during first 75 exposure days | ||
| Peak treatment moment at least 3 days | 386 (67.0) | NA |
| Peak treatment moment at least 5 days | 277 (48.1) | NA |
| Peak treatment moment at least 10 days | 93 (16.1) | NA |
| Ever had a major surgery during first 75 exposure days | 144 (25) | NA |
| Prophylaxis | ||
| Regular prophylaxis† | ||
| No. patients who started regular prophylaxis | 412 (71.5) | NA |
| Age start regular prophylaxis (mo.) | 16.7 (12.4-24.4) | 0.95-96.9 |
| No. exposure days at start prophylaxis | 15 (7-25) | 2-74 |
| Dose of FVIII at start of prophylaxis (IU/kg) | 44.6 (32.3-50.0) | 18.9-235.3 |
| Frequency of FVIII at start of prophylaxis, no. infusions per week | 1 (1-2) | 1-7 |
| Start of regular prophylaxis | ||
| No. patients who started prophylaxis <15 exposure days | 208 (50.5) | NA |
| No. patients who started prophylaxis <5 exposure days | 79 (19.2) | NA |
| Regular prophylaxis at least twice a week | ||
| No. patients who started regular prophylaxis at least twice a week | 316 (54.9) | NA |
| Age start regular prophylaxis at least twice a week (mo.) | 18.7 (12.9-27.2) | 1.0-96.9 |
| No. exposure days at start prophylaxis at least twice a week | 22 (14-40) | 2-74 |
| Intention to start prophylaxis‡ | ||
| No. patients who had an intention to start prophylaxis | 470 (81.6) | NA |
| Age at intention to start prophylaxis (mo.) | 14.6 (10.2-21.4) | 0-96.5 |
| No. exposure days at intention to start prophylaxis | 5 (2-12) | 0-74 |
| Characteristics . | No. (%) or median (IQR) . | Range . |
|---|---|---|
| Age at first exposure (mo.) | 9.8 (5.3-13.4) | 0-55.8 |
| Age at 75th exposure day (mo.)* | 26.0 (19.5-34.5) | 2.9-115.0 |
| Duration between 1st and 75th exposure day (mo.)* | 15.6 (10.2-23.8) | 2.6-84.8 |
| Intensity of treatment | ||
| Treatment characteristics at first treatment | ||
| Reason of first exposure to FVIII | ||
| Hemorrhage, including head trauma | 488 (84.7) | NA |
| Prophylaxis | 68 (11.8) | NA |
| Surgery | 20 (3.5) | NA |
| Peak treatment moment at first exposure to FVIII | ||
| Peak treatment moment at least 3 days | 149 (25.9) | NA |
| Peak treatment moment at least 5 days | 98 (17) | NA |
| Peak treatment moment at least 10 days | 40 (6.9) | NA |
| Major surgery at first exposure to FVIII | 13 (2.3) | NA |
| Treatment characteristics at subsequent exposure days | ||
| Peak treatment moment during first 75 exposure days | ||
| Peak treatment moment at least 3 days | 386 (67.0) | NA |
| Peak treatment moment at least 5 days | 277 (48.1) | NA |
| Peak treatment moment at least 10 days | 93 (16.1) | NA |
| Ever had a major surgery during first 75 exposure days | 144 (25) | NA |
| Prophylaxis | ||
| Regular prophylaxis† | ||
| No. patients who started regular prophylaxis | 412 (71.5) | NA |
| Age start regular prophylaxis (mo.) | 16.7 (12.4-24.4) | 0.95-96.9 |
| No. exposure days at start prophylaxis | 15 (7-25) | 2-74 |
| Dose of FVIII at start of prophylaxis (IU/kg) | 44.6 (32.3-50.0) | 18.9-235.3 |
| Frequency of FVIII at start of prophylaxis, no. infusions per week | 1 (1-2) | 1-7 |
| Start of regular prophylaxis | ||
| No. patients who started prophylaxis <15 exposure days | 208 (50.5) | NA |
| No. patients who started prophylaxis <5 exposure days | 79 (19.2) | NA |
| Regular prophylaxis at least twice a week | ||
| No. patients who started regular prophylaxis at least twice a week | 316 (54.9) | NA |
| Age start regular prophylaxis at least twice a week (mo.) | 18.7 (12.9-27.2) | 1.0-96.9 |
| No. exposure days at start prophylaxis at least twice a week | 22 (14-40) | 2-74 |
| Intention to start prophylaxis‡ | ||
| No. patients who had an intention to start prophylaxis | 470 (81.6) | NA |
| Age at intention to start prophylaxis (mo.) | 14.6 (10.2-21.4) | 0-96.5 |
| No. exposure days at intention to start prophylaxis | 5 (2-12) | 0-74 |
NA, not applicable.
In noninhibitor patients who reached the study end point and had available data on exposure days (n = 340).
The start of regular prophylaxis was defined as the moment on which at least 3 consecutive prophylactic FVIII exposure days are given for a period of at least 2 weeks.
The intention to start prophylaxis was defined as the moment on which at least 1 prophylactic infusion was given or at the time of any surgical procedures to facilitate venous access (implantation of a central venous access device or creation of an arteriovenous fistula).
Inhibitor development
The overall cumulative incidence of inhibitors was 32.0% (95% CI, 28.1-35.9). The cumulative incidence of high titer inhibitor development was 22.2% (95% CI, 18.7-25.8) (Figure 2).
Cumulative incidence of inhibitor development according to cumulative number of factor VIII exposure days for all inhibitors, high-titer, and low-titer inhibitors.
Cumulative incidence of inhibitor development according to cumulative number of factor VIII exposure days for all inhibitors, high-titer, and low-titer inhibitors.
Of the 179 patients with inhibitors, 118 (65.9%) had high titer inhibitors and 61 (34.1%) had low titer inhibitors. Table 2 summarizes the characteristics of the patients with inhibitors. Inhibitor development occurred in patients after a median of 14.5 exposure days (interquartile range [IQR], 9.75-20.0 days) at a median age of 15.5 months (age range, 8 days-6.8 years).
Inhibitor characteristics
| Characteristics . | All inhibitors . | Range . | High-titer* inhibitors . | Range . | Low-titer* inhibitors . | Range . |
|---|---|---|---|---|---|---|
| No. patients (%) | 179 (100) | NA | 118 (66) | NA | 61 (34) | NA |
| No. exposure days at inhibitor development (IQR) | 14.5 (9.75-20)† | 3->75‡ | 15 (9.5-19)† | 3->75‡ | 14 (9.5-24.0) | 3-66 |
| Peak inhibitor titer (BU/mL) (IQR) | 18.0 (3.0-103.0) | 0.5-6351.0 | 50.5 (18.4-308.5) | 5.4-6351.0 | 2 (1.1-3.0) | 0.5-4.4 |
| Age at inhibitor development, mo. (IQR) | 15.5 (10.8-19.6) | 8d-6.8y | 14.0 (9.4-18.0) | 8d-6.8y | 17.1 (13.5-23.9) | 20d-3.2y |
| Duration between first exposure day and inhibitor development, mo. (IQR) | 4.3 (1.6-8.2)† | 7d-5.9y | 3.5 (1.4-7.4)† | 7d-5.9y | 5.7 (3.1-10.3) | 8d-2.4y |
| Characteristics . | All inhibitors . | Range . | High-titer* inhibitors . | Range . | Low-titer* inhibitors . | Range . |
|---|---|---|---|---|---|---|
| No. patients (%) | 179 (100) | NA | 118 (66) | NA | 61 (34) | NA |
| No. exposure days at inhibitor development (IQR) | 14.5 (9.75-20)† | 3->75‡ | 15 (9.5-19)† | 3->75‡ | 14 (9.5-24.0) | 3-66 |
| Peak inhibitor titer (BU/mL) (IQR) | 18.0 (3.0-103.0) | 0.5-6351.0 | 50.5 (18.4-308.5) | 5.4-6351.0 | 2 (1.1-3.0) | 0.5-4.4 |
| Age at inhibitor development, mo. (IQR) | 15.5 (10.8-19.6) | 8d-6.8y | 14.0 (9.4-18.0) | 8d-6.8y | 17.1 (13.5-23.9) | 20d-3.2y |
| Duration between first exposure day and inhibitor development, mo. (IQR) | 4.3 (1.6-8.2)† | 7d-5.9y | 3.5 (1.4-7.4)† | 7d-5.9y | 5.7 (3.1-10.3) | 8d-2.4y |
Values are medians (IQR) or numbers (%). NA, not applicable.
High-titer inhibitor was defined as a clinically relevant inhibitor with a peak titer of at least 5 BU/mL, and alow-titer inhibitor was defined as a clinically relevant inhibitor with a peak titer of less than 5 BU/mL.
Unknown in 1 high-titer inhibitor patient.
The patient with inhibitor development after 75 exposure days was a newborn who underwent a surgical procedure after birth with subsequent daily “prophylaxis” 500 IU until inhibitor detection at age 3 months.
Intensive FVIII treatment
The crude and adjusted relative risks for inhibitor development according to intensive FVIII treatment are presented in Table 3.
Intensive treatment and inhibitor development
| Characteristics . | All inhibitors . | High-titer inhibitors . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. exposure days . | N inh/N . | % . | Crude HR (95% CI) . | P value . | aHR (95% CI) . | P value . | N inh/N . | % . | Crude HR (95% CI) . | P value . | aHR (95% CI) . | P value . | |
| Treatment characteristics of first exposure | |||||||||||||
| Reason of first exposure to FVIII* | |||||||||||||
| Hemorrhage | 151/488 | 32.6 | 1 | 1 | 98/488 | 21.1 | 1 | 1 | |||||
| Prophylaxis | 19/68 | 28.8 | 0.86 (0.53-1.4) | .55 | 0.91 (0.53-1.6) | .73 | 13/68 | 22.4 | 0.91 (0.51-1.6) | .75 | 0.98 (0.51-1.9) | .95 | |
| Minor and major surgery | 7/20 | 37.1 | 1.1 (0.52-2.4) | .76 | 1.2 (0.54-2.6) | .69 | 5/20 | 27.1 | 1.2 (0.50-3.1) | .65 | 1.2 (0.48-3.1) | .68 | |
| Major surgery at first exposure*† | |||||||||||||
| No major surgery | 169/561 | 31.7 | 1 | 1 | 111/561 | 22.0 | 1 | 1 | |||||
| Venous access surgery | 2/4 | 50.0 | 1.7 (0.41-7.0) | .46 | 1.8 (0.43-7.7) | .42 | 1/4 | 33.3 | 1.3 (0.18-9.3) | .81 | 1.1 (0.14-8.2) | .94 | |
| Surgery for other indications | 6/10 | 60.0 | 2.2 (0.95-5.0) | .066 | 2.1 (0.77-5.8) | .14 | 4/10 | 41.7 | 2.2 (0.79-6.0) | .13 | 1.9 (0.55-6.6) | .31 | |
| Peak treatment moment at first exposure* | |||||||||||||
| None | 107/423 | 26.9 | 1 | 1 | 63/423 | 16.6 | 1 | 1 | |||||
| 3-5 days | 20/55 | 37.0 | 1.5 (0.93-2.4) | .099 | 1.4 (0.87-2.3) | .15 | 14/55 | 26.8 | 1.8 (1.0-3.2) | .052 | 1.7 (0.95-3.1) | .074 | |
| 5-10 days | 29/58 | 50.7 | 2.1 (1.4-3.2) | .00037 | 2.0 (1.3-3.0) | .0026 | 20/58 | 38.2 | 2.5 (1.5-4.1) | .00047 | 2.4 (1.4-4.1) | .0015 | |
| ≥10 days | 21/40 | 52.5 | 2.1 (1.3-3.3) | .0029 | 1.7 (1.0-2.9) | .047 | 19/40 | 49.5 | 3.1 (1.9-5.3) | .000015 | 2.7 (1.5-4.9) | .0014 | |
| Treatment characteristics of subsequent exposure days | |||||||||||||
| Peak treatment moment ≥3 days*† | 18 242 | 1.5 (1.1-2.0) | .015 | 1.5 (1.0-2.2) | .039 | 1.7 (1.2-2.6) | .0066 | 1.6 (1.0-2.6) | .046 | ||||
| Peak treatment moment ≥5 days*† | 12 975 | 1.5 (1.1-2.0) | .0078 | 1.4 (0.97-2.0) | .071 | 1.6 (1.1-2.3) | .012 | 1.3 (0.83-2.1) | .25 | ||||
| Peak treatment moment ≥ 10 days*† | 4282 | 1.7 (1.2-2.5) | .0051 | 1.3 (0.77-2.2) | .32 | 2.1 (1.4-3.3) | .00057 | 1.7 (0.96-3.1) | .068 | ||||
| Surgery*† | 7361 | 0.93 (0.64-1.4) | .73 | 1.0 (0.60-1.8) | .87 | 0.76 (0.46-1.3) | .29 | 0.84 (0.38-1.9) | .68 | ||||
| Type of surgery*† | |||||||||||||
| No or before surgery | 22 396 | 1 | 1 | 1 | 1 | ||||||||
| After surgery for venous access | 5266 | 0.78 (0.48-1.3) | .31 | 0.84 (0.51-1.4) | .50 | 0.61 (0.32-1.2) | .14 | 0.66 (0.34-1.3) | .23 | ||||
| After surgery for other indications | 1660 | 1.5 (0.83-2.6) | .19 | 1.4 (0.74-2.6) | .30 | 1.2 (0.54-2.5) | .69 | 0.95 (0.41-2.2) | .91 | ||||
| Unknown indication | 435 | 0.47 (0.066-3.4) | .46 | 0.40 (0.056-2.9) | .37 | 0.72 (0.099-5.2) | .74 | 0.63 (0.085-4.6) | .65 | ||||
| Duration between exposure days‡,§ | .18|| | .93|| | .030|| | .42|| | |||||||||
| >50 | 1119 | 1 | 1 | 1 | 1 | ||||||||
| 10-50 | 799 | 1.2 (0.68-2.0) | 1.0 (0.64-1.6) | 0.99 (0.50-2.0) | 0.99 (0.49-2.0) | ||||||||
| <10 | 1238 | 1.3 (0.87-2.1) | 1.2 (0.68-2.0) | 1.7 (1.0-2.9) | 1.3 (0.71-2.2) | ||||||||
| Dose of FVIII product (IU/kg) §,¶ | .0019|| | .11|| | .0087|| | .49|| | |||||||||
| <35 | 640 | 1 | 1 | 1 | 1 | ||||||||
| 35-50 | 1093 | 2.4 (1.2-4.9) | 2.4 (1.2-5.2) | 2.4 (1.0-5.7) | 2.4 (0.95-5.8) | ||||||||
| >50 | 1423 | 3.0 (1.5-6.0) | 2.3 (1.0-4.8) | 3.0 (1.3-6.9) | 1.8 (0.72-4.7) | ||||||||
| Characteristics . | All inhibitors . | High-titer inhibitors . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. exposure days . | N inh/N . | % . | Crude HR (95% CI) . | P value . | aHR (95% CI) . | P value . | N inh/N . | % . | Crude HR (95% CI) . | P value . | aHR (95% CI) . | P value . | |
| Treatment characteristics of first exposure | |||||||||||||
| Reason of first exposure to FVIII* | |||||||||||||
| Hemorrhage | 151/488 | 32.6 | 1 | 1 | 98/488 | 21.1 | 1 | 1 | |||||
| Prophylaxis | 19/68 | 28.8 | 0.86 (0.53-1.4) | .55 | 0.91 (0.53-1.6) | .73 | 13/68 | 22.4 | 0.91 (0.51-1.6) | .75 | 0.98 (0.51-1.9) | .95 | |
| Minor and major surgery | 7/20 | 37.1 | 1.1 (0.52-2.4) | .76 | 1.2 (0.54-2.6) | .69 | 5/20 | 27.1 | 1.2 (0.50-3.1) | .65 | 1.2 (0.48-3.1) | .68 | |
| Major surgery at first exposure*† | |||||||||||||
| No major surgery | 169/561 | 31.7 | 1 | 1 | 111/561 | 22.0 | 1 | 1 | |||||
| Venous access surgery | 2/4 | 50.0 | 1.7 (0.41-7.0) | .46 | 1.8 (0.43-7.7) | .42 | 1/4 | 33.3 | 1.3 (0.18-9.3) | .81 | 1.1 (0.14-8.2) | .94 | |
| Surgery for other indications | 6/10 | 60.0 | 2.2 (0.95-5.0) | .066 | 2.1 (0.77-5.8) | .14 | 4/10 | 41.7 | 2.2 (0.79-6.0) | .13 | 1.9 (0.55-6.6) | .31 | |
| Peak treatment moment at first exposure* | |||||||||||||
| None | 107/423 | 26.9 | 1 | 1 | 63/423 | 16.6 | 1 | 1 | |||||
| 3-5 days | 20/55 | 37.0 | 1.5 (0.93-2.4) | .099 | 1.4 (0.87-2.3) | .15 | 14/55 | 26.8 | 1.8 (1.0-3.2) | .052 | 1.7 (0.95-3.1) | .074 | |
| 5-10 days | 29/58 | 50.7 | 2.1 (1.4-3.2) | .00037 | 2.0 (1.3-3.0) | .0026 | 20/58 | 38.2 | 2.5 (1.5-4.1) | .00047 | 2.4 (1.4-4.1) | .0015 | |
| ≥10 days | 21/40 | 52.5 | 2.1 (1.3-3.3) | .0029 | 1.7 (1.0-2.9) | .047 | 19/40 | 49.5 | 3.1 (1.9-5.3) | .000015 | 2.7 (1.5-4.9) | .0014 | |
| Treatment characteristics of subsequent exposure days | |||||||||||||
| Peak treatment moment ≥3 days*† | 18 242 | 1.5 (1.1-2.0) | .015 | 1.5 (1.0-2.2) | .039 | 1.7 (1.2-2.6) | .0066 | 1.6 (1.0-2.6) | .046 | ||||
| Peak treatment moment ≥5 days*† | 12 975 | 1.5 (1.1-2.0) | .0078 | 1.4 (0.97-2.0) | .071 | 1.6 (1.1-2.3) | .012 | 1.3 (0.83-2.1) | .25 | ||||
| Peak treatment moment ≥ 10 days*† | 4282 | 1.7 (1.2-2.5) | .0051 | 1.3 (0.77-2.2) | .32 | 2.1 (1.4-3.3) | .00057 | 1.7 (0.96-3.1) | .068 | ||||
| Surgery*† | 7361 | 0.93 (0.64-1.4) | .73 | 1.0 (0.60-1.8) | .87 | 0.76 (0.46-1.3) | .29 | 0.84 (0.38-1.9) | .68 | ||||
| Type of surgery*† | |||||||||||||
| No or before surgery | 22 396 | 1 | 1 | 1 | 1 | ||||||||
| After surgery for venous access | 5266 | 0.78 (0.48-1.3) | .31 | 0.84 (0.51-1.4) | .50 | 0.61 (0.32-1.2) | .14 | 0.66 (0.34-1.3) | .23 | ||||
| After surgery for other indications | 1660 | 1.5 (0.83-2.6) | .19 | 1.4 (0.74-2.6) | .30 | 1.2 (0.54-2.5) | .69 | 0.95 (0.41-2.2) | .91 | ||||
| Unknown indication | 435 | 0.47 (0.066-3.4) | .46 | 0.40 (0.056-2.9) | .37 | 0.72 (0.099-5.2) | .74 | 0.63 (0.085-4.6) | .65 | ||||
| Duration between exposure days‡,§ | .18|| | .93|| | .030|| | .42|| | |||||||||
| >50 | 1119 | 1 | 1 | 1 | 1 | ||||||||
| 10-50 | 799 | 1.2 (0.68-2.0) | 1.0 (0.64-1.6) | 0.99 (0.50-2.0) | 0.99 (0.49-2.0) | ||||||||
| <10 | 1238 | 1.3 (0.87-2.1) | 1.2 (0.68-2.0) | 1.7 (1.0-2.9) | 1.3 (0.71-2.2) | ||||||||
| Dose of FVIII product (IU/kg) §,¶ | .0019|| | .11|| | .0087|| | .49|| | |||||||||
| <35 | 640 | 1 | 1 | 1 | 1 | ||||||||
| 35-50 | 1093 | 2.4 (1.2-4.9) | 2.4 (1.2-5.2) | 2.4 (1.0-5.7) | 2.4 (0.95-5.8) | ||||||||
| >50 | 1423 | 3.0 (1.5-6.0) | 2.3 (1.0-4.8) | 3.0 (1.3-6.9) | 1.8 (0.72-4.7) | ||||||||
Associations between inhibitor development and duration, dose, and type of surgery were adjusted with multivariable Cox proportional hazards regression. Associations between inhibitor development and surgery were adjusted with marginal structural models with inverse probability weighting.
Inh/N, number of inhibitor patients per total number of patients.
Adjusted for ethnicity, F8 genotype, family history, product type, and prophylaxis.
In 1 patient, the indication for surgery at first treatment was unknown.
Adjusted for ethnicity, F8 genotype, family history, dose, and product type.
In patients who were never treated with regular prophylaxis.
P for trend.
Adjusted for ethnicity, F8 genotype, family history, duration between ED, product type, and body weight.
Features of the first FVIII exposure.
Reason for first FVIII treatment.
Most patients’ first FVIII treatments were for hemorrhages (85%). At the first FVIII treatment, the estimated aHR for the development of inhibitors after major surgical procedures for reasons other than implantation of venous access devices compared with treatment of other reasons than surgery was 2.1, but this result was not statistically significant (95% CI, 0.77-5.8).
Peak treatment moments.
At the first treatment, 25.9% of patients were treated with FVIII for a bleed or surgery on at least 3 consecutive days. Peak treatment moments of 3 to 5 days at the first FVIII treatment were associated with a 40% higher risk for inhibitor development (aHR, 1.4; 95% CI, 0.87-2.3). Peak treatment moments of 5 to 10 days and peak treatment moments of more than 10 days were associated with an almost twice higher risk for inhibitor development (aHR, 2.0; 95% CI, 1.3-3.0 and aHR, 1.7; 95% CI, 1.0-2.9, respectively).
Features of subsequent exposure days to FVIII.
Peak treatment moment.
A total of 277 (48.1%) patients had at least 1 major peak treatment moment of at least 5 days during the first 75 exposure days or before inhibitor development. In 194 patients, the indication was a hemorrhage; in 83 patients, it was a surgical procedure. After the occurrence of a peak treatment moment of at least 5 days, patients had a 41% higher risk for inhibitor development (aHR, 1.4; 95% CI, 0.97-2.0). After peak treatment moments of at least 10 days, the risk was increased by 30% (aHR, 1.3; 95% CI, 0.77-2.2). These findings did not reach statistical significance.
Surgery.
After a major surgical procedure, the risk for inhibitor development was equal to that before surgery (aHR, 1.0; 95% CI, 0.60-1.8). The aHR for inhibitor development after surgical procedures for reasons other than for central venous access was 1.4 (95% CI, 0.74-2.6), whereas the aHR after surgery for central venous access was 0.84 (95% CI, 0.51-1.4). However, this finding was not statistically significant.
Duration between exposure days.
The duration as a measure for frequency of FVIII exposures was not independently associated with the risk for inhibitor development.
Mean FVIII dose.
Compared with a mean dose of FVIII of 5 consecutive exposure days of <35 IU/kg, the risk for inhibitor development was 2.4 (95% CI, 1.2-5.2) times higher when the mean dose was between 35 and 50 IU/kg, and it was 2.3 (95% CI, 1.0-4.8) times higher when the mean dose was more than 50 IU/kg.
Prophylaxis.
Characteristics of patients who started regular prophylaxis.
A total of 412 patients (71.5%) started regular prophylaxis within the first 75 exposure days. Patients started prophylaxis at a median age of 16.7 months (IQR, 12.4-24.4 months) and after a median number of 15 exposure days (IQR, 7-25 days). Twenty-five patients started prophylactic treatment from the first exposure onward. The crude and adjusted relative risks for inhibitor development according to the treatment regimen (on demand or prophylaxis) are presented in Table 4.
Prophylaxis and inhibitor development
| . | Regular prophylaxis . | Intention to start prophylaxis . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Determinant . | No. ED . | All inhibitors . | High-titer inhibitors . | . | All inhibitors . | High-titer inhibitors . | ||||||||||||
| Crude HR (95% CI) . | P value . | aHR (95% CI) . | P value . | Crude HR (95% CI) . | P value . | aHR (95% CI) . | P value . | N ED . | Crude HR (95% CI) . | P value . | aHR (95% CI) . | P value . | Crude HR (95% CI) . | P value . | aHR (95% CI) . | P value . | ||
| Regular prophylactic infusions | 18 846 | 0.68 (0.47-0.99) | .043 | 0.70 (0.44-1.1)* | .14 | 0.58 (0.36-0.92) | .021 | 0.61 (0.35-1.1)* | .093 | 23 426 | 0.63 (0.46-0.88) | .0060 | 0.66 (0.46-0.94) | .020 | 0.56 (0.38-0.83) | .0039 | 0.57 (0.36-0.88) | .012 |
| Dose, frequency of prophylaxis | ||||||||||||||||||
| 1/wk, <30 IU/kg | 2422 | 0.84 (0.46-1.5) | .58 | 0.76 (0.37-1.6)* | .46 | 0.92 (0.46-1.8) | .82 | 0.99 (0.44-2.2)* | .98 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 1/wk, >30 IU/kg | 7704 | 0.58 (0.35-0.94) | .027 | 0.54 (0.29-1.0)* | .050 | 0.48 (0.26-0.89) | .020 | 0.55 (0.26-1.2)* | .12 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| >1/wk, <30 IU/kg | 2243 | 0.39 (0.14-1.1) | .075 | 0.36 (0.11-1.2)* | .086 | 0.42 (0.13-1.4) | .16 | 0.47 (0.13-1.7)* | .25 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| >1/wk, >30 IU/kg | 6477 | 0.86 (0.51-1.5) | .58 | 1.11 (0.48-2.5)* | .81 | 0.57 (0.28-1.2) | .13 | 0.53 (0.23-1.3)* | .15 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Start of prophylaxis before 15 ED† | 10 527 | 0.69 (0.47-1.0) | .057 | NA§ | NA | 0.64 (0.40-1.0) | .059 | NA§ | NA | 19 509 | 0.64 (0.46-0.88) | .0068 | NA§ | NA | 0.57 (0.39-0.85) | .0056 | NA§ | NA |
| Start of prophylaxis before 5 ED† | 3919 | 0.81 (0.50-1.3) | .37 | NA§ | NA | 0.69 (0.38-1.2) | .20 | NA§ | NA | 10 981 | 0.67 (0.47-0.96) | .031 | NA§ | NA | 0.60 (0.39-0.94) | .024 | NA§ | NA |
| Start of prophylaxis before age 11 mo.‡ | 3618 | 0.68 (0.38-1.2) | .19 | NA§ | NA | 0.65 (0.33-1.3) | .23 | NA§ | NA | 6929 | 0.71 (0.47-1.1) | .11 | NA§ | NA | 0.69 (0.42-1.1) | .14 | NA§ | NA |
| Effect of prophylaxis according to no. ED|| | ||||||||||||||||||
| 1-10 | NA | 1.0 (0.48-2.2) | NA | 0.80 (0.37-1.7) | NA | 1.2 (0.46-2.9) | NA | 0.97 (0.39-2.5) | NA | NA | 0.65 (0.36-1.2) | NA | NA | NA | 0.75 (0.36-1.56) | NA | 0.79 (0.37-1.7) | NA |
| 11-20 | NA | 0.95 | NA | 0.93 | NA | 0.86 | NA | 0.86 | NA | NA | 0.82 | NA | NA | NA | 0.74 | NA | 0.69 | NA |
| 21-30 | NA | 0.22 | NA | 0.17 | NA | 0.13 | NA | 0.15 | NA | NA | 0.34 | NA | 0.34 | NA | 0.21 | NA | 0.20 | NA |
| 31-40 | NA | 0.27 | NA | 0.43 | NA | 0.084 | NA | 0.26 | NA | NA | 0.21 | NA | 0.47 | NA | 0.066 | NA | 0.12 | NA |
| 41-75 | NA | 0.32 | NA | 1.3 | NA | 0.080 | NA | 0.15 | NA | NA | 0.38 | NA | 0.97 | NA | 0.11 | NA | 0.40 | NA |
| Effect of prophylaxis in different F8 genotypes | N ED | N ED | ||||||||||||||||
| Low-risk genotype | 10 153 | 0.30 (0.11-0.83) | .020 | 0.61 (0.19-2.0)¶ | .41 | 0.14 (0.028-0.72) | .018 | 0.17 (0.028-0.98)¶ | .047 | 10 153 | 0.47 (0.21-1.1) | .069 | 0.42 (0.16-1.1)¶ | .079 | 0.39 (0.13-1.2) | .088 | 0.32 (0.062-1.6)¶ | .17 |
| High-risk genotype | 16 263 | 0.94 (0.61-1.4) | .77 | 0.85 (0.51-1.4)¶ | .52 | 0.83 (0.49-1.4) | .49 | 0.83 (0.45-1.6)¶ | .57 | 16 263 | 0.70 (0.48-1.0) | .069 | 0.78 (0.51-1.2)¶ | .25 | 0.64 (0.40-1.0) | .055 | 0.73 (0.44-1.2)¶ | .21 |
| . | Regular prophylaxis . | Intention to start prophylaxis . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Determinant . | No. ED . | All inhibitors . | High-titer inhibitors . | . | All inhibitors . | High-titer inhibitors . | ||||||||||||
| Crude HR (95% CI) . | P value . | aHR (95% CI) . | P value . | Crude HR (95% CI) . | P value . | aHR (95% CI) . | P value . | N ED . | Crude HR (95% CI) . | P value . | aHR (95% CI) . | P value . | Crude HR (95% CI) . | P value . | aHR (95% CI) . | P value . | ||
| Regular prophylactic infusions | 18 846 | 0.68 (0.47-0.99) | .043 | 0.70 (0.44-1.1)* | .14 | 0.58 (0.36-0.92) | .021 | 0.61 (0.35-1.1)* | .093 | 23 426 | 0.63 (0.46-0.88) | .0060 | 0.66 (0.46-0.94) | .020 | 0.56 (0.38-0.83) | .0039 | 0.57 (0.36-0.88) | .012 |
| Dose, frequency of prophylaxis | ||||||||||||||||||
| 1/wk, <30 IU/kg | 2422 | 0.84 (0.46-1.5) | .58 | 0.76 (0.37-1.6)* | .46 | 0.92 (0.46-1.8) | .82 | 0.99 (0.44-2.2)* | .98 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 1/wk, >30 IU/kg | 7704 | 0.58 (0.35-0.94) | .027 | 0.54 (0.29-1.0)* | .050 | 0.48 (0.26-0.89) | .020 | 0.55 (0.26-1.2)* | .12 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| >1/wk, <30 IU/kg | 2243 | 0.39 (0.14-1.1) | .075 | 0.36 (0.11-1.2)* | .086 | 0.42 (0.13-1.4) | .16 | 0.47 (0.13-1.7)* | .25 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| >1/wk, >30 IU/kg | 6477 | 0.86 (0.51-1.5) | .58 | 1.11 (0.48-2.5)* | .81 | 0.57 (0.28-1.2) | .13 | 0.53 (0.23-1.3)* | .15 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Start of prophylaxis before 15 ED† | 10 527 | 0.69 (0.47-1.0) | .057 | NA§ | NA | 0.64 (0.40-1.0) | .059 | NA§ | NA | 19 509 | 0.64 (0.46-0.88) | .0068 | NA§ | NA | 0.57 (0.39-0.85) | .0056 | NA§ | NA |
| Start of prophylaxis before 5 ED† | 3919 | 0.81 (0.50-1.3) | .37 | NA§ | NA | 0.69 (0.38-1.2) | .20 | NA§ | NA | 10 981 | 0.67 (0.47-0.96) | .031 | NA§ | NA | 0.60 (0.39-0.94) | .024 | NA§ | NA |
| Start of prophylaxis before age 11 mo.‡ | 3618 | 0.68 (0.38-1.2) | .19 | NA§ | NA | 0.65 (0.33-1.3) | .23 | NA§ | NA | 6929 | 0.71 (0.47-1.1) | .11 | NA§ | NA | 0.69 (0.42-1.1) | .14 | NA§ | NA |
| Effect of prophylaxis according to no. ED|| | ||||||||||||||||||
| 1-10 | NA | 1.0 (0.48-2.2) | NA | 0.80 (0.37-1.7) | NA | 1.2 (0.46-2.9) | NA | 0.97 (0.39-2.5) | NA | NA | 0.65 (0.36-1.2) | NA | NA | NA | 0.75 (0.36-1.56) | NA | 0.79 (0.37-1.7) | NA |
| 11-20 | NA | 0.95 | NA | 0.93 | NA | 0.86 | NA | 0.86 | NA | NA | 0.82 | NA | NA | NA | 0.74 | NA | 0.69 | NA |
| 21-30 | NA | 0.22 | NA | 0.17 | NA | 0.13 | NA | 0.15 | NA | NA | 0.34 | NA | 0.34 | NA | 0.21 | NA | 0.20 | NA |
| 31-40 | NA | 0.27 | NA | 0.43 | NA | 0.084 | NA | 0.26 | NA | NA | 0.21 | NA | 0.47 | NA | 0.066 | NA | 0.12 | NA |
| 41-75 | NA | 0.32 | NA | 1.3 | NA | 0.080 | NA | 0.15 | NA | NA | 0.38 | NA | 0.97 | NA | 0.11 | NA | 0.40 | NA |
| Effect of prophylaxis in different F8 genotypes | N ED | N ED | ||||||||||||||||
| Low-risk genotype | 10 153 | 0.30 (0.11-0.83) | .020 | 0.61 (0.19-2.0)¶ | .41 | 0.14 (0.028-0.72) | .018 | 0.17 (0.028-0.98)¶ | .047 | 10 153 | 0.47 (0.21-1.1) | .069 | 0.42 (0.16-1.1)¶ | .079 | 0.39 (0.13-1.2) | .088 | 0.32 (0.062-1.6)¶ | .17 |
| High-risk genotype | 16 263 | 0.94 (0.61-1.4) | .77 | 0.85 (0.51-1.4)¶ | .52 | 0.83 (0.49-1.4) | .49 | 0.83 (0.45-1.6)¶ | .57 | 16 263 | 0.70 (0.48-1.0) | .069 | 0.78 (0.51-1.2)¶ | .25 | 0.64 (0.40-1.0) | .055 | 0.73 (0.44-1.2)¶ | .21 |
In total, 576 patients were treated on 29 757 EDs to FVIII.
ED, exposure day; N, number; NA, not applicable.
Adjusted for ethnicity, F8 gene mutation type, family history of hemophilia and inhibitors, duration between first and current exposure day, duration between exposure days, dose, FVIII product type, peak treatment moments, and surgery. The inverse probability of treatment weights for regular prophylaxis had a mean of 1.0230, median of 0.8503, IQR of 0.5935-1.1043, and a range of 0.11-100.91. The inverse probability of treatment weights for intention to start prophylaxis had a mean of 1.0087, median of 0.9798, IQR of 0.7364-1.1184, and a range of 0.08-11.66.
Patients who started prophylaxis later than 15 or 5 EDs were censored at start of prophylaxis.
Patients who started prophylaxis later than age 11 months were censored at start of prophylaxis.
The aHRs for early and late start of prophylaxis were not available as the currently available statistical methods do not allow correct adjustment for confounding and censoring in this specific situation.
HRs of prophylaxis compared with on-demand treatment during the number of exposure days, categorized in 10-day periods.
Adjusted for ethnicity, family history of hemophilia and inhibitors, duration between first and current ED, duration between exposure days, dose, FVIII product type, and surgery.
Regular prophylaxis.
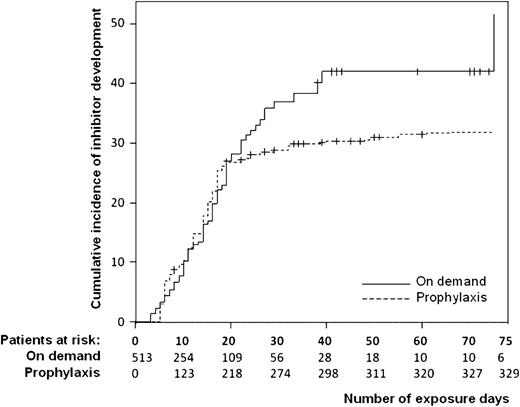
Patients who started prophylaxis within the first 75 exposure days had a 32% lower risk for inhibitor development compared with patients treated on demand (crude HR, 0.68; 95% CI, 0.47-0.99). After adjustment for confounding factors, the HR was similar. Prophylaxis was associated with a 42% lower risk for the development of high-titer inhibitors (crude HR, 0.58; 95% CI, 0.36-0.92). The time-dependent Kaplan-Meier graph (Figure 3) demonstrates that the inhibitor incidences of patients receiving prophylaxis and patients receiving on-demand treatment did not differ during the first 20 exposure days. After 20 exposure days, however, there was a markedly lower inhibitor incidence among those receiving prophylaxis.
Regular prophylaxis and the cumulative incidence of inhibitor development. The relative risk for regular prophylaxis was 1.0 in the period from 1 to 10 exposure days, 0.95 from 11 to 20 exposure days, 0.22 from 21 to 30 exposure days, 0.27 from 31 to 40 exposure days, and 0.32 from 41 to 75 exposure days.
Regular prophylaxis and the cumulative incidence of inhibitor development. The relative risk for regular prophylaxis was 1.0 in the period from 1 to 10 exposure days, 0.95 from 11 to 20 exposure days, 0.22 from 21 to 30 exposure days, 0.27 from 31 to 40 exposure days, and 0.32 from 41 to 75 exposure days.
Intention to start prophylaxis.
In total, in 470 patients (81.6%) there was an intention to start prophylaxis. The median number of exposure days at the intention to start prophylaxis was 5 days (IQR, 2-12 days). The crude relative risk for the intention to start prophylaxis was 0.63 (95% CI, 0.46-0.88; supplemental Figure 1). For high-titer inhibitor development, the crude relative risk was 0.56 (95% CI, 0.38-0.83). After adjustment for confounding factors, the relative risks were similar.
Timing of start of prophylaxis.
Of the 412 patients who started regular prophylaxis, 208 (50%) started prophylaxis before a maximum of 15 exposure days of on-demand treatment. These patients had a 31% lower risk for inhibitor development than the other patients (crude HR, 0.69; 95% CI, 0.47-1.0).
Dose and frequency of prophylaxis.
No clear trend was observed in the effect of prophylaxis according to increasing dose or frequency of prophylaxis (Table 4).
Prophylaxis in patients with high-risk and low-risk F8 genotypes.
In patients with low-risk F8 gene mutation types, the aHR for inhibitor development was 0.61 (95% CI, 0.19-2.0); in patients with high-risk F8 gene mutations, it was 0.85 (95% CI, 0.51-1.4) (Table 4). For high titer inhibitor development, this difference was more evident (aHR, 0.17; 95% CI, 0.028-0.98 and aHR, 0.83; 95% CI, 0.45-1.6, respectively).
Sensitivity analyses.
The results of the sensitivity analyses showed similar results, indicating that the results on prophylaxis are robust (supplemental Results).
Discussion
In this multicenter cohort study among 606 consecutive previously untreated patients with severe hemophilia A born between 2000 and 2010, the incidence of inhibitor development was 32%, and the incidence of high titer inhibitor development was 22%. High-dose intensive FVIII treatment of bleeding or surgery was associated with an increased risk for the development of inhibitors. Correspondingly, prophylactic FVIII treatment was associated with a decreased risk for inhibitor development. A new and rather surprising finding was that during the first 20 exposure days, patients receiving prophylaxis had exactly the same inhibitor risks as the patients treated on demand. In this study, the frequency and dose of prophylaxis were not associated with the risk for inhibitor development.
A strength of our study was that we avoided selection bias by making every effort to include consecutive patients from a 10-year birth cohort and by carefully excluding patients who were referred from other nonparticipating hemophilia centers because of inhibitor development.
At the moment of data analysis, some patients were still at risk for inhibitor development. Survival analyses enabled us to calculate unbiased cumulative incidences for the entire cohort. A major asset of our study is that we thoroughly checked whether the findings were robust in several sensitivity analyses. In this observational study, potentially high-risk patients may have been given prophylaxis to benefit from an assumed protective effect on inhibitor development. Collection of detailed information on all 75 exposure days allowed us to adjust the associations for potential confounding factors. Because the Cox proportional hazard model with time-dependent variables can give biased results, we used marginal structural models with an inverse probability of treatment weighting to avoid this bias.27
After adjustment for all measured potential confounding factors, the relative risks did not change substantially. Thus, either confounding factors may not be important, or the adjusted relative risks may still be biased, because of residual confounding by unmeasured confounding factors or by model misspecification of the inverse probability of treatment weights.28,29 Although we made careful efforts to specify the correct model, we cannot exclude that, in the future, a better model might prove to perform better on these data.
A low inhibitor incidence during prophylaxis is expected, as the immunologic danger theory proposes that immune tolerance should occur on exposure to infused FVIII, unless FVIII is accompanied by alarm signals that trigger the maturation of dendritic cells.17 These alarm signals may be endogenous substances released by tissues, injured by hemorrhages and surgery, thereby explaining the observed increased inhibitor risk during intensive treatment periods.
Although there is a plausible pathogenetic mechanism, the relationship between intensive treatment and inhibitor development may be partially attributable to reverse causation; intensive treatment may not be the cause but actually may be the effect of as-yet undetected inhibitors. We cannot exclude that this (partially) explained the reported association. However, the clear relationship between peak treatment moments at the start of treatment and a higher inhibitor incidence indicates that intensive treatment preceded development of the inhibitors.
Our findings of an increased inhibitor risk after intensive FVIII treatment and a decreased risk with prophylaxis corroborate the results of earlier studies.18,,,,-23 However, our study is the first to show that during the first 20 exposure days, prophylaxis and on-demand treatment confer the same inhibitor risk. Furthermore, the initial frequency and dose of prophylaxis were not associated with the risk for inhibitor development. This finding may seem to contrast with the conclusions of a previous study that reported a very low inhibitor risk in patients treated with an early-onset, low-dose prophylaxis regimen.22,23 In the latter study, however, very low doses of FVIII product were used, and further study is needed to confirm these findings.
It is currently not clear which patients may profit from the potentially protective effect of prophylaxis on inhibitor development. Apparently, there are 3 types of patients with severe hemophilia A: (1) patients in whom inhibitors will never develop; (2) patients in whom inhibitor development depends on the treatment regimen; and (3) patients in whom inhibitors will develop in all situations. The results of this study suggest that the potentially protective effect of prophylaxis may be more pronounced in patients with low-risk F8 genotypes than in patients with high-risk F8 genotypes, suggesting that the patients with high-risk F8 genotypes are more likely to be type 3 patients and, thus, are not susceptible to the protective effect of prophylaxis.
The current data provide no base for advice regarding how early prophylaxis should be started to minimize the risk for inhibitors. We propose to primarily adjust the onset, frequency, and dose of prophylaxis to achieve its main aim, which is the prevention of hemorrhages and joint damage. According to current guidelines, prophylaxis should be started at least as early as the occurrence of the first joint hemorrhage.30,-32 To minimize the risk for inhibitor development, the dose and frequency of prophylaxis may be adjusted to avoid intensive FVIII treatment of bleeding and surgery for venous access.
Our data suggest that starting prophylaxis in patients in whom inhibitors are predestined to develop may not have any effect on the occurrence of inhibitors. Identifying those patients who might benefit from prophylaxis is a challenge that remains to be tackled in additional studies.
In conclusion, our findings suggest that in previously untreated patients with severe hemophilia A, high-dose intensive factor VIII treatment increases the risk for inhibitor development on the one hand, and on the other hand, prophylactic factor VIII treatment decreases the risk for inhibitor development, especially in patients with low-inhibitor-risk F8 mutations.
Presented in abstract form at the XXIII International Society of Thrombosis and Haemostasis Congress, Kyoto, Japan, July 23-28, 2011; and the World Federation of Haemophilia World Congress 2012, Paris, France, July 8-12, 2012.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to acknowledge study coordinator Ella Smink. The authors also thank Emma Smid and Mojtaba Hashemi for their support in data cleaning. The authors are grateful to Yves Guillaume, Kate Khair, Karin Lindvall, Monique Spoor, and Bep Verkerk for their assistance in this study. The authors thank Roswita Neumann and Gertrud Schroeder from Bayer Healthcare and Bruce Ewenstein and Hartmut Ehrlich from Baxter Healthcare for supporting this study with unrestricted research grants.
Authorship
Contributions: S.C.G., J.G.v.d.B., and H.M.v.d.B. designed the research, analyzed and interpreted the data, and wrote the manuscript; S.C.G., J.G.v.d.B., and R.A.M. designed the sensitivity analyses; and K.F., G.A., M.C., E.C., H.C., K.K., R. Liesner, P.P., H.P., C.A., J.O., B.N., R.P.G., M.E.M., A.R., M.W., N.C., and R. Ljung collected data and critically reviewed the manuscript.
Conflict-of-interest disclosures: The RODIN Study was supported by unrestricted research grants from Baxter Bioscience (Deerfield, IL) and Bayer Healthcare. S.C.G. and H.M.v.d.B. have reported receiving unrestricted research support from CSL Behring, Novo Nordisk, Wyeth, Baxter, and Bayer. K.F. has received speaker fees from Baxter, Pfizer, Novo Nordisk, and Biotest; performed consultancy for Bayer, Baxter, Biogen, and Novo Nordisk; and has received research support from Bayer, Wyeth/Pfizer, Baxter, and Novo Nordisk. M.D.C. has received research support and honoraria (speaker fees/participation in advisory boards) from Baxter, Bayer, Biogen, CSL Behring, Novo Nordisk, Octapharma, and Pfizer (none of these relate to the present study). K.K. has received research support, speaker fees, and consultation fees from Baxter, Bayer, Biotest, CSL Behring, Pfizer, and Novo Nordisk. R. Liesner has received support for meetings from Baxter, Bayer, Novo Nordisk, and Octapharma and has received consultancy fees from Pfizer, Bayer, Baxter, and Novo Nordisk. M.E.M. has received fees as a speaker at meetings organized by Bayer, Baxter, Pfizer, CSL Behring, Novo Nordisk, Kedrion, and Grifols, and acted as a consultant for Bayer, Baxter, Pfizer, CSL Behring, Novo Nordisk, Kedrion, and Grifols. M.W. has received honoraria from Bayer, Baxter, and Novo Nordisk, and acted as a consultant on an advisory board for Novo Nordisk. R. Ljung has received research grant, speaker fees, and consultation fees from Bayer, Baxter, Novo Nordisk, and Octapharma during the last 5 years. J.G.v.d.B. has received unrestricted research/educational funding for various projects from the following companies: Bayer Schering Pharma, Baxter, CSL Behring, Novo Nordisk, and Wyeth. In addition, she has been a consultant to Baxter and Wyeth, and she has been a teacher on educational activities of Bayer Schering Pharma. The remaining authors declare no competing financial interests.
A complete list of the members of the PedNet and RODIN Study Group appears in supplemental Material.
Correspondence: Johanna G. van der Bom, Department of Clinical Epidemiology, Leiden University Medical Center, Albinusdreef 2, PO Box 9600, 2300 RC, Leiden, The Netherlands; e-mail: j.g.vanderbom@lumc.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal