Although direct oral anticoagulants do not need laboratory testing for dose adjustment, there are instances when laboratory measurement of the drug anticoagulant effect may be useful. They include before initiation of treatment, before surgical or invasive procedures, on the occasion of hemorrhagic or thrombotic events, and whenever immediate reversal of anticoagulation is needed. Choice of tests should be primarily based on their prompt availability. Accordingly, the dilute-thrombin or the ecarin clotting times are best suited for dabigatran and the prothrombin time or the anti-FXa for rivaroxaban.
The direct oral anticoagulants (DOAC), which include the thrombin inhibitor dabigatran and the anti-Xa agents rivaroxaban, apixaban, and edoxaban, are in clinical use in many countries (Figure 1). Clinical trials have shown that DOAC, unlike vitamin K antagonists (VKA), are effective and safe when administered without dose adjustment based on laboratory testing.1 However, it is a misconception to believe that the laboratory will not have a role in the management of patients treated with DOAC. This review is aimed at discussing:
The situations where the laboratory may help clinicians with decision making in the management of treated patients;
The most appropriate tests to be used; and
The interpretation of results.
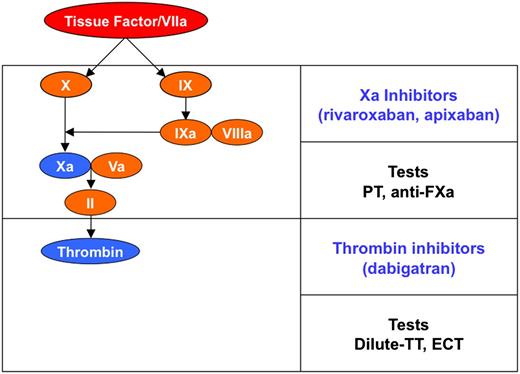
Schematic representation of coagulation. DOACs and tests suggested for the measurement of their anticoagulant effect. ECT, ecarin clotting time; PT, prothrombin time; TT, thrombin clotting time.
Schematic representation of coagulation. DOACs and tests suggested for the measurement of their anticoagulant effect. ECT, ecarin clotting time; PT, prothrombin time; TT, thrombin clotting time.
Need for testing
Although the experience is still limited, one may identify situations when the laboratory measurement for DOAC is potentially useful and others that would require further evaluation (Table 1).
Situations when the measurement of the DOAC anticoagulant effect is potentially useful and others that would require consideration
| Potentially useful . | Others . |
|---|---|
| At baseline (before initiation of treatment) | When chronic anticoagulation is achieved (1-2 weeks after initiation) |
| Before surgical or invasive procedures | During clinical visits |
| During adverse events (hemorrhage or thrombosis) | Soon before and after introducing additional drugs |
| Need for immediate reversal of anticoagulation | Low or high body weight |
| Potentially useful . | Others . |
|---|---|
| At baseline (before initiation of treatment) | When chronic anticoagulation is achieved (1-2 weeks after initiation) |
| Before surgical or invasive procedures | During clinical visits |
| During adverse events (hemorrhage or thrombosis) | Soon before and after introducing additional drugs |
| Need for immediate reversal of anticoagulation | Low or high body weight |
Potentially useful
At baseline.
Laboratory (and clinical) evaluation is useful for excluding an underlying bleeding tendency or renal insufficiency and to determine if the test(s) that will be used for measuring the drug effect is (are) normal at baseline. In addition to the prothrombin time (PT) and partial thromboplastin time (APTT), other tests to be included are blood cell counts and others that will depend on which drug is used for treatment (see below). Assessment of creatinine clearance is of paramount importance as DOAC are largely excreted via the kidney.1 Therefore, even mild renal insufficiency may lead to drug accumulation during treatment, thus increasing the bleeding risk. Assessment of creatinine clearance is indicated not only before starting treatment but also at regular intervals during treatment, as kidney function may deteriorate rapidly, especially in the elderly.
Preoperatively.
Owing to their short half-life, the effect of DOAC may be rapidly dissipated by discontinuation of the treatment before surgical or invasive procedures. However, there may be patients with mild, perhaps unrecognized renal insufficiency in whom the clearance may be delayed.2 Preoperative laboratory testing of the drug anticoagulant effect may therefore help to prevent the risk of life-threatening hemorrhage2,3 and expensive therapeutic interventions aimed at reversing anticoagulation.2
Adverse events.
Patients referred to emergency departments with thrombosis or hemorrhage should be tested to see whether they are under- or overanticoagulated. Laboratory testing may also help in the event of life-threatening (intracranial) hemorrhage to help decide whether immediate anticoagulation reversal is needed and to judge subsequently whether neutralization is achieved. There are not yet antidotes for DOAC, and current recommendations for reversing their effects are based on the experience accumulated with traditional anticoagulants (ie, infusion of prothrombin complex concentrates [PCC] or recombinant FVIIa]. Although the experience with these procoagulants in the reversal of DOAC effects is still limited, a recent study4 suggested a role for laboratory testing in assessing drug neutralization. Healthy subjects were treated with dabigatran or rivaroxaban for 2.5 days before reversal of anticoagulation by PCC or saline infusion. Laboratory testing showed that PCC were able to neutralize the effect of rivaroxaban but not that of dabigatran.4 It is still unknown whether these results reflect what occurs in vivo and to what extent laboratory testing may help to judge reversal. However, if different drugs will have different antidotes and/or reversal strategies, physicians in emergency departments need to be able to assess the levels of anticoagulation and the specific DOAC being used by patients. Simple laboratory detection (eg, in the urine) of the type of drug5 might be useful.
Others
Coagulation tests might be considered upon attainment of stable anticoagulation (1-2 weeks after initiation) to provide the level of the anticoagulation achieved chronically. This information might be useful to interpret subsequent results.
Drug anticoagulant levels could be measured occasionally at the time of medical visits to assess adherence to treatment. However, it should be realized that given the short DOAC half-life (8-15 hours), a dose missed a few days earlier than testing might not be detected in the laboratory.
Since DOAC may interact with other drugs,6 comparison of their anticoagulant effects measured before and after the introduction of additional drugs may help to confirm the degree of known drug-to-drug interactions or to unravel unknown interactions.
DOAC are prescribed at fixed dosage, so individuals with extreme body weights may benefit from dose adjustment to avoid under- or overanticoagulation. This issue was not evaluated in phase 3 trials and warrants assessment when DOAC will be used in real life. Information based on laboratory testing and clinical events recorded during treatment could help when making decisions about these patients.
Which test(s) for which drug
The following sections are aimed at discussing the choice of tests for the currently available drugs. The choice is based on the limited experience and literature data accumulated to date and on the following practical considerations:
Prompt test availability;
Linearity and adequacy of test response to increasing dosage; and
Amenability to standardization (ie, comparability of results between laboratories using the same method but different reagents). Standardization is mandatory for patients on VKA, but it is also important for DOAC. Assuming that future clinical studies will determine specific cutoff values able to alert clinicians to bleeding risk, these values could be generalized to all laboratories, regardless of the reagent used for testing, only if the tests are standardized.
Dabigatran
Dabigatran prolongs the clotting times of tests based on thrombin generation and fibrinogen-to-fibrin conversion. Among them, the most promising are the thrombin time (TT) and ecarin clotting time (ECT).7 TT reflects fibrinogen-to-fibrin conversion and is performed by measuring plasma clotting times upon addition of thrombin. TT is readily available in most laboratories; the clotting time prolongation is linearly and dose-dependently related to dabigatran concentrations.8 However, responsiveness to increasing dosage is excessive: TT prolongations induced by the typical 175 ng/mL dabigatran plasma concentration (observed in patients taking 150 mg twice daily)9,10 may be 10 times the baseline value.8 Hence, a normal TT should rule out a dabigatran anticoagulant effect, but the degree of prolongation poorly reflects drug concentration. Recently, a dilute TT adequately responsive to dabigatran (ie, prolongation up to 2 times the baseline value at 175 ng/mL dabigatran) (Table 2) has become available and may be used for dabigatran measurement.11,12
Expected plasma concentrations of dabigatran or rivaroxaban after treatment
| Drug . | Cpeak (range) . | Ctrough (range) . |
|---|---|---|
| Dabigatran* | 175 ng/mL (117-275 ng/mL) | 91 ng/mL (61-143 ng/mL) |
| Rivaroxaban† | 215 ng/mL (22-535 ng/mL) | 32 ng/mL (6-239 ng/mL) |
| Drug . | Cpeak (range) . | Ctrough (range) . |
|---|---|---|
| Dabigatran* | 175 ng/mL (117-275 ng/mL) | 91 ng/mL (61-143 ng/mL) |
| Rivaroxaban† | 215 ng/mL (22-535 ng/mL) | 32 ng/mL (6-239 ng/mL) |
Steady-state geometric mean dabigatran plasma concentration (25th-75th percentile range) measured around 2 hours (Cpeak) or 12 hours (Ctrough) after 150 mg dabigatran administration twice daily.10 Dilute thrombin clotting time or ECT at Cpeak are approximately prolonged 2 or 3 times the baseline value, respectively.8 Between-reagent variability should be considered when interpreting results.
Steady-state geometric mean rivaroxaban plasma concentration (90% prediction interval) measured around 2 hours (Cpeak) or 24 hours (Ctrough) after 20 mg rivaroxaban administration once daily.17 PT at Cpeak is approximately prolonged 1.5 times the baseline value.16,19 Between-reagent variability should be considered when interpreting results.16,19,26,30
Ecarin is a commercially available snake venom that converts FII into meizothrombin. The dabigatran-induced inhibition of meizothrombin is measured by synthetic substrates or clotting assays.13 ECT proved to be linearly and dose-dependently related to dabigatran concentrations: ECT prolongations induced by 175 ng/mL amount to 3 times the baseline value8 (Table 2). Presently, ECT is not readily available in most laboratories but could be easily implemented in many coagulation platforms.13,14
The APTT is another test that can be used for dabigatran; the APTT prolongation in response to 175 ng/mL dabigatran amounts to 1.7 times the baseline value but is not linearly related to the concentration.8 Furthermore, the between-reagent variability associated with the APTT makes the interpretation of results obtained with one reagent not necessarily applicable to others. The PT is relatively insensitive to dabigatran8 and should not be used for this drug.
In summary, the dilute-TT or ECT are the tests of choice for dabigatran. The first is readily available even in an emergency and can be easily implemented in all coagulometers. The second (although not yet widely available) could become available in clinical laboratories as the ecarin venom is commercially available. Both dilute-TT and ECT are fairly responsive to dabigatran8 and might be easier to standardize than the APTT.
Rivaroxaban
Rivaroxaban inhibits FXa and prolongs the clotting times of tests assessing coagulation downstream from FXa. Among them, the most promising are the anti-FXa and PT tests.7
Anti-FXa is based on the measurement of residual FXa with synthetic substrates upon mixing plasma with FXa. This test, presently used for low-molecular-weight heparins, has been modified to be fairly responsive to rivaroxaban.15
PT prolongations are adequate to detect rivaroxaban activity within the range of concentrations observed in treated patients.16 On average, PT is prolonged 1.5 times the baseline value at 215 ng/mL rivaroxaban16 (ie, the plasma concentration observed in patients taking 20 mg once daily)17 (Table 2). Hence, prolongations are greater in patients who have received an overdose of the drug.
In summary, the PT or the anti-FXa assay can be used for patients on rivaroxaban. However, the anti-FXa is not readily available in most laboratories, especially at night. Moreover, standardization as assessed in a collaborative survey is poor: the average interlaboratory variation of results obtained while measuring the same rivaroxaban-spiked plasmas by means of local methods was high (ie, 25%).18 Conversely, the PT is readily available even in an emergency and its standardization could be obtained as described19,20 by expressing results as PT ratio (patient-to-normal) corrected by means of a sensitivity index. Such an index can be determined for working thromboplastins relative to an international standard. The international normalized ratio (used for VKA) should not be used for rivaroxaban because it increases the drug-induced between-thromboplastin variability.19
Apixaban
Limited experience is available for apixaban and laboratory testing. While the PT is in general poorly responsive, the anti-FXa assay proved to be highly correlated and adequately responsive to the apixaban plasma concentrations.21 The problems of availability and standardization of the anti-FXa assay discussed above for rivaroxaban also apply to apixaban.
Timing of testing
Onset/offset actions of DOAC are relatively fast. Peak plasma levels are reached 2 hours after ingestion of dabigatran8,10 or rivaroxaban.17 Rivaroxaban plasma concentration (in patients taking 20 mg once daily) is as low as 32 ng/mL17 at 24 hours and dabigatran plasma concentration (in patients taking 150 mg twice daily) is reduced to 91 ng/mL after 12 hours10 (Table 2). Consequently, the interpretation of test results is heavily dependent on the timing of blood sampling. It is still unclear whether the peak or trough drug concentration should be evaluated when the choice of timing for blood drawing is feasible.
Expression of results
Results of coagulation tests (ie, dilute-TT, ECT, and PT) can be expressed as clotting time ratio (patient-to-normal). Alternatively, clotting times can be converted into drug concentration equivalent by interpolation of patient clotting times from a dose-response curve, constructed by plotting clotting times for calibration plasmas spiked with known amounts of drugs versus their target concentrations. This result expression has been proposed for dilute-TT12 and anti-FXa assays.18 Whether clotting time prolongation or drug concentration equivalent is the preferred way of expressing results has not yet been established.
Alert values
Test values able to alert clinicians to bleeding or thrombotic risk have not yet been established because the experience to date is based mainly on normal plasma spiked in vitro with increasing amounts of each DOAC. Accurate determination of alert values requires clinical observations in treated patients combined with standardized laboratory testing and will be undertaken when DOAC are more widely used.
DOAC effect on common hemostatic parameters
Owing to their mode of action, it is anticipated that DOAC affect the measurement of some of the most common hemostatic parameters. Here some examples:
Antithrombin activity is overestimated in patients on rivaroxaban or dabigatran when the target enzyme used for testing is FXa or thrombin, respectively.22,23 This can be avoided by using FXa or thrombin as a target enzyme when the drug used for treatment is dabigatran or rivaroxaban, respectively.
Fibrinogen, when measured as clotting activity, is underestimated in patients on dabigatran.23 The degree of underestimation is reagent dependent and is likely due to different types or concentrations of thrombin used for testing.
Tests for activated protein C resistance may be affected by DOAC when testing is based on paired APTT with or without activated protein C.23,24 Because of this effect, plasmas from heterozygous FV Leiden carriers might resemble wild-types when patients are treated with DOAC.23,24
FXIII when measured by chromogenic substrates may be underestimated in patients on dabigatran,25 simply because FXIII is activated by thrombin.
Although few data are available, it is possible that measurements of proteins C and S, performed with clotting techniques,26 individual coagulation factors,26,27 and the search for lupus anticoagulants,28 may also be affected by DOAC.
All of the above considerations point to the conclusion that caution should be exerted in the interpretation of results of hemostatic parameters measured for patients on DOAC.
Conclusions
Although DOAC do not require laboratory testing for dose adjustment, there are occasions when laboratory investigations may be useful. Many tests can be used, and the choice should be primarily based on their prompt availability. In the future, there will be millions of patients worldwide treated with DOAC. Some of them may bleed anywhere and anytime. This situation requires the availability of simple assays that can be run in both large and small hospitals. To this end, the dilute-TT or ECT tests are best suited for dabigatran and the PT or anti-FXa for rivaroxaban. The choice between TT and ECT or between PT and anti-FXa depends on clinical observations that are still lacking. Owing to the rather large between-reagent variability, laboratories should define their own reagent responsiveness to DOAC.29 Results should be interpreted with caution if responsiveness is unknown.30
Authorship
Contribution: A.T. prepared the manuscript, and no other individuals were involved in the writing or editing of the manuscript.
Conflict-of-interest disclosure: A.T. received speaker’s fees on the occasion of educational meetings organized by Instrumentation Laboratory.
Correspondence: A. Tripodi, Via Pace 9, 20122-Milano, Italy; e-mail: armando.tripodi@unimi.it.