In this issue of Blood, Itonaga and colleagues report the outcomes of and discuss treatment options for patients with relapse of adult T-cell leukemia (ATL) after allogeneic hematopoietic stem cell transplantation (HSCT). Their findings suggest an association of postrelapse induction of a graft-versus-ATL effect with long-term cure.1
Adult T-cell leukemia (ATL) is characterized by the presence of malignant CD3+ dim CD4+ CD8−; CD25+–expressing T cells in the peripheral blood and in lymphoid and other tissues. The prognosis for ATL with standard therapies is poor. The survival data in the initial publication defining subgroups of patients with ATL that received aggressive chemotherapy showed a median survival of 6.2 months for acute type, 10.2 months for lymphoma type, and 24.3 months for chronic type ATL.2 Although there have been responses in select patients receiving aggressive chemotherapy, azidothymidine plus interferon α, and monoclonal antibodies, the optimal chemotherapy regimen has only improved the median survival to 13 months.
Allogeneic HSCT has been reported to lead to long-term disease-free survival. For example, Ishida and colleagues recently reported a retrospective study of HSCT for ATL, where they demonstrated a 3-year overall survival (OS) of 36% in 586 patients, including individuals who were not in complete remission (CR) at the time of transplant with some manifesting primary refractory disease.3 The apparent long-term cure after allogeneic transplant in such patients where prior therapies had failed suggests the possibility of a potent graft-versus-ATL effect.
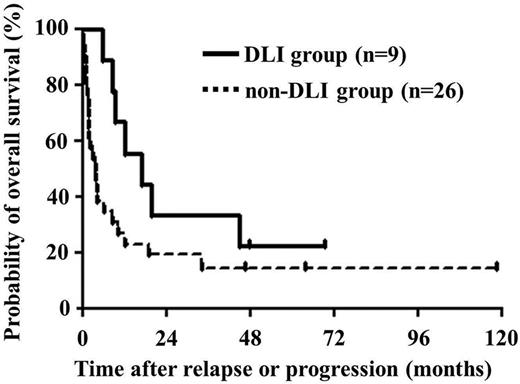
As with most diseases for which HSCT can be curative, relapse of ATL is the primary cause of posttransplant treatment failure. Understanding the disease-specific biology and natural history of posttransplant relapse and defining optimal ways to detect and treat posttransplant relapse are the primary goals of an active international effort (see figure).4 In this regard, the report by Itonaga and colleagues advances our knowledge of relapsed ATL after HSCT.1 They conducted a retrospective study of 35 patients with progression or relapse of ATL after HSCT. The 3-year OS after relapse was 19% with a median survival time of 6.2 months after relapse or progression. The first treatment attempt included withdrawal of immunosuppression (WIS) in 29 of 35 patients. Although this was ineffective in most, complete remission was observed in 2 of 29 patients who also developed acute graft-versus-host disease (aGVHD) after WIS. Most notable was the demonstration of complete remission in 4 of 9 patients who received subsequent donor lymphocyte infusion (DLI) with or without prior cytoreductive therapy, which suggested a DLI-induced graft-versus-ATL effect, for which there is growing evidence (see figure).5,6 Patients received multiple doses of DLI until attaining the best response and among the patients experiencing a CR, all developed or had worsening of chronic graft-versus-host disease (cGVHD). Importantly, in 3 patients who responded to DLI (2 of whom had long-term sustainable remissions), relapses of ATL were seen in the skin and these patients may have had lower levels of disease burden where a graft-versus-ATL effect may be more likely. The successful induction of a graft-versus-ATL effect, however, was never without the development of GVHD. Cytoreductive therapy alone was effective only for patients with local relapse. Cytoreductive therapy when used as pre-DLI therapy, however, was associated with improved response to DLI.
Overall survival after relapse or progression. Median survival times after relapse or progression were 16.9 and 3.9 months in patients treated with and without DLI, respectively. This figure is adapted from the article by Itonaga et al that begins on page 219.
Overall survival after relapse or progression. Median survival times after relapse or progression were 16.9 and 3.9 months in patients treated with and without DLI, respectively. This figure is adapted from the article by Itonaga et al that begins on page 219.
What may limit treatment of relapsed ATL includes the fact that, as shown by Itonaga and colleagues, the median time from transplantation to relapse or progression was 2.8 months, with some patients having demonstrated progression by as early as 15 days after transplant. Intensive chemotherapy to combat relapse in the posttransplant period is often not feasible due to posttransplant co-morbidities and high risk of treatment-related morbidity and mortality. In addition, patients with rapid disease progression after HSCT may not respond to additional attempts to induce a graft-versus-ATL effect because such a response may require time to reach the full effect. Itonaga et al's observation of improved outcomes in individuals with low-level disease raises the possibility that detection of posttransplant minimal residual disease might allow for intervention at an earlier time point when recurrent ATL might be more amenable to therapy. In this regard, improved methods of minimal residual disease detection in ATL such as the use of PCR to detect clonal T-cell receptor β or γ gene rearrangements and clonal HTLV-1 integration to monitor clonality may be of value. Other markers of disease recurrence may include specialized flow cytometric analysis to help identify CD3 dim/CD25+ cells, quantitation of whole blood HTLV-1 viral load, which is of value because almost all HTLV-1 is in the leukemia cell, and measurement of soluble interleukin-2 receptor (sIL-2R) as a surrogate marker for malignant cells.7
Improvement on outcomes after transplantation is likely dependent on identification of those patients who are at highest risk of relapse and consideration of enrollment of such patients on relapse prevention trials. Alternatively, novel approaches for use in the immediate post-HSCT period for relapse prevention or early treatment of relapse using targeted antibody-based therapies8 show promise, and development of molecular-based therapies is on the horizon.
In summary, Itonaga and colleagues provide greatly needed insight into the outcomes and treatment options for patients with ATL who relapse after transplant. While the graft-versus-ATL effect shows promise toward cure, this is not without the risk of GVHD. Ultimately, there is a need for improved monitoring strategies for posttransplant relapse with a focus on development of disease-specific prophylactic or preemptive trials to improve outcomes for patients with ATL.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■