Abstract
Adult T-cell leukemia/lymphoma (ATL) relapse is a serious therapeutic challenge after allogeneic hematopoietic stem cell transplantation (allo-SCT). In the present study, we retrospectively analyzed 35 patients who experienced progression of or relapsed persistent ATL after a first allo-SCT at 3 institutions in Nagasaki prefecture (Japan) between 1997 and 2010. Twenty-nine patients were treated by the withdrawal of immune suppressants as the initial intervention, which resulted in complete remission (CR) in 2 patients. As the second intervention, 9 patients went on to receive a combination of donor lymphocyte infusion and cytoreductive therapy and CR was achieved in 4 patients. Of 6 patients who had already had their immune suppressants discontinued before the relapse, 3 patients with local recurrence received local cytoreductive therapy as the initial treatment, which resulted in CR for more than 19 months. Donor lymphocyte infusion–induced remissions of ATL were durable, with 3 cases of long-term remission of more than 3 years and, interestingly, the emergence or progression of chronic GVHD was observed in all of these cases. For all 35 patients, overall survival after relapse was 19.3% at 3 years. The results of the present study suggest that induction of a graft-versus-ATL effect may be crucial to obtaining durable remission for ATL patients with relapse or progression after allo-SCT.
Key Points
ATL patients who relapsed after allogeneic HSCT have a very high mortality rate and present a serious therapeutic challenge.
No large study exists that assesses the role of salvage therapies for relapsed ATL after HSCT; this is the first report summarizing the outcome.
Introduction
Adult T-cell leukemia/lymphoma (ATL) is a peripheral T-cell neoplasm caused by a specific retrovirus, human T-cell lymphotropic virus type I (HTLV-1).1-4 Patients with the aggressive type of ATL (the acute, lymphoma, and unfavorable chronic types) generally have a poor prognosis because of chemotherapy resistance and predisposition to opportunistic infections.5-10 In Japan, allogeneic hematopoietic stem cell transplantation (allo-SCT) has been explored as an alternative treatment that can provide long-term remission11,12 ; overall survival (OS) at 3 years has been reported to be approximately 33%-45% in these patients.13-20 However, the relapse rate after allo-SCT is approximately 40%13 and relapsed patients have a very poor prognosis. Treatment options include withdrawal of immune suppressants (IS), chemotherapy, local radiation therapy, lymphocyte infusions (DLIs) from the original donor, and second allo-SCT, but there are limited data describing the outcome of each treatment.12,17,19,21,22
It has been shown that the graft-versus-ATL (GVATL) effect plays an important role in the prevention of relapse13,19,21,23,24 and therapy that could induce the GVATL reaction may improve postrelapse outcome. DLI, a therapy that would induce a GVL reaction, has gained a prominent role in the management of leukemia patients who relapse after allo-SCT.25-28 The best responses to DLI occur in patients with chronic myelogenous leukemia, which yields complete remission (CR) in approximately 80% of patients with relapsed chronic myelogenous leukemia after transplantation. However, the benefit of DLI for relapsed acute leukemia is often limited, partly because of the rapid growth of leukemia cells and poor response to the GVL reaction. Considering the similar characteristics of ATL cells, the role of GVATL-based therapy (ie, withdrawal of IS and DLI) as salvage therapy remains controversial.
In the present study, we retrospectively analyzed 35 patients, including 9 who received DLIs for progression of or relapsed ATL after allo-SCT, and found that the outcomes of those treated with salvage chemotherapy and treatments intended to induce an immune-mediated GVATL effect could be promising for at least some of them.
Methods
Patients and donors
Eighty-one patients with ATL received allo-SCT between September 1997 and December 2010 at 3 institutions in Nagasaki prefecture (Japan). Thirty-five of 81 patients experienced persistence of ATL or relapse of ATL after allo-SCT and these patients were candidates for salvage treatment. Data on these 35 patients were collected and updated as of July 2011.
Before transplantation, all 35 patients received conventional chemotherapy. Because transplantation was performed following the protocol of each institution, the conditioning regimen and prophylaxis for GVHD varied among institutions.
Five related donors showed a positive result for the anti–HTLV-1 Ab. The PBMCs of these donors were subjected to Southern blot analysis to examine monoclonal integration of the HTLV-1 provirus into the genome and all 5 donors were confirmed as carriers of HTLV-1.
Our study was approved by the ethical committees of the participating hospitals. The outline was followed and clinical information about the patients was available to be retrospectively analyzed when informed consent was obtained according to the Declaration of Helsinki.
Treatment for the relapse or progression of ATL after allo-SCT
The relapse was defined by reappearance of abnormal lymphocytes in peripheral blood or local failure diagnosed by biopsies. In 22 cases, HTLV-1 provirus load in peripheral blood was monitored using quantitative PCR, however, an increase in HTLV-1 provirus load was not used to diagnose a relapse. When relapse or progression of persistent ATL after allo-SCT was confirmed, IS was withdrawn if the patients were still receiving IS for the prophylaxis or treatment for GVHD. Indication for DLI was determined by attending physicians following the policy of each institution. Collected cells were given intravenously on the same day of the collection or cryopreserved for later use. Salvage chemotherapy and/or local radiation therapy were also administered before DLI at the discretion of the attending physician. Donor leukocytes from the original donor were obtained by leukocyte apheresis. Cells were infused without further manipulation. Calculation of the number of T cells infused was performed by FACScan analysis of buffy coat cells using anti-CD3–specific mAbs. No donor received G-CSF mobilization.
Definition of therapeutic response and GVHD
Response to treatmentation was divided into 4 categories: CR, partial remission (PR), stable disease (SD), and progressive disease (PD). Responses were defined as follows: CR, disappearance of all disease; PR, ≥ 50% reduction of measurable disease; SD, failure to attain CR or PR and no PD; and PD, new or increased lesions.
Statistical analysis
Descriptive statistics were used for summarizing variables related to patient demographics and transplant characteristics. Comparisons among the groups were performed by use of the χ2 statistic or Fisher exact test as appropriate for categorical variables and the Mann-Whitney U test for continuous variables. OS was calculated from the first day of relapse or progression of persistent ATL to the date of death or the last follow-up. The analysis included the overall study population of 35 patients to evaluate the impact of DLI-based strategies on outcome. The Kaplan-Meier method was used to estimate OS after relapse. The 95% confidence interval of 3-year OS was calculated. The following variables were analyzed by Fisher exact test to determine significantly associated factors for the response of DLI: donor type (HLA-matched related or alternative), HTLV-1 serostatus of donor (positive or negative), chemotherapy or radiotherapy (pre-DLI or post-DLI), time of allo-SCT (1997-2003 or 2004-2010), disease status at allo-SCT, disease status at DLI, T-cell dose, GVHD at relapse/progression (present or absent), GVHD after withdrawing IS (present or absent), post-DLI GVHD (progressive or stable), and time from transplantation to relapse (within 6 months or longer than 6 months). All tests were 2-sided and P < .05 was considered significant in all analyses. All statistical analyses were performed with Prism Version 5.0 software (GraphPad).
Results
Characteristics of patients and disease status at transplantation
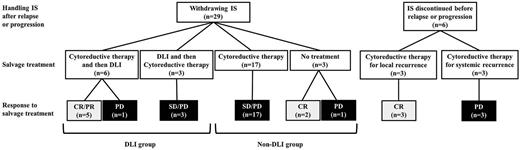
Patient characteristics including transplantation procedures and clinical outcomes of allo-SCT are summarized in Table 1. Nine of 35 patients (25.7%) received DLI as a part of the treatment for relapse or progression of persistent ATL after allo-SCT (DLI group), whereas 26 patients (74.3%) received other types of treatment without DLI (non-DLI group). Among other characteristics, the 2 groups were well matched in terms of age, HTLV-1 serostatus of donor, disease status at allo-SCT, acute GVHD, chronic GVHD, and intervals from allo-SCT to relapse. All DLI recipients showed that the subtype at diagnosis was the acute type. In the non-DLI group, one patient received conditioning containing antithymocyte globulin. No patient received an ex vivo T cell–depleted graft. Nine patients who experienced relapse after cord blood transplantation were treated without DLI. The clinical outcome after relapse or progression is summarized in Figure 1.
Patient characteristics and disease status at transplantation
| Characteristic . | DLI performed . | P . | |
|---|---|---|---|
| Yes . | No . | ||
| No. of patients | 9 | 26 | |
| Median age at allo-SCT, y | 54 (range, 41-62) | 51 (range, 39-63) | .496 |
| Sex, n | |||
| Male | 8 | 10 | .018 |
| Female | 1 | 16 | |
| Subtype of ATL at diagnosis, n | |||
| Acute | 9 | 20 | .304 |
| Lymphoma | 0 | 6 | |
| Type of donor, n | |||
| HLA-matched related | 7 | 13 | .072 |
| HLA-mismatched related | 1 | 0 | |
| HLA-matched unrelated, BM | 1 | 4 | |
| Unrelated, cord blood | 0 | 9 | |
| Source of stem cells, n | |||
| BM | 5 | 11 | .109 |
| Peripheral blood | 4 | 6 | |
| Cord blood | 0 | 9 | |
| HTLV-1 serostatus of donor, n | |||
| HTLV-1 Ab positive | 1 | 4 | 1.000 |
| HTLV-1 Ab negative | 8 | 22 | |
| Status at allo-SCT, n | |||
| CR | 3 | 6 | .930 |
| Partial remission | 2 | 6 | |
| Primary induction failure | 2 | 8 | |
| Relapse | 2 | 6 | |
| Conditioning for allo-SCT, n | |||
| Myeloablative | .456 | ||
| TBI-based | 3 | 7 | |
| Non TBI-based | 0 | 4 | |
| Reduced intensity | |||
| Fludarabine-based | 6 | 15 | |
| GVHD prophylaxis, n | |||
| Cyclosporine | 4 | 2 | .063 |
| Tacrolimus | 0 | 5 | |
| Cyclosporine + short-term methotrexate | 3 | 12 | |
| Tacrolimus + short-term methotrexate | 2 | 7 | |
| GVHD after allo-SCT, n | |||
| Acute | .490 | ||
| No | 6 | 13 | |
| Grade 1 | 0 | 3 | |
| Grade 2-4 | 3 | 10 | |
| Chronic | .239 | ||
| No | 3 | 17 | |
| Yes | 3 | 5 | |
| Not evaluated | 3 | 4 | |
| Attaining CR after allo-SCT, n | |||
| Yes | 7 | 23 | .586 |
| No | 2 | 3 | |
| Interval from allo-SCT to relapse, mo (range) | 2.8 (0.4-100.7) | 3.6 (0.4-45.9) | .836 |
| Time of allo-SCT | |||
| 1997-2003 | 2 | 8 | 1.000 |
| 2004-2010 | 7 | 18 | |
| Characteristic . | DLI performed . | P . | |
|---|---|---|---|
| Yes . | No . | ||
| No. of patients | 9 | 26 | |
| Median age at allo-SCT, y | 54 (range, 41-62) | 51 (range, 39-63) | .496 |
| Sex, n | |||
| Male | 8 | 10 | .018 |
| Female | 1 | 16 | |
| Subtype of ATL at diagnosis, n | |||
| Acute | 9 | 20 | .304 |
| Lymphoma | 0 | 6 | |
| Type of donor, n | |||
| HLA-matched related | 7 | 13 | .072 |
| HLA-mismatched related | 1 | 0 | |
| HLA-matched unrelated, BM | 1 | 4 | |
| Unrelated, cord blood | 0 | 9 | |
| Source of stem cells, n | |||
| BM | 5 | 11 | .109 |
| Peripheral blood | 4 | 6 | |
| Cord blood | 0 | 9 | |
| HTLV-1 serostatus of donor, n | |||
| HTLV-1 Ab positive | 1 | 4 | 1.000 |
| HTLV-1 Ab negative | 8 | 22 | |
| Status at allo-SCT, n | |||
| CR | 3 | 6 | .930 |
| Partial remission | 2 | 6 | |
| Primary induction failure | 2 | 8 | |
| Relapse | 2 | 6 | |
| Conditioning for allo-SCT, n | |||
| Myeloablative | .456 | ||
| TBI-based | 3 | 7 | |
| Non TBI-based | 0 | 4 | |
| Reduced intensity | |||
| Fludarabine-based | 6 | 15 | |
| GVHD prophylaxis, n | |||
| Cyclosporine | 4 | 2 | .063 |
| Tacrolimus | 0 | 5 | |
| Cyclosporine + short-term methotrexate | 3 | 12 | |
| Tacrolimus + short-term methotrexate | 2 | 7 | |
| GVHD after allo-SCT, n | |||
| Acute | .490 | ||
| No | 6 | 13 | |
| Grade 1 | 0 | 3 | |
| Grade 2-4 | 3 | 10 | |
| Chronic | .239 | ||
| No | 3 | 17 | |
| Yes | 3 | 5 | |
| Not evaluated | 3 | 4 | |
| Attaining CR after allo-SCT, n | |||
| Yes | 7 | 23 | .586 |
| No | 2 | 3 | |
| Interval from allo-SCT to relapse, mo (range) | 2.8 (0.4-100.7) | 3.6 (0.4-45.9) | .836 |
| Time of allo-SCT | |||
| 1997-2003 | 2 | 8 | 1.000 |
| 2004-2010 | 7 | 18 | |
TBI indicates total body irradiation.
Clinical course of posttransplantation patients with relapse or progression of ATL after allo-SCT.
Clinical course of posttransplantation patients with relapse or progression of ATL after allo-SCT.
Clinical characteristics and outcome of patients who received DLI
The characteristics of 9 patients in the DLI group are summarized in Table 2. In the DLI group, after allo-SCT, ATL was noticed before or at the time of hematopoietic recovery in 2 patients (unique patient number [UPN] 1 and UPN 5). The other 7 patients attained or maintained CR with hematopoietic reconstitution after allo-SCT. The median time from transplantation to relapse or progression was 2.8 months (range, 0.4-100.7) and the median time from relapse or progression to DLI was 1.2 months (range, 0.4-8.0). All 9 patients had their IS terminated as the first intervention, but no patients achieved CR by the discontinuation of IS alone. One patient (UPN 1) achieved SD by the mere discontinuation of IS. Six patients received cytoreductive therapy (chemotherapy or local radiation therapy) before the first DLI (pre-DLI therapy). No patients achieved CR by any pre-DLI therapy. The response to pre-DLI therapy could not be evaluated in 1 patient (UPN 1) because the interval between pre-DLI therapy and the first DLI was not long enough for evaluation (the patient received pre-DLI therapy for 3 days before the initial DLI).
DLI and patient outcome
| UPN . | Age, y* . | Sex, recipient/donor . | Type of donor . | Status at allo-SCT . | Year of allo-SCT . | Time of relapse after allo-SCT, mo . | Tumor lesion at relapse . | Pre-DLI therapy . | Status at DLI . | Time of DLI after relapse, mo . | T-cell dose, × 106/kg . | Response to DLI . | GVHD after DLI (type of cGVHD) . | OS after relapse, mo . | Cause of death . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial . | Total . | |||||||||||||||
| 1 | 56 | M/F | R-PBSC | CR | 2005 | 1.0 | Skin | Oral VP-16 | NE | 8.0 | 5.5 | 5.5 | CR | Progression (extensive) | 45.0 | Infection |
| 2 | 63 | M/M | R-PBSC | CR | 2004 | 2.4 | Skin, hypodermis | Half-dose CHOP | PD | 0.6 | 10.0 | 110.0 | PR | Emergence (extensive) | 16.9 | PD |
| 3 | 41 | M/M | R-BM | PIF | 2004 | 2.8 | CNS | IT | PR | 0.4 | 10.0 | 40.0 | CR | Absent | 19.9 | PD |
| 4 | 53 | M/M | R-BM | PR | 2009 | 100.7 | Skin | RT | SD | 1.0 | 12.0 | 221.0 | CR | Emergence (limited) | 47.7+ | - |
| 5 | 55 | M/F | R-PBSC | Relapse | 2002 | 0.4 | PB, liver, pleural effusion | Half-dose CHOP | PR | 0.9 | 10.0 | 10.0 | CR | Emergence (extensive) | 69.4+ | - |
| 6 | 63 | M/F | R-BM | PR | 2008 | 3.1 | PB | No | PD | 2.3 | 6.9 | 33.0 | PD | Absent | 8.7 | PD |
| 7 | 44 | F/F | R-PBSC | Relapse | 1997 | 10.6 | Bone, liver | No | PD | 1.2 | 5.0 | 15.0 | SD | No progression | 9.2 | PD |
| 8 | 55 | M/M | UR-BM | CR | 2010 | 11.0 | Lymph nodes | No | PD | 1.4 | 5.0 | 65.0 | SD | Progression (extensive) | 5.7 | PD |
| 9 | 53 | M/F | R-BM | PIF | 2009 | 1.1 | Hypodermis | RT | PD | 5.0 | 8.2 | 40.8 | PD | Progression (extensive) | 12.1 | PD |
| UPN . | Age, y* . | Sex, recipient/donor . | Type of donor . | Status at allo-SCT . | Year of allo-SCT . | Time of relapse after allo-SCT, mo . | Tumor lesion at relapse . | Pre-DLI therapy . | Status at DLI . | Time of DLI after relapse, mo . | T-cell dose, × 106/kg . | Response to DLI . | GVHD after DLI (type of cGVHD) . | OS after relapse, mo . | Cause of death . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial . | Total . | |||||||||||||||
| 1 | 56 | M/F | R-PBSC | CR | 2005 | 1.0 | Skin | Oral VP-16 | NE | 8.0 | 5.5 | 5.5 | CR | Progression (extensive) | 45.0 | Infection |
| 2 | 63 | M/M | R-PBSC | CR | 2004 | 2.4 | Skin, hypodermis | Half-dose CHOP | PD | 0.6 | 10.0 | 110.0 | PR | Emergence (extensive) | 16.9 | PD |
| 3 | 41 | M/M | R-BM | PIF | 2004 | 2.8 | CNS | IT | PR | 0.4 | 10.0 | 40.0 | CR | Absent | 19.9 | PD |
| 4 | 53 | M/M | R-BM | PR | 2009 | 100.7 | Skin | RT | SD | 1.0 | 12.0 | 221.0 | CR | Emergence (limited) | 47.7+ | - |
| 5 | 55 | M/F | R-PBSC | Relapse | 2002 | 0.4 | PB, liver, pleural effusion | Half-dose CHOP | PR | 0.9 | 10.0 | 10.0 | CR | Emergence (extensive) | 69.4+ | - |
| 6 | 63 | M/F | R-BM | PR | 2008 | 3.1 | PB | No | PD | 2.3 | 6.9 | 33.0 | PD | Absent | 8.7 | PD |
| 7 | 44 | F/F | R-PBSC | Relapse | 1997 | 10.6 | Bone, liver | No | PD | 1.2 | 5.0 | 15.0 | SD | No progression | 9.2 | PD |
| 8 | 55 | M/M | UR-BM | CR | 2010 | 11.0 | Lymph nodes | No | PD | 1.4 | 5.0 | 65.0 | SD | Progression (extensive) | 5.7 | PD |
| 9 | 53 | M/F | R-BM | PIF | 2009 | 1.1 | Hypodermis | RT | PD | 5.0 | 8.2 | 40.8 | PD | Progression (extensive) | 12.1 | PD |
cGVHD indicates chronic GVHD; R-PBSC, related peripheral blood stem cell; R-BM, related BM; UR-BM, unrelated BM; PB, peripheral blood; PIF, primary induction failure; NE, not evaluated; VP-16, etoposide; IT, intrathecal injection of cytarabine, methotrexate and prednisone; and RT, local radiation therapy.
Age indicates age at time of relapse or progression.
A total of 20 DLIs were performed in 9 patients. The median of initial and total T-cell doses was 8.2 × 106/kg (range, 5.0-12.0) and 40.0 × 106/kg (range, 5.5-221.0), respectively. The median follow-up duration after relapse/progression and initial DLI was 16.9 months (range, 11.8-148.4) and 16.3 months (range, 4.3-68.1), respectively. Basically, the DLIs were repeated if CR was not obtained and 7 patients received multiple infusions with sequential T-cell dose escalation. Five of 6 patients who received pre-DLI therapy showed clinical response to DLI (4 CRs and 1 PR). In 3 CR patients who received multiple infusions, the effect of first DLI was PR in 2 patients (UPN 3 and 5) and SD in 1 patient (UPN 4). Four patients (UPN 6, 7, 8, and 9) did not experience any response even after the administration of multiple DLIs with escalated T-cell doses. The total number of DLIs given to UPN 2 and UPN 4 were 3 and 4, respectively. Two patients (UPN 4 and 5) were alive in CR at the time of analysis with follow-up times from relapse or progres-sion of 47.7 and 69.4 months, respectively. The other 2 patients who achieved CR died of bacterial infection without relapse (UPN 1) and of progression of ATL (UPN 3) at 45.0 and 19.9 months after relapse, respectively. One patient with PR after DLI (UPN 2) experienced a relapse at 1.2 months from the first DLI and died of ATL at 16.9 months from the first relapse after allo-SCT. Among patients with CR or PR after DLI, the median remission duration was 37.0 months (range, 1.2-68.5).
One patient (UPN 9) who received pre-DLI therapy and 3 patients (UPN 6, 7, and 8) who did not receive pre-DLI therapy did not achieve any remission. These 4 patients died of ATL within 9 months after the first DLI.
Factors significantly associated with clinical responses to DLI were the administration of pre-DLI therapy (P = .018) and absence of GVHD at relapse or progression of ATL (P = .018). The distribution of the T-cell dose of the first DLI tended to be skewed toward higher numbers in patients who responded (median 10.0 × 106/kg, range 5.5-12.0 × 106/kg) relative to those without a response (median 6.0 × 106/kg, range 5.0-8.2 × 106/kg; P = .0617). The other factors, including year of transplantation, were not significantly associated with the response. Interestingly, 3 of 5 patients who achieved remission by DLIs had skin involvement at the time of relapse or progression, but this association was not significant (P = .1667).
Six patients experienced the emergence or progression of GVHD after DLIs. Three of 4 CR patients (UPN 1, 4, and 5) had chronic GVHD with oral lichenoid change as a common symptom and had been kept in continuous remission for more than 2 years after the first DLI. Two patients (UPN 1 and 9) developed chronic GVHD, including bronchiolitis obliterans and cryptogenic organizing pneumonia, respectively. In 4 patients (UPN 1, 2, 5, and 8), IS was resumed for the treatment of chronic GVHD after DLI.
Treatments for patients in the non-DLI group
Of 26 patients who did not receive DLI, 6 patients had IS discontinued before the relapse or progression of persistent ATL after allo-SCT and all received cytoreductive therapy. Three of these 6 patients (UPN 10, 11, and 12) achieved CR and were alive at the time of analysis, with follow-up times from relapse or progression of 63.9, 118.6, and 19.0 months, respectively (Table 3). The common characteristic of these 3 patients is that they all experienced local relapse (ie, CNS relapse, localized lymph node relapse, or localized bone relapse) and received local cytoreductive therapies (ie, local radiation therapy or intrathecal injections).
Outcome of patients maintaining CR for more than 1 year after relapse without DLI
| UPN . | Age at time of relapse, y . | Sex, recipient/donor . | Type of donor . | Status at allo-SCT . | Year of allo-SCT . | Time of relapse after allo-SCT, mo . | Tumor lesion at relapse . | IS at relapse . | GVHD at relapse . | GVHD after withdrawing IS (type of cGVHD) . | Response to withdrawing IS . | Salvage treatment . | Response to salvage treatment . | OS after relapse, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 57 | F/M | R-BM | PIF | 2005 | 4.6 | CNS | None | Absent | PD | IT only | CR | 63.9+ | |
| 11 | 55 | F/F | R-BM | PR | 2000 | 14.3 | Bone | None | Absent | PD | RT | CR | 118.6+ | |
| 12 | 63 | F/F | UR-CB | PIF | 2007 | 45.9 | Lymph nodes | None | Absent | PD | RT | CR | 19.0+ | |
| 13 | 50 | F/M | R-BM | PR | 2005 | 0.9 | Lymph nodes | CsA | Absent | Emergence (limited) | CR | Not done | - | 46.7+ |
| UPN . | Age at time of relapse, y . | Sex, recipient/donor . | Type of donor . | Status at allo-SCT . | Year of allo-SCT . | Time of relapse after allo-SCT, mo . | Tumor lesion at relapse . | IS at relapse . | GVHD at relapse . | GVHD after withdrawing IS (type of cGVHD) . | Response to withdrawing IS . | Salvage treatment . | Response to salvage treatment . | OS after relapse, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 57 | F/M | R-BM | PIF | 2005 | 4.6 | CNS | None | Absent | PD | IT only | CR | 63.9+ | |
| 11 | 55 | F/F | R-BM | PR | 2000 | 14.3 | Bone | None | Absent | PD | RT | CR | 118.6+ | |
| 12 | 63 | F/F | UR-CB | PIF | 2007 | 45.9 | Lymph nodes | None | Absent | PD | RT | CR | 19.0+ | |
| 13 | 50 | F/M | R-BM | PR | 2005 | 0.9 | Lymph nodes | CsA | Absent | Emergence (limited) | CR | Not done | - | 46.7+ |
R-BM indicates related BM; UR-CB, unrelated cord blood; PIF, primary induction failure; CsA, cyclosporine A; IT, intrathecal injection of cytarabine, methotrexate and prednisone; and RT, local radiation therapy.
For 20 patients with IS at the time of relapse or progression, withdrawal of IS was performed as a first intervention. A total of 5, 1, and 2 patients developed severe acute GVHD (grade II-IV), limited type chronic GVHD, and extensive type chronic GVHD after the withdrawal of IS, respectively. Discontinuation of IS alone resulted in CR for 2 patients, along with the emergence of GVHD. One of these patients (UPN 13) remained in CR for 46.7 months with limited type chronic GVHD (oral lichenoid change), although the other eventually died of acute GVHD. Seventeen patients received either chemotherapy (n = 16) or local radiation therapy (n = 1) as an initial therapy after discontinuation of IS. These salvage treatments resulted in SD in 2 patients and PD in 15 patients. These 17 patients could not receive DLI for the following reasons: in 5 patients who had cord blood as the donor source of SCT, DLI was not applicable; in 4 patients, attempts at collecting lymphocytes from an unrelated donor was unsuccessful; in 3 patients, severe GVHD (acute GVHD grade II-IV or extensive type chronic GVHD) emerged after the discontinuation of IS and DLI was not warranted; and in 5 patients, disease progressed very rapidly and resulted in death within 1 month after the relapse. Eleven of 17 patients received additional salvage treatments after the initial cytoreductive therapy, including second allo-SCT from unrelated cord blood (n = 1). All 17 patients died of disease progression; the patient who received the second cord blood transplantation died of disease progression at day 8 after transplantation.
OS
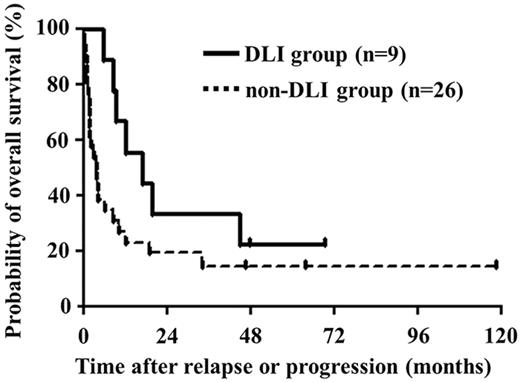
For all patients, the median survival time after relapse or progression was 6.2 months and estimated OS after relapse or progression was 19.3% (95% confidence interval [CI]: 8.2%-33.9%) at 3 years. Median survival times after relapse or progression were 16.9 months in the DLI group and 3.9 months in the non-DLI group (Figure 2). Estimated OS rates after relapse or progression were 33.3% (95% CI, 7.8%-62.3%) and 14.4% (95% CI, 4.1%-30.9%) at 3 years in DLI and non-DLI groups, respectively.
OS after relapse or progression. Median survival times after relapse or progression were 16.9 and 3.9 months in patients treated with and without DLI, respectively.
OS after relapse or progression. Median survival times after relapse or progression were 16.9 and 3.9 months in patients treated with and without DLI, respectively.
Discussion
Relapse of ATL after allo-SCT is one of the main causes of treatment failure after allo-SCT and remains a significant therapeutic challenge. In the present study, the most common initial intervention for these patients was the withdrawal of IS. The remission (CR + PR) rate by this procedure alone was only 7% (2 of 29), suggesting that mere discontinuation of IS may not provide enough effect for ATL. However, the fact that one of these patients achieved long-term durable remission strongly suggested the existence of the GVATL effect; similar observations have been reported from other groups.13,19,21,24 In the present study, administration of donor lymphocytes was performed for patients who did not achieve CR by the withdrawal of IS. DLI with or without cytoreductive therapy induced remission (CR + PR) for 5 of 9 patients with median OS of 16.9 months (OS from relapse or progression). Considering the extremely poor outcomes of relapsed ATL patients after allo-SCT, a treatment strategy including DLI seems to be a potential therapeutic option to obtain a response that may lead to a long-term durable remission. However, generally, the prognosis of patients with relapsed ATL or progressive ATL after allo-SCT is still not satisfactory, even with the salvage treatments containing DLI.
It has been reported previously that achieving hematologic remission with DLI is generally a difficult task, especially in patients with a high tumor burden and rapidly proliferating leukemic cells.25-28,31,32 Therefore, debulking with cytoreductive therapy before DLI was thought to be advantageous.33 In this analysis, DLI brought CR in 4 patients who responded to pre-DLI therapy. Using pre-DLI therapy, 2 patients (UPN 3 and 5) and 1 patient (UPN 4) obtained PR and SD, respectively, and 1 patient (UPN 1) achieved SD after discontinuation of IS. Five patients (UPN 2, 6, 7, 8, and 9) who did not receive pre-DLI treatment were all in PD before the DLI, and the responses were either SD or PD. These results suggest that the patients with better disease control will be more likely to benefit from DLI. Our data imply that once patients obtain any remission by cytoreductive therapy, DLI is the treatment of first choice if possible.
The regimens for pre-DLI therapy are yet to be established. Such regimens are challenging, as the condition of relapsed patients may vary widely because they often suffer from preexisting transplantation-related complications (eg, infections, organ damage, and GVHD). We believe that intensity of CHOP (vincristine, cyclophosphamide, doxorubicin, and prednisone) is sufficient in this setting because the primary purpose of pre-DLI chemotherapy is to slow down the speed of progression.
In acute lymphoblastic leukemia, it has been suggested that the administration of DLI at the time of detecting minimal residual disease (MRD) could be more effective.31 However, in ATL, detecting MRD using HTLV-1 provirus load34 is often limited because the provirus load itself may not reflect the true amount of leukemia cells (HTLV-1–infected donor derived T cells may also exist). ATL cell–specific inverse PCR is capable of detecting real MRD, but it has not been put into clinical practice.
GVHD was closely correlated with disease response to DLI. Four patients (UPN 1, 4, 5, and 13) with long-term remission experienced emergence or progression of GVHD after DLI and no patient showed response to DLI without GVHD, suggesting that GVHD exerts a potent GVATL effect. In the present study, the types of GVHD observed after DLI were chronic, mostly with oral lichenoid changes. Kami et al reported 2 cases of ATL with posttransplantation relapses who received a single DLI and gained CR but died of exacerbation of the preexisting chronic GVHD.12 In addition, Kamimura et al reported 2 cases of successful DLI in ATL relapse after allo-SCT and also observed exacerbation of chronic GVHD with oral lichenoid changes.22 Together with our observations, chronic GVHD seems to be associated with GVATL. Similarly, in the case of acute myeloid leukemia, it has been reported that the development of chronic GVHD was associated with superior outcome, whereas the development of acute GVHD had a negative effect on survival.28 Furthermore, it has also been reported that in the case of nonmyeloablative transplantation for acute myeloid leukemia, relapse rates are lower in patients developing chronic GVHD.35 Because the development of mild to moderate acute GVHD, not chronic GVHD, has been reported to be associated with a lower risk of disease progression and confers a beneficial influence on the survival of patients who received allo-SCT,23 the type of GVHD that is associated with durable remission may be different in the settings of allo-SCT and DLI. A definitive assessment of the contribution of the GVATL effect linked with chronic GVHD for the relapse or progression of ATL would require a study with a larger number of patients.
It is of interest that the site of ATL at relapse involved the skin in 3 of 5 patients who responded to DLIs and 2 of 3 patients with skin lesions maintained long-term remissions. It is possible that the relapse in skin is a nonaggressive (ie, low-level) disease and DLI may be effective against these type of diseases. Aggressiveness of ATL at the time of relapse may be a factor determining the effect of DLI. In addition, it is known that GVL-based therapy could induce durable responses in posttransplantation relapsed patients with cutaneous T-cell lymphoma, which is another type of mature T-cell lymphoma.36-40 Based on these previous results and together with our findings, it is suggested that mature T-cell lymphomas with skin lesions are good targets for GVL-based therapy.
The results of the present study found several factors associated with the efficacy of GVATL-based therapy. However, the number of patients in our study was limited and the treatment protocols used in this cohort of patients were not uniform, so it is possible that these factors exerted a bias and affected the results. Our findings should be interpreted carefully and should be confirmed in prospective studies.
Reinduction chemotherapy alone to treat posttransplantation relapse of ATL has been reported to be largely ineffective. In the present study, however, local cytoreductive therapy alone induced long-durable remission in 3 patients with local recurrence. Interestingly, Simone et al41 have recently reported that local radiotherapy is useful for the management of ATL patients who have not received allo-SCT. For local relapse, whether posttransplantation or not, local cytoreductive therapy should be considered as one of the treatment options.
In conclusion, the results of our analysis suggest a role of GVATL-based therapy for patients with progression of or relapsed persistent ATL after allo-SCT, but also point out the limitations of GVATL-based therapy. Further experimental and clinical research is required to enhance the GVATL effect to overcome the obviously high capacity of leukemic cells that escape from an allogeneic immune reaction. Such research may lead to more effective and less toxic methods of using adoptive GVATL-based therapy for this disease.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the many hematologists in the Department of Hematology, Atomic Bomb Disease and Hibakusya Medicine Unit, Atomic Bomb Disease Institute, Nagasaki University Graduate School of Biomedical Sciences, for the diagnosis and treatment of patients with ATL.
This study was supported in part by a grant-in-aid from the Ministry of Health and Welfare of Japan and a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Authorship
Contribution: H.I., J.T., T.F., and Y. Miyazaki conceived and designed the study; H.I., H. Tsushima, J.T., T.F., H. Taniguchi, S.S., K.A., Y.S., E.M., R.Y., Y.O., D.I., Y.I., S.Y., T.H., Y. Moriuchi, and Y. Miyazaki collected and analyzed the data; H.I., H. Tsushima, and Y. Miyazaki performed the statistical analysis, wrote the manuscript, and created the figures and tables; and all authors critically reviewed the manuscript and read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hideki Tsushima, MD, PhD, Department of Hematology, Nagasaki University Hospital, 1-7-1 Sakamoto, Nagasaki 852-8501, Japan; e-mail: tsushima@nagasaki-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal