Abstract
Binding of Glu-plasminogen (the native, circulating form of the zymogen) to cells results in enhancement of its activation. Cell-associated plasmin proteolytic activity is a key component of physiologic and pathologic processes requiring extracellular matrix degradation. Recently, we developed antiplasminogen mAbs that recognize receptor-induced binding sites (RIBS) in Glu-plasminogen and, therefore, preferentially react with cell-associated Glu-plasminogen in the presence of soluble Glu-plasminogen. Here we have used FACS with a representative antiplasminogen receptor-induced binding site mAb, mAb49, to examine whether plasminogen associates with peripheral blood cells in blood. Plasminogen binding to neutrophils, monocytes, B-lymphocytes, T-lymphocytes, and platelets was clearly detected. Treatment of whole blood with lipopolysaccharide or 12-0 tetradecanoylphorbol-13-acetate up-regulated plasminogen binding to neutrophils and in vivo treatment with all-trans retinoic acid decreased plasminogen binding to acute promyelocytic leukemia blasts. Our results demonstrate that mAb49 can be used to monitor cell-bound plasminogen in blood under both normal and pathologic conditions.
Introduction
Plasminogen binds to nucleated blood cells and platelets in a specific, saturable, and reversible manner.1,2 Based on the plasminogen concentration in plasma (2μM)3 and the affinity for its receptors (∼ 1μM),4 approximately 50% of plasminogen binding sites on peripheral blood cells within the vasculature are predicted to be occupied by plasminogen. Recently, we developed antiplasminogen receptor-induced binding site (RIBS) mAbs that preferentially recognize cell-associated plasminogen in the presence of soluble plasminogen.5 Therefore, we have investigated whether the representative antiplasminogen RIBS mAb, mAb49, can detect plasminogen bound to live cells in blood. Here we demonstrate that plasminogen binds to peripheral blood cells in normal whole blood and that modulation of cell-associated plasminogen occurs during inflammation and blood cell diatheses, including acute promyelocytic leukemia (APL).
Methods
Proteins
Glu-plasminogen was from Chromogenix (Mölndal). mAb49 against plasminogen was raised and characterized in our laboratory.5 mAbs to CD15, CD14, CD33, CD19, CD2, GPIIb-IIIa, Glycophorin A, and HLDR were from Immunotech or Coulter. FITC- and PE-conjugated goat anti–mouse mAbs were from Sera-Lab.
Cells
Neutrophils, monocytes, lymphocytes, and red blood cells were isolated from blood collected into heparin (5 U/mL), theophylline (10mM), and prostaglandin E1α (10 U/mL; Sigma-Aldrich) as described.6
Blast cells from peripheral blood were analyzed from a patient with acute nonlymphoblastic leukemia, categorized according to the French-American-British classification.7 Blood drawing was approved by the Institute Català de la Salut Institutional Review Board. NB4 cells were provided by Dr M. Lanotte (Hôpital St Louis, Paris, France). The human cell line Nalm6 was provided by Dr J. Inglés-Esteve (IDIBELL, Barcelona). Other cell lines were from the ATCC and cultured as described.8
FACS analysis
Cells were washed with PBS containing 1% BSA and 0.1% sodium azide (PBA), incubated with PBA containing 10% heat-inactivated normal rabbit serum, washed again, and incubated with mAb49 (130nM) or isotype control, washed, and then stained with FITC-goat anti–mouse IgG, which was detected in a flow cytometry analyzer (Coulter EPICS XL-MCL).
Plasminogen binding to cells in whole peripheral blood collected into EDTA was determined as in the preceeding paragraph with the following exceptions. Cells were incubated in 10% heat-inactivated human AB serum in PBS, washed with PBA, and incubated with anti–mouse IgG conjugated to PE, washed and incubated with FITC-conjugated antibodies to specific leukocyte antigens. Cells were incubated in Ortho-mune Lysing Reagent (Ortho Diagnostic Systems), centrifuged, and resuspended in PBA containing 7-aminoactynomycion D (Invitrogen) at 1 mg/mL.
Radiolabeled antiplasminogen RIBS mAb binding to cells
Reagents
HEPES, 12-0 tetradecanoylphorbol-13-acetate (PMA), and BSA were from Sigma-Aldrich. LPS was from Calbiochem-Behring. All-trans-retinoic acid was from Hoffmann-La Roche.
Results and discussion
Detection of plasminogen bound to the surfaces of normal peripheral blood cells
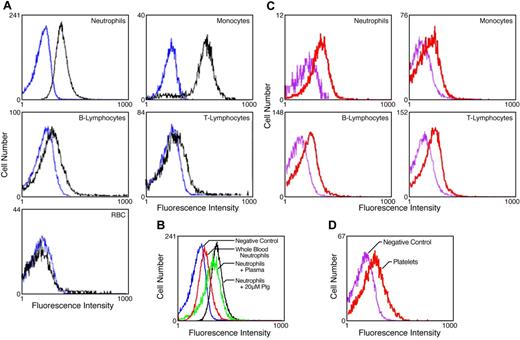
We tested whether cell-associated Glu-plasminogen was detected by mAb49 on peripheral blood cells. First, isolated peripheral blood cells were preincubated with plasminogen. In FACS analysis with mAB49, fluorescent populations of neutrophils, monocytes, B-lymphocytes, and T-lymphocytes were clearly detected, compared with cells incubated without plasminogen (Figure 1A), consistent with the abilities of these cells to bind plasminogen.1 In contrast, no plasminogen-dependent binding of mAb49 was detected on red blood cells (Figure 1A), which do not appreciably bind plasminogen.1
Detection of plasminogen bound to the surfaces of normal peripheral blood cells. (A) Isolated peripheral blood cells were preincubated with either 10μM plasminogen (black tracings) or buffer (blue tracings) and analyzed in FACS analyses with mAb49 as described in “Methods.” (B) Isolated neutrophils were incubated with 20μM plasminogen (black tracing), autologous plasma (green tracing), or buffer (blue tracing) and analyzed in FACS with mAb49. In addition, FACS analyses were performed with mAb49 in whole blood (red tracing), gated for neutrophils using CD15 as described in “Methods.” (C) FACS analyses was performed with mAb49 (red tracing) or isotype control (purple tracing) in whole blood gated for the indicated cell types: CD15 for neutrophils, CD14 for monocytes, CD2 for T lymphocytes, and CD19 for B lymphocytes. (D) FACS analyses were performed with mAb49 (red tracing) or isotype control (purple tracing) in whole blood gated for platelets with anti–GPIIb-IIIa mAb.
Detection of plasminogen bound to the surfaces of normal peripheral blood cells. (A) Isolated peripheral blood cells were preincubated with either 10μM plasminogen (black tracings) or buffer (blue tracings) and analyzed in FACS analyses with mAb49 as described in “Methods.” (B) Isolated neutrophils were incubated with 20μM plasminogen (black tracing), autologous plasma (green tracing), or buffer (blue tracing) and analyzed in FACS with mAb49. In addition, FACS analyses were performed with mAb49 in whole blood (red tracing), gated for neutrophils using CD15 as described in “Methods.” (C) FACS analyses was performed with mAb49 (red tracing) or isotype control (purple tracing) in whole blood gated for the indicated cell types: CD15 for neutrophils, CD14 for monocytes, CD2 for T lymphocytes, and CD19 for B lymphocytes. (D) FACS analyses were performed with mAb49 (red tracing) or isotype control (purple tracing) in whole blood gated for platelets with anti–GPIIb-IIIa mAb.
Second, we compared FACS signals with mAb49 when purified neutrophils were preincubated with either Glu-plasminogen or autologous plasma. Under both conditions, positive signals were obtained compared with neutrophils incubated with buffer (Figure 1B). In addition, positive FACS signals with mAb49 were detected after gating whole blood for neutrophils (Figure 1B).
Third, we performed FACS analysis with mAb49 and compared signals after gating for monocytes, T-lymphocytes, B-lymphocytes, neutrophils, and platelets in whole blood. A clear positive signal was observed with each of these cell types, compared with isotype control (Figure 1C-D).
Detection of plasminogen bound to cells under pathologic conditions
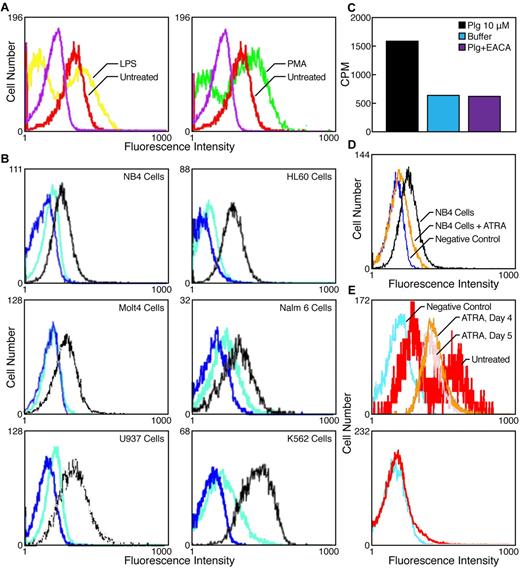
We evaluated the ability of mAb49 to probe cell-bound plasminogen under pathologic conditions. As a model of inflammation, we incubated whole blood with LPS. The FACS signal with mAb49 was markedly higher on neutrophils from LPS-treated compared with untreated blood (Figure 2A left panel). As differentiation of cells is associated with the response to inflammation, we examined the effects of stimulation of whole blood with PMA, a differentiation-inducing agonist that induces increased binding of plasminogen to cells.8 PMA treatment of whole blood also markedly increased plasminogen binding to neutrophils as detected with mAb49 (Figure 2A right panel).
Detection of plasminogen bound to cells under pathologic conditions. (A) Peripheral blood was incubated for 6 hours with 10 μg/mL LPS (yellow tracing) or untreated (red tracing; left panel) or 1nM PMA (green tracing) or untreated (red tracing; right panel, as indicated), and FACS analysis was performed with antiplasminogen mAb49 as described in “Methods.” Purple tracings indicate FACS analyses of untreated blood with isotype control. Neutrophils were gated with anti-CD15 mAb. (B) FACS analyses with mAb49 of cell lines of different lineages incubated with 10μM plasminogen (black tracings) or without plasminogen (blue tracings). In other controls, the cell lines were treated with carboxypeptidase B (200 U/mL) before adding plasminogen (teal tracings). The cell events are different for each cell type because the calibration of the flow cytometer changes in each experiment. (C) Molt4 cells (2.5 × 106 cells/mL) were incubated with 10μM plasminogen for 1 hour at 37°C in either the absence (black bar) or presence of 0.2M EACA (violet bar) or without plasminogen or EACA (blue bar). Radiolabeled mAb49 (20nM) was then added and incubated for 30 minutes, and cell-bound radioactivity was separated from free radioactivity as described in “Methods.” In controls, direct binding of mAb49 to immobilized plasminogen (absorbance OD405 = 0.18 ± 0.003) was not decreased in the presence of EACA (absorbance OD405 = 0.22 ± 0.002). Thus, these results suggest that mAb49 binding to the cells reports the full picture of cell bound plasminogen. (D) NB4 cells were incubated with 1μM all-trans-retinoic acid (ATRA) for 48 hours, washed and preincubated with plasminogen (10μM), followed by FACS analyses with mAb49 (orange tracing). FACS analyses with mAb49 of untreated NB4 cells preincubated with either plasminogen (black tracing) or buffer (blue tracing) detected by antiplasminogen mAb49 is also shown. (E) Top panel: FACS analysis using mAb49 of peripheral blood from a patient containing promyelocytic blast cells (CD33+; HLDR−) at day 0 of ATRA treatment (red tracing) and after ATRA treatment for either 4 days (orange tracing) or 5 days (pink tracing). As a negative control, FACS analysis with an isotype control antibody was performed on blood containing promyelocytic blast cells (CD33+; HLDR−) at day 0 (turquoise tracing). Bottom panel: FACS analysis using mAb49 (red tracing) or isotype control (turquoise tracing) of blood from a patient with an M1 leukemia.
Detection of plasminogen bound to cells under pathologic conditions. (A) Peripheral blood was incubated for 6 hours with 10 μg/mL LPS (yellow tracing) or untreated (red tracing; left panel) or 1nM PMA (green tracing) or untreated (red tracing; right panel, as indicated), and FACS analysis was performed with antiplasminogen mAb49 as described in “Methods.” Purple tracings indicate FACS analyses of untreated blood with isotype control. Neutrophils were gated with anti-CD15 mAb. (B) FACS analyses with mAb49 of cell lines of different lineages incubated with 10μM plasminogen (black tracings) or without plasminogen (blue tracings). In other controls, the cell lines were treated with carboxypeptidase B (200 U/mL) before adding plasminogen (teal tracings). The cell events are different for each cell type because the calibration of the flow cytometer changes in each experiment. (C) Molt4 cells (2.5 × 106 cells/mL) were incubated with 10μM plasminogen for 1 hour at 37°C in either the absence (black bar) or presence of 0.2M EACA (violet bar) or without plasminogen or EACA (blue bar). Radiolabeled mAb49 (20nM) was then added and incubated for 30 minutes, and cell-bound radioactivity was separated from free radioactivity as described in “Methods.” In controls, direct binding of mAb49 to immobilized plasminogen (absorbance OD405 = 0.18 ± 0.003) was not decreased in the presence of EACA (absorbance OD405 = 0.22 ± 0.002). Thus, these results suggest that mAb49 binding to the cells reports the full picture of cell bound plasminogen. (D) NB4 cells were incubated with 1μM all-trans-retinoic acid (ATRA) for 48 hours, washed and preincubated with plasminogen (10μM), followed by FACS analyses with mAb49 (orange tracing). FACS analyses with mAb49 of untreated NB4 cells preincubated with either plasminogen (black tracing) or buffer (blue tracing) detected by antiplasminogen mAb49 is also shown. (E) Top panel: FACS analysis using mAb49 of peripheral blood from a patient containing promyelocytic blast cells (CD33+; HLDR−) at day 0 of ATRA treatment (red tracing) and after ATRA treatment for either 4 days (orange tracing) or 5 days (pink tracing). As a negative control, FACS analysis with an isotype control antibody was performed on blood containing promyelocytic blast cells (CD33+; HLDR−) at day 0 (turquoise tracing). Bottom panel: FACS analysis using mAb49 (red tracing) or isotype control (turquoise tracing) of blood from a patient with an M1 leukemia.
We next evaluated the ability of mAb49 to detect plasminogen binding to cell lines (known to bind plasminogen) derived from blood cell cancers. A positive signal in FACS analysis was clearly observed when promyelocytic leukemic (NB4), myeloid (HL60), lymphoid (Molt4 and Nalm6), monocytoid (U937), and erythro-myeloid precursor cells (K562) were preincubated with plasminogen (Figure 2B).
Treatment of cells with carboxypeptidase B removes C-terminal lysines and decreases the ability of cells to bind plasminogen.6,9 Therefore, we preincubated the cell lines with carboxypeptidase B, before addition of plasminogen and FACS analysis with mAb49. After treatment with carboxypeptidase B, binding of mAb49 was markedly reduced, verifying that mAb49 was reporting the association of plasminogen with the cells (Figure 2B).
To further address whether mAb49 reports the full picture of cell bound plasminogen, we examined radiolabeled mAb49 binding to cells in the presence of ϵ-aminocaproic acid (EACA) that fully blocks the specific binding of plasminogen to cells.4 When representative Molt4 cells were incubated with plasminogen, substantial binding of mAb49 was observed (Figure 2C). When cells were incubated with plasminogen plus EACA, binding of mAb49 was reduced to the level of cells incubated without plasminogen (Figure 2C). Thus, these results suggest that the conformational change that is recognized by mAb49 occurs when plasminogen is bound to all plasminogen receptors expressed by these cells. mAb49 most likely reports a conformational change that renders Glu-plasminogen more accessible to plasmic cleavage to the more readily activated Lys-plasminogen form.5 This is a necessary step in cell-dependent promotion of plasminogen activation.10
The NB4 promyelocytic cell line has been studied as a model for APL.11,12 APL is associated with severe bleeding complications that are responsible for the early mortality of this disease and have been related to disseminated intravascular coagulation and/or to hyperfibrinolysis.13 NB4 cells display a very high number of plasminogen binding sites, and these cells have a high capacity to promote plasminogen activation on their surfaces.11,12 Treatment of APL patients with all-trans-retinoic acid (ATRA) decreases bleeding in these patients13 and also reduces the ability of NB4 cells to bind plasminogen and promote plasminogen activation.11,12 Thus, we tested whether mAb49 could detect effects of ATRA on plasminogen binding to NB4 cells. The positive FACS signal observed with untreated NB4 cells, preincubated with plasminogen, was markedly decreased after treatment of NB4 cells with ATRA for 48 hours (Figure 2D). Recently, a major contribution of the plasminogen receptor, S100A10, to ATRA-dependent plasminogen binding to NB4 cells has been demonstrated.12 mAb49 can thus be used to detect plasminogen bound to S100A10, as well as to other plasminogen receptors that may participate.
We studied peripheral blood from a patient with APL with a high content of blast cells (80%), both before and after ATRA treatment in vivo. A strong FACS signal with mAb49 was detected before ATRA treatment compared with the isotype control (Figure 2E top panel). After both a 4-day and a 5-day treatment with ATRA, blast cells exhibited a markedly decreased FACS signal, compared with untreated cells (Figure 2E). In other controls, FACS analysis of M1 blast cells (which have not been reported to bind plasminogen) with mAb49 did not show a positive signal compared with isotype control. The increased plasminogen binding to the APL blasts in blood may play a role in the hyperfibrinolysis observed in APL patients.14 Furthermore, down-regulation of plasminogen binding by ATRA treatment is consistent with decreased hyperfibrinolytic bleeding in these patients after ATRA treatment.13
Our results demonstrate that mAb49 can be used to monitor cell-bound plasminogen in whole blood under normal and pathologic conditions and has potential for use in future studies as new roles of cell-associated plasminogen in disease etiology and progression are identified.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr M. Lanotte (Hôpital St Louis, Paris, France) for kindly providing the human promyelocytic NB4 cell line and Dr J. Inglés-Esteve (IDIBELL, Barcelona) for kindly providing the human cell line Nalm6.
This work was supported by Fondo Investigación Sanitaria (03/0459 and 08/0956; J.F.), the National Center for Research Resources and National Institutes of Health (grants HL38272, HL45934, and HL081046, L.A.M.; grant HL50398, R.J.P.), and Department of Veterans Affairs (R.J.P.). This is publication no. 21641 from The Scripps Research Institute.
National Institutes of Health
Authorship
Contribution: J.F. designed experiments, analyzed data, and wrote the manuscript; M.J. and P.F. designed experiments, performed research, analyzed data, and participated in manuscript drafting, R.J.P. analyzed data and participated in manuscript drafting, and L.A.M. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lindsey A. Miles, Department of Cell Biology, The Scripps Research Institute, 10550 N Torrey Pines Rd, SP30-3020, La Jolla, CA 92037; e-mail: lmiles@scripps.edu.