Abstract
The mechanisms responsible for increased cardiovascular risk associated with HIV-1 infection are incompletely defined. Using flow cytometry, in the present study, we examined activation phenotypes of monocyte subpopulations in patients with HIV-1 infection or acute coronary syndrome to find common cellular profiles. Nonclassic (CD14+CD16++) and intermediate (CD14++CD16+) monocytes are proportionally increased and express high levels of tissue factor and CD62P in HIV-1 infection. These proportions are related to viremia, T-cell activation, and plasma levels of IL-6. In vitro exposure of whole blood samples from uninfected control donors to lipopolysaccharide increased surface tissue factor expression on all monocyte subsets, but exposure to HIV-1 resulted in activation only of nonclassic monocytes. Remarkably, the profile of monocyte activation in uncontrolled HIV-1 disease mirrors that of acute coronary syndrome in uninfected persons. Therefore, drivers of immune activation and inflammation in HIV-1 disease may alter monocyte subpopulations and activation phenotype, contributing to a pro-atherothrombotic state that may drive cardiovascular risk in HIV-1 infection.
Introduction
Immune activation and inflammation are recognized predictors of HIV-1 disease progression and mortality.1-3 HIV-1 infection is also associated with an increased risk of venous and arterial thrombosis.4-8 In a recent clinical trial, plasma levels of the proinflammatory cytokine IL-6, C-reactive protein (CRP), D-dimers, and soluble CD14 (sCD14),were independent predictors of mortalities including deaths related to cardiovascular events.2,3,9 Patients randomized to receive intermittent antiretroviral therapy were at greater risk, particularly for cardiovascular events, suggesting that dynamic changes in levels of inflammation associated with viral replication increase the risk of atherothrombosis in HIV-1–infected patients.
Inflammation is an important contributor to atherosclerosis and coronary artery disease (CAD).10,11 Previous work suggests that systemic immune activation may be particularly relevant in patients who transition from stable CAD to unstable acute coronary syndrome (ACS).12-17 Although chronic immune activation and inflammation are associated with progressive HIV-1 disease,1 the drivers of inflammation and morbidity—and their mechanisms in HIV-1 disease—are incompletely defined.
Blood monocytes and tissue macrophages have been implicated in the initiation, progression, and thrombotic complications of atherosclerosis because they can mediate inflammation, are found within atherosclerotic plaques, and can drive coagulation through mechanisms that include the expression of tissue factor (TF).10,11,18,19
Monocytes are not a uniform cell population and can be segregated by CD14 and CD16 expression levels into 3 distinct subsets. Intermediate monocytes express high levels of CD14 and CD16 (CD14++CD16+) and are functionally distinguishable from classic CD14++CD16− monocytes based on homing marker expression, antigen presentation capabilities, and levels of proinflammatory cytokines produced after stimulation with bacterial TLR ligands.20-23 In contrast, nonclassic (CD14+CD16++) monocytes can recognize viral products and home to the vascular endothelium via expression of CX3CR1.22,24
Increased proportions of CD16-expressing monocytes have been reported in patients with inflammatory conditions such as sepsis25 and rheumatoid arthritis.26,27 In the present study, we report that the proportions of CD14+CD16++ and CD14++CD16+ monocytes are significantly increased in HIV-1 infection, as are the proportions of each monocyte subset expressing the procoagulant TF. In HIV-1–infected patients, the proportions of both intermediate and nonclassic monocytes are related to the magnitude of viremia, markers of T-cell activation (CD38 and HLA-DR), and plasma IL-6 levels. We also demonstrate that nonclassic, but not intermediate or classic, monocytes can be activated by HIV-1 to express TF in vitro. Plasma levels of D-dimer products of fibrinolysis and the bacterial lipopolysaccharide (LPS) receptor sCD14 were directly related to the proportion of intermediate (CD14++CD16+) monocytes in HIV-1 infection and, in patients with controlled viremia, plasma LPS levels were correlated directly with the proportion of intermediate monocytes. Finally, in HIV-1–uninfected patients with ACS, the proportions of intermediate and nonclassic monocytes, and the levels of TF on these cells, are increased in patterns similar to those seen in HIV-1–infected patients with uncontrolled viremia. Monocyte activation, with resultant procoagulant expression, is linked to cardiovascular disease (CVD) in HIV-1–uninfected persons and to predictors of morbidity and mortality in HIV-1–infected persons. These activated monocytes may play an important role in CVD risk in both HIV-1–infected and HIV-1–uninfected persons.
Methods
Patients
These studies were performed in compliance with policies of the institutional review board at Case Western Reserve University/University Hospital/Case Medical Center. Blood samples were obtained after informed consent in accordance with the Declaration of Helsinki. HIV-1–uninfected subjects (n = 23) were healthy donors from the general Case Western Reserve University/University Hospital population. HIV-1–infected donors (n = 57) were recruited from the Special Immunology Unit of University Hospitals/Case Medical Center and were divided into 2 populations based on viremia (viral load [VL] < 400 copies/mL and VL > 400 copies/mL). ACS patients (n = 10) had unstable symptoms consistent with cardiac ischemia and either elevated serum levels of cardiac troponins or dynamic electrocardiographic changes; these patients were recruited from the in-patient cardiology service and blood was drawn before cardiac catheterization. Stable CAD controls (n = 16) had 2 or more conventional cardiac risk factors, but did not have clinically apparent CAD. The majority (13 of 16) had evidence of coronary artery calcification by computed tomography, suggesting subclinical CAD. Age and cholesterol levels between ACS patients and stable CAD patients did not differ significantly. Framingham risk scores could be computed for 25 HIV-1–infected patients with controlled viremia, 7 HIV-1–infected patients with uncontrolled viremia, all ACS patients, and 10 of 16 stable CAD patients using sex, age, total cholesterol, high density lipoprotein level, diabetes, and smoking status as determined by chart review. To avoid the use of arbitrary blood pressure measurements, patients with blood pressure of 140-149/90-99 mmHg were considered to have hypertension and patients with blood pressure between 120-129/80-84 mmHg were considered not to have hypertension. This Framingham-based risk score was calculated using the online calculator at www.mdcalc.com and is expressed as the risk of incident coronary heart disease within a 10-year period.28 Framingham-based risk scores were not different for any of the 4 groups in which we were able to calculate them (P > .43 for each). Demographics for all patients in this study are listed in Table 1.
Cell preparation and incubations
Blood was drawn into EDTA-coated tubes. Whole blood (250 μL) was incubated in 15-mL polypropylene Falcon tubes (BD Biosciences) for 3 hours with individual TLR ligands. LPS from Escherichia coli (50 ng/mL), imiquimod (5 μg/mL), and single-stranded PolyU complexed with the cationic lipid LyovecR (10 μg/mL) were purchased from Invivogen.
Aldrithiol-2 (AT-2)–treated HIV-1 was kindly provided by Jeffrey Lifson (National Cancer Institute, Frederick MD). HIV-1 (MN) CL.4/SUPT1 lot P4095 is a CXCR4 tropic HIV-1 strain and HIV-1 ADA-M/SUPT1-CCR5 CL.30 lot P4101 is a CCR5 tropic virus. Each virus was tested at 3 capsid concentrations, 50, 150, and 500 ng/mL, and the 150 ng/mL concentration was found to be optimal for TF induction. Empty vesicles at a total protein concentration that was equal to the total protein in the viral preparations were used as a control.
Flow cytometry
Monocytes and T cells were identified by size, granularity, and expression of CD14 and CD16 or CD3, CD8, and CD4, respectively. CD14 expression was based on an isotype and population gating strategy with both the lymphocyte population and the isotype serving as the lower limit for determining which cells were CD14+ versus CD14−. The upper limit of CD14+ and lower limit for CD14++ expression is based on the CD14++ population. Expression of CD16 was based on a conservative isotype gating strategy and was confirmed by fluorescence intensity of the CD16− lymphocyte population. Fluorescence minus 1 and isotype gating strategies were used to identify expression of activation markers.
Cell-surface molecule expression was monitored by staining cells with the following fluorochrome-labeled Abs: anti-TF FITC (American Diagnostica), anti-CD14 Pacific Blue, anti-CD16 phycoerythrin (PE), anti-CD62 P-selectin PE-cy5 (all BD Pharmingen), anti-CX3CR1 allophycocyanin (APC; BioLegend), anti-CD69 PE-cy7, and anti–HLA-DR APC-cy7 (both BD Biosciences). Positive expression of these surface proteins was determined based on isotype control staining. To ensure that monocyte populations were not contaminated by lymphocytes, preliminary experiments using an exclusion gate that included anti-CD3 FITC, anti-CD20 FITC, anti-CD56 FITC (BD Pharmingen) were performed.
T-cell activation was measured using anti-CD38 PE, anti–HLA-DR FITC, anti-CD3 APC (BD Biosciences), anti-CD8 APC-cy7, anti-CD4 Pacific Blue (both BD Pharmingen), and the appropriate isotype control mAbs.
Whole blood samples were incubated for 15 minutes on ice with FACS Lyse buffer (BD Biosciences) and then washed in wash buffer (PBS with 1% BSA and 0.1% sodium azide). Cells were then stained for 30 minutes in the dark on ice and then washed in wash buffer, fixed in 1% formaldehyde, and analyzed using a MACS Quant flow cytometer (Miltenyi Biotec). MACS Quant Version 2.21031.1 software (Miltenyi Biotec) and Prism Version 5.0 software (GraphPad) were used to organize and analyze the data.
Measurement of D-dimers, IL-6, sCD14, and CRP
Fresh whole blood samples were collected in EDTA-containing tubes and centrifuged for 15 minutes at 495g. Plasma was removed and frozen at −80°C until thawed once and analyzed in batch. Levels of D-dimers were measured using the Asserachrom D-DI immunoassay (Diagnostica Stago). Soluble CD14, CRP, and IL-6 levels were measured using Quantikine ELISA kits (R&D Systems).
Measurement of LPS
Plasma samples were diluted to 10% or 20% with endotoxin-free water and then heated to 85°C for 15 minutes to denature plasma proteins. Plasma levels of LPS were then quantified with a commercially available limulus amebocyte lysate assay (QCL-1000; Lonza) according to the manufacturer's protocol. Samples were run in triplicate, backgrounds were subtracted, and mean values were reported.
Statistical methods
Conventional measures of central location and dispersion are used to describe the data. We performed pairwise comparisons using the Mann-Whitney U test and evaluated correlations between pairs of continuous variables using the Spearman rank correlation. Given the considerable differences in conventional risk factors between each of the HIV-1–infected groups and the healthy control group, we fitted multiple linear regression models that included age, race (coded as white/non-white), sex, and current smoking status as covariates to assess the independent contribution of subject group to the differences observed in the distribution of total and TF+ monocytes. Analyses were done using SPSS Version 20.0 software (IBM) and Stata MP Version 11.2 software. All comparisons were 2-sided without formal correction for multiple comparisons and P < .05 was considered statistically significant.
Results
We have demonstrated previously that monocyte expression of TF is increased in HIV-1 infection.29 The present study was designed to explore further the role of monocyte activation and TF expression as potential mediators of increased CVD risk. Because 3 monocyte subsets have been delineated, distinguishable by their responsiveness to bacterial and viral products,20-23 we assessed the proportional representation of these subsets and their relative state of activation in HIV-1 disease. Initial studies were performed on fresh whole-blood samples obtained from 57 HIV-1–infected patients and 23 controls not known to be HIV-1 infected. We divided the HIV-1–infected population into 2 groups, 1 with controlled viremia (plasma HIV-1 RNA < 400 copies/mL, n = 39) and 1 with uncontrolled viremia (plasma HIV-1 RNA > 400 copies/mL, n = 18). The median CD4+ T-cell count was 578 cells/μL for the patients with controlled viremia and 398 cells/μL for the uncontrolled group. All patients with controlled viremia and 44% of the uncontrolled viremia patient group were receiving antiretroviral therapy. A more complete description of these groups is provided in Table 1.
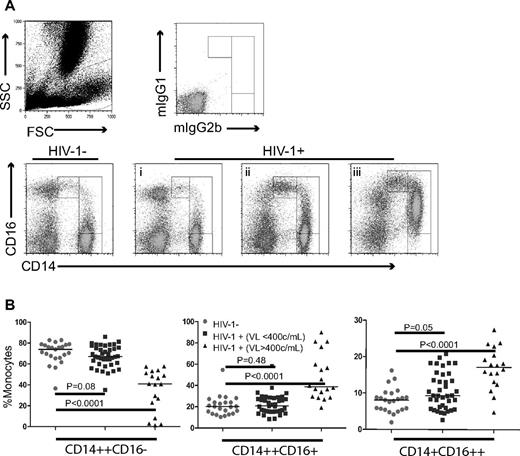
Monocytes and their subsets were identified by flow cytometry based on forward and side scatter characteristics and by expression of CD14 and CD16.22 All monocyte subsets expressed high levels of HLA-DR. Based on previous reports20-23 and preliminary experiments that contained a lymphocyte exclusion gate (CD3, CD20, and CD56), the populations were ensured to be monocytes. The gating strategy used to identify classic (CD14++CD16−), intermediate (CD14++CD16+), and nonclassic monocytes (CD14+CD16++) is shown in Figure 1A. Representative dot plots from 1 control and 3 HIV-1–infected patients (Figure 1A) and summary figures (Figure 1B) are shown. The proportions of nonclassic monocytes (CD14+CD16++) were significantly increased in HIV-1–infected patients with uncontrolled viremia (median = 17.1%, interquartile range [IQR] = 13.0%-27.5%, P < .001), but not in patients with controlled viremia (median = 9.3%, IQR = 6.0%-15.2%, P = .05) compared with these proportions in healthy controls (median = 8.1%, IQR = 5.5%-9.9%). After controlling for age, sex, race, and smoking, the difference between aviremic patients and controls was also not significant (adjusted P = .07).
Proportions of monocyte subsets are altered in HIV-1 disease. Whole blood samples were obtained from 57 HIV-1–infected donors and 23 healthy controls and the relative proportions of monocyte subsets were analyzed by flow cytometry. (A) Three monocyte subsets were identified by size and granularity and by CD14 and CD16 expression, and representative dot plots show monocyte subsets from 1 healthy control and 3 HIV-1–infected patients (patients i, ii, and iii). All 3 patients were male and were receiving antiretroviral therapy. Patients i and ii had VL < 400 copies/mL and CD4+ T-cell counts of 857 and 456 cells/μL, respectively. Patient iii was viremic (333 706 copies/mL) and had a CD4+ T-cell count of 132 cells/μL. (B) Summary data of monocyte subsets among healthy controls and HIV-1–infected patients with controlled (< 400 copies/mL) or uncontrolled (> 400 copies/mL) viremia.
Proportions of monocyte subsets are altered in HIV-1 disease. Whole blood samples were obtained from 57 HIV-1–infected donors and 23 healthy controls and the relative proportions of monocyte subsets were analyzed by flow cytometry. (A) Three monocyte subsets were identified by size and granularity and by CD14 and CD16 expression, and representative dot plots show monocyte subsets from 1 healthy control and 3 HIV-1–infected patients (patients i, ii, and iii). All 3 patients were male and were receiving antiretroviral therapy. Patients i and ii had VL < 400 copies/mL and CD4+ T-cell counts of 857 and 456 cells/μL, respectively. Patient iii was viremic (333 706 copies/mL) and had a CD4+ T-cell count of 132 cells/μL. (B) Summary data of monocyte subsets among healthy controls and HIV-1–infected patients with controlled (< 400 copies/mL) or uncontrolled (> 400 copies/mL) viremia.
The proportion of intermediate (CD14++CD16+) monocytes was increased in the viremic group (median = 38.8%, IQR = 29.6%-59.8%, P < .0001), but not in the controlled viremia group (median = 20.7%, IQR = 15.6%-28.2%) compared with healthy controls (median = 20.2%, IQR = 13.6%-24.1%). When adjusted for age, race, sex, and smoking, both the viremic patients (P = .001) and the controlled viremia group (P = .014) had increased proportions of intermediate monocytes.
CVD risk in HIV-1 infection is inversely related to CD4+ T-cell count30 and is increased in patients who experience uncontrolled viral replication during intermittent antiretroviral therapy,2 potentially linking the pathogenesis of CVD to immune deficiency and viral replication. Therefore, in the present study, we compared monocyte subset proportions with CD4+ T-cell counts, plasma HIV-1 RNA levels, and expression of the activation markers CD38 and HLA-DR on CD8+ T lymphocytes, which has been linked to disease course.1 In all HIV-1–infected patients, the proportion of intermediate (CD14++CD16+) monocytes was directly correlated with viremia (r = 0.59, P < .0001) and the proportion of activated CD8+ T cells (r = 0.67, P < .0001) and inversely correlated with CD4+ T-cell count (r = −0.424, P = .001). In addition, in all patients, the proportion of nonclassic CD14+CD16++ monocytes was directly correlated with viremia (r = 0.372, P = .004) and the proportion of activated CD8+ T cells (r = 0.317, P = .023), but not with CD4+ T-cell counts (r = −0.218, P = .104). Because viremia, CD4+ count, and T-cell activation are often correlated, we investigated whether any of these indices was related to the proportions of monocyte subsets independently. The proportion of CD14++CD16+ intermediate monocytes in these patients was independently associated with the magnitude of viremia and the proportions of activated CD8+ T cells (P = .013, and P = .004, respectively). In addition, the proportion of nonclassic CD14+CD16++ monocytes was independently associated with the proportion of activated CD8+ T cells (P = .027).
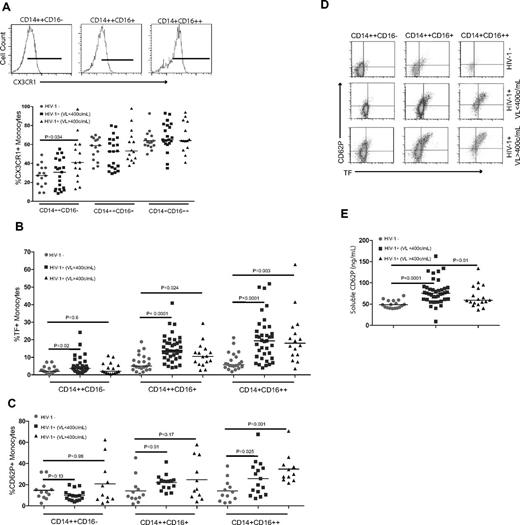
Monocytes can home to the vascular endothelium using several surface receptors, including the fractalkine receptor (CX3CR1).20,22,24 We confirmed that CX3CR1 is most often and most highly expressed on the CD14+CD16++ monocyte subset, with comparatively less expression on the CD14++CD16+ subset and lower levels on the CD14++CD16− subset (Figure 2A). Viremic patients had proportionally more CD14+CD16− cells that expressed CX3CR1 than did uninfected controls (median = 41.0%, IQR = 25.8%-60.3% versus median = 27.3%, IQR = 18.4%-35.4%, respectively, P = .034). Because monocyte subsets differentially home to the vascular endothelium, where they may contribute to thrombus formation, we next investigated whether any of these subsets were enriched for surface expression of the procoagulant TF or the adhesion molecule P-selectin (CD62P). Compared with findings among healthy controls, the proportions of intermediate and nonclassic monocytes that express TF were increased in both the controlled (P < .001 for both) and uncontrolled (P < .02 and P < .003) viremia patient populations (Figure 2B), although the difference in TF+ intermediate monocytes between viremic HIV-1–infected patients and controls became nonsignificant after controlling for age, sex, race, and smoking history (adjusted P = .096). In both patient populations, the nonclassic monocytes had the greatest proportion of TF+ cells.
Nonclassic and intermediate monocytes from HIV-1–infected patients are enriched for cells that express TF and P-selectin. Monocyte subsets were identified in whole blood samples from HIV-1–infected patients and HIV-1–uninfected controls. Surface expression of CX3CR1, TF, and CD62P-selectin was measured on monocyte subsets by flow cytometry. (B) Summary data of TF expression. (C) Summary data of CD62P expression. (D) Representative dot plots of TF and CD62P expression. (E) Plasma samples from all donors were thawed and levels of soluble CD62P were measured in batch by ELISA.
Nonclassic and intermediate monocytes from HIV-1–infected patients are enriched for cells that express TF and P-selectin. Monocyte subsets were identified in whole blood samples from HIV-1–infected patients and HIV-1–uninfected controls. Surface expression of CX3CR1, TF, and CD62P-selectin was measured on monocyte subsets by flow cytometry. (B) Summary data of TF expression. (C) Summary data of CD62P expression. (D) Representative dot plots of TF and CD62P expression. (E) Plasma samples from all donors were thawed and levels of soluble CD62P were measured in batch by ELISA.
The proportion of cells that expressed CD62P was significantly higher in the nonclassic monocyte populations from patients with controlled (median = 25.7%, IQR = 13.4%-36.7%) and uncontrolled viremia (median = 30.0%, IQR = 24.9%-38.8%) compared with controls (median = 11.7%, IQR = 5.8%-19.5%, P = .025 and .001, respectively, Figure 2C). Monocytes that expressed TF also frequently expressed CD62P (Figure 2D) and, not surprisingly, the proportions of nonclassic monocytes that were both TF+ and CD62P+ were significantly increased in patients with controlled (median = 15.3%, IQR = 7.8%-20.6%) and uncontrolled (median = 15.0%, IQR = 11.3%-37.2%) viremia compared with the proportion in controls (median = 7.6%, IQR = 4.4%-11.4%, P = .02 and .002, respectively). The increased proportional representation and activated, procoagulant phenotype of nonclassic monocytes in HIV-1–infected patients with controlled and uncontrolled viremia may provide insights into the increased risk for thrombosis in these patients.
sCD62P is shed from activated platelets and endothelial cells,31 can induce monocyte TF expression in vitro,32 and is a strong independent predictor of venous thrombosis in HIV-1–infected patients.33 Plasma levels of sCD62P were higher in the controlled viremia (median = 75.9 ng/mL, IQR = 61.2-88.9 ng/mL, P < .0001) and the uncontrolled viremia (median = 59.2 ng/mL, IQR = 53.1-87.5 ng/mL, P = .01) patients than among controls (median = 48.4 ng/mL, IQR = 41.5-58.3 ng/mL, Figure 2E). Among the HIV-1–infected patients, there was a strong correlation between the plasma levels of sCD62P and the proportions of intermediate (r = 0.515, P = .007) and nonclassic (r = 0.534, P = .005) monocytes that expressed CD62P.
Increased plasma levels of the inflammatory cytokine IL-6, the acute-phase protein CRP, and D-dimer products of fibrinolysis are known to predict CVD risk in HIV-1–uninfected populations34 and were also predictive of all-cause and cardiovascular-related mortality in HIV-1–infected patients in the SMART study.2 As expected, plasma levels of IL-6 (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and D-dimers (supplemental Figure 1B) were significantly higher in both HIV-1–infected patient populations than among healthy controls. CRP levels were increased only in the uncontrolled viremia population and not in the controlled viremia patients compared with levels in controls (supplemental Figure 1C). The proportion of intermediate monocytes in HIV-1–infected patients was correlated with plasma levels of D-dimers (r = 0.369, P = .005) and CRP (r = 0.283, P = .033). Plasma IL-6 levels were correlated with both the proportion of CD14++CD16+ monocytes (r = 0.294, P = .026) and the proportion of CD14+CD16++ monocytes (r = 0.268, P = .043) in HIV-1–infected patients.
We also measured levels of sCD14, an LPS coreceptor shed from monocytes on activation,35,36 because these levels also predict mortality in HIV-1 infection.3 Levels of sCD14 were significantly higher in both patient populations than in controls (P < .0001, supplemental Figure 1D). Plasma levels of D-dimers and sCD14 were directly correlated in the HIV-1–infected population (r = 0.304, P = .021), again suggesting a link between monocyte activation and coagulation in HIV-1 infection.
Microbial translocation has been linked to inflammation and immune activation in HIV-1 disease.37,38 Because classic and intermediate monocyte subsets produce high levels of IL-8, IL-6, and IL-10 in response to LPS exposure,22 we assessed the relationship between plasma LPS levels and monocyte activation. Plasma levels of LPS were significantly increased in both the controlled viremia (median = 81.6 pg/mL, IQR = 61.8-96.1 pg/mL) and uncontrolled viremia patients (median = 87.2 pg/mL, IQR = 65.1-117.4 pg/mL) compared with levels of LPS in the plasma of controls (median = 38.1 pg/mL, IQR = 20.3-62.4 pg/mL, P < .0001 for both comparisons, Figure 3A). We found a strong correlation between plasma LPS levels and CD8+ T-cell activation (r = 0.393, P = .004) in the HIV-1–infected population, which is consistent with a previous study.37 Levels of plasma LPS were not significantly correlated with proportions of CD14++CD16+ monocytes in the entire HIV-1–infected patient population (r = 0.216, P = .106, Figure 3B). However, in patients with controlled viremia, there was a significant correlation between plasma LPS levels and the proportion of intermediate monocytes (r = 0.336, P = .037, Figure 3C), but this correlation was not seen in patients with uncontrolled viremia (r = 0.005, P = .984, Figure 3D), suggesting that in patients with controlled viremia, systemic translocation of microbial products may drive intermediate monocyte expansion, whereas in subjects with sustained HIV-1 replication, other factors such as HIV-1 itself contribute more to this phenotype.
Plasma levels of LPS are increased in HIV-1–infected patients and are correlated with the proportion of circulating intermediate monocytes. Plasma samples from all donors were thawed and levels of LPS were measured using the limulus lysate assay. (A) Plasma levels of LPS were significantly increased in HIV-1–infected patients with controlled (< 400 copies/mL) and uncontrolled (> 400 copies/mL) viremia compared with levels in uninfected controls. (B) There was a modest and not significant correlation between plasma LPS levels and the proportion of CD14++CD16+monocytes in the entire HIV-1–infected population. (C) There was a direct and significant correlation between plasma levels of LPS and the proportion of CD14++CD16+ intermediate monocytes in patients with controlled viremia, but this correlation was not seen in patients with uncontrolled viremia (D).
Plasma levels of LPS are increased in HIV-1–infected patients and are correlated with the proportion of circulating intermediate monocytes. Plasma samples from all donors were thawed and levels of LPS were measured using the limulus lysate assay. (A) Plasma levels of LPS were significantly increased in HIV-1–infected patients with controlled (< 400 copies/mL) and uncontrolled (> 400 copies/mL) viremia compared with levels in uninfected controls. (B) There was a modest and not significant correlation between plasma LPS levels and the proportion of CD14++CD16+monocytes in the entire HIV-1–infected population. (C) There was a direct and significant correlation between plasma levels of LPS and the proportion of CD14++CD16+ intermediate monocytes in patients with controlled viremia, but this correlation was not seen in patients with uncontrolled viremia (D).
Monocyte subsets are differentially responsive to TLR stimulation with intermediate monocytes, especially those activated by bacterial TLR ligands (eg, LPS) and nonclassic monocytes by viral products through TLR7 and TLR8 ligation.22 Because both viral replication and systemic translocation of bacterial elements are characteristic of chronic HIV-1 infection, in the present study, we assessed the relative responsiveness of these subsets to HIV-1 and other microbial elements in vitro. Whole blood samples from healthy, uninfected donors were incubated for 3 hours alone or in the presence of individual TLR ligands (LPS:TLR4, imiquimod:TLR7, or single-stranded PolyU:TLR8) or AT-2–inactivated HIV-1 (MN4095 or P4101). Exposure to LPS resulted in significant increases in TF expression on all monocyte subsets (Figure 4), whereas only CD14+CD16++ monocytes increased TF expression after incubation with imiquimod or single-stranded PolyU (Figure 4). Likewise, exposure of whole blood samples to either the CXCR4 using HIV-1 MN4095 or the CCR5 tropic HIV-1 P4101 induced TF only on CD14+CD16++ monocytes (Figure 4).
Exposure of whole blood samples to HIV-1 results in increased surface expression of TF on nonclassic (CD14+CD16++) monocytes, but not on the intermediate (CD14++CD16+ CD14+CD16+) or CD14++CD16− monocytes. Whole blood was obtained from HIV-1–uninfected subjects and exposed to LPS (50 ng/mL), imiquimod (5 μg/mL), single-stranded PolyU complexed with the cationic lipid LyovecR (ssPolyU; 10 μg/mL), or AT-2–inactivated HIV-1 (X4 or R5 tropic, 150 ng/mL) for 3 hours. Surface expression of TF was measured on monocyte subsets by flow cytometry. Exposure to LPS resulted in a significant increase in TF on all monocyte subsets, but exposure to imiquimod, ssPolyU, or HIV-1 resulted in increased TF expression on only the CD14+CD16++ subset.
Exposure of whole blood samples to HIV-1 results in increased surface expression of TF on nonclassic (CD14+CD16++) monocytes, but not on the intermediate (CD14++CD16+ CD14+CD16+) or CD14++CD16− monocytes. Whole blood was obtained from HIV-1–uninfected subjects and exposed to LPS (50 ng/mL), imiquimod (5 μg/mL), single-stranded PolyU complexed with the cationic lipid LyovecR (ssPolyU; 10 μg/mL), or AT-2–inactivated HIV-1 (X4 or R5 tropic, 150 ng/mL) for 3 hours. Surface expression of TF was measured on monocyte subsets by flow cytometry. Exposure to LPS resulted in a significant increase in TF on all monocyte subsets, but exposure to imiquimod, ssPolyU, or HIV-1 resulted in increased TF expression on only the CD14+CD16++ subset.
Persons with atherosclerosis are at risk for the ACS, in which active atherothrombosis occurs at a site of atherosclerotic plaque rupture or superficial endothelial cell erosion. Activation of monocytes/macrophages is thought to be central to the pathogenesis of atherosclerosis and appears to play an important role in the progression to ACS.17,39-41 Therefore, to provide a context in which the degree of monocyte activation in HIV-1–infected patients could be judged, we next examined monocyte subsets and their activation phenotype in patients presenting to the cardiac catheterization laboratory with ACS. None of these subjects was known to be HIV-1 infected. We also studied a second control cohort composed of subjects with stable CAD (none of whom was known to be HIV-1 infected) with 2 or more known cardiac risk factors. The majority of these patients had evidence of subclinical CAD (coronary calcification by cardiac CT scan), but none had a clinical history of CAD or symptoms of ACS. Although individual cardiac risk factors varied, ACS and stable CAD patients were similar in age and background cardiovascular risk as computed by Framingham Risk Score assessment (Table 1).
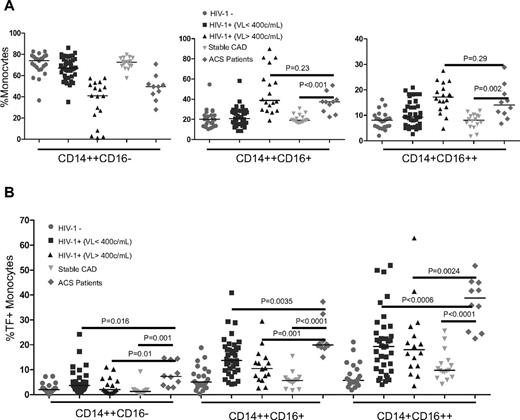
The proportions of both intermediate and nonclassic monocytes were increased in the ACS patient group (n = 10; median = 37.4%, IQR = 28.1%-39.7% and median = 14.0%, IQR = 10.8%-18.4%, respectively) compared with the proportions in the stable CAD controls (n = 16; median = 19.1%, IQR = 17.5%-23.3% and median = 8.1%, IQR = 6.0%-9.8%, P < .001 and .002, respectively), and proportions were similar to those in HIV-1–infected subjects with uncontrolled viremia (P = .23 and P = .29, respectively, Figure 5A). The proportions of intermediate and nonclassic monocytes that expressed TF were also increased in the ACS subjects (median = 20.0%, IQR = 19.1%-29.6%, and median = 38.8%, IQR = 25.2%-45.0%, respectively) compared with the proportions of TF+ cells in the stable CAD controls (median = 5.1%, IQR = 3.0%-9.2%, and median = 5.7%, IQR = 4.6%-10.2%, respectively, P < .0001 for both, Figure 5B). The proportions of TF-expressing cells in the intermediate (P = .001) and nonclassic (P = .002) monocyte subsets in the ACS patients were higher than among the patients with both controlled (P = .003 and P = .0006, respectively) and uncontrolled viremia (P = .001 and P = .002). Markers of T-cell activation (CD38 and HLA-DR) were low on CD8+ lymphocytes in the ACS patients (median = 7.9%, IQR = 5.3%-11.7%), clearly distinguishing these subjects from viremic HIV-1–infected patients (median = 39.9%, IQR = 29.7%-48.9%, P < .0001) and suggesting that despite a similar monocyte phenotype, the underlying determinants of T-cell activation status are different in these 2 groups.
HIV-1–uninfected patients with ACS also have altered monocyte subset proportions and increased monocyte expression of TF. Whole blood samples were collected from 10 HIV-1–uninfected ACS patients and 16 patients without AC, but with a similar CVD risk profile (stable CAD controls). Proportions of monocyte subsets (A) and monocytes (B) that express TF were analyzed by flow cytometry. Proportions of intermediate and nonclassic monocytes were increased in ACS patients compared with controls; TF expression was also increased on these cells.
HIV-1–uninfected patients with ACS also have altered monocyte subset proportions and increased monocyte expression of TF. Whole blood samples were collected from 10 HIV-1–uninfected ACS patients and 16 patients without AC, but with a similar CVD risk profile (stable CAD controls). Proportions of monocyte subsets (A) and monocytes (B) that express TF were analyzed by flow cytometry. Proportions of intermediate and nonclassic monocytes were increased in ACS patients compared with controls; TF expression was also increased on these cells.
Discussion
Because HIV-1–infected patients are now living longer, CVD is becoming more prevalent in this population.5-8 Inflammation is a recognized contributor to atherosclerosis11 and, because immune activation and inflammation are increased in HIV-1 disease, it is plausible that these processes may accelerate the progression of CVD in HIV-1–infected patients.
Immune activation in HIV-1 disease is likely driven by multiple mechanisms, including HIV-1 itself, increased replication of other viral copathogens such as CMV42-44 in the setting of HIV-1–related immune deficiency, and systemic translocation of microbial products across a damaged gut mucosal barrier. We and others have found increased levels of LPS and bacterial DNA in the plasma of HIV-1–infected persons.37,38,45 These microbial products can activate innate immune receptors such as TLRs, resulting in production of inflammatory cytokines and cellular activation.46 We have shown that high levels of LPS and bacterial DNA are correlated directly with indices of immune activation and inversely with CD4+ T-cell restoration after administration of combination antiretroviral therapy,37,38 linking microbial translocation to both immune activation and impaired immune homeostasis in HIV-1 infection.
Monocytes and tissue macrophages, which express multiple TLRs, have been implicated in CVD because they can mediate inflammation, plaque destabilization, and coagulation.10,11,18,19 Recently, in a substudy of the SMART trial, the monocyte activation marker sCD14 was shown to be an independent predictor of mortality in HIV-1 infection.3 We demonstrated previously that monocytes of HIV-1–infected patients express high levels of the procoagulant TF and that TF expression is related to viremia and to plasma levels of both sCD14 and D-dimer products of fibrinolysis.29 TF can initiate the extrinsic clotting pathway,47-49 potentially linking monocyte activation to coagulation and thrombosis in HIV-1 disease.
Three monocyte subsets that are functionally and phenotypically distinguishable have been identified.22 In the present study, we report striking alterations in the proportions of these monocyte subsets in HIV-1 infection, expanding on previous studies50,51 and demonstrating that the proportions of nonclassic (CD14+CD16++) monocytes are correlated directly with viremia and markers of T-cell activation. We also show for the first time that both intermediate and nonclassic monocytes from HIV-1–infected patients are enriched for cells that express TF, readily distinguishable from findings among healthy controls. Proportions of nonclassic monocytes that express TF are increased in patients with controlled or uncontrolled viremia, suggesting that even during successful antiretroviral therapy, these vascular homing monocytes may increase risk for coagulation in HIV-1 disease. We also report for the first time that expression of CD62P is increased on CD14+CD16++ monocytes from HIV-1–infected donors. This may play a role in transition of these monocytes into foam cells, as has previously been reported for the monocytic cell line U937 on exposure to ox-LDL.52
Intermediate monocytes produce high levels of IL-6 and IL-8 in response to TLR4 stimulation.22 We found that in HIV-1 infection, the proportions of intermediate monocytes are related to plasma levels of LPS, suggesting that microbial translocation may, at least partially, drive expansion of these cells. Some of these CD14++CD16+ monocytes were directly related to plasma levels of D-dimers in our HIV-1–infected population, suggesting a link between intermediate monocytes and intravascular coagulation. Plasma levels of sCD14 were also correlated directly with D-dimer levels, also linking monocyte activation and coagulation.
Nonclassic monocytes tended to have the highest levels of TF expression among all monocyte subsets, a novel and potentially clinically important observation because these monocytes also readily home and become tethered to the vascular endothelium via CX3CR1. Therefore, we speculate that this subpopulation may be particularly prothrombotic in vivo.22 We also show for the first time that CD14+CD16++ monocytes, but not other monocyte subsets, can be activated directly by HIV-1 to express surface TF. We suspect that HIV-1–mediated induction of TF is not HIV-1 coreceptor mediated, but rather is likely though innate immune receptor recognition because all monocyte subsets express CXCR4 and CCR5,24 but only nonclassic monocytes increased TF expression after HIV-1 exposure. Experiments with small-molecule inhibitors of CCR5 (maraviroc) or CXCR4 (AMD3100) indicate that interaction between R5 or X4 using HIV-1 and coreceptors is necessary for induction of TF on nonclassic monocytes (not shown). This same monocyte subpopulation is also responsive to imiquimod and single-stranded PolyU complexed with the cationic lipid LyovecR (Figure 4), ligands for TLR7 and TLR8 that also can be activated by genomic sequences of HIV-1.53 Alternatively and, in our view less likely, nonclassic monocytes might be uniquely responsive to a soluble mediator induced by HIV-1 exposure. Additional studies are needed to identify the precise mechanisms of TF up-regulation by HIV-1.
The results of the present study suggest that HIV-1 infection results in a potentially prothrombotic state of monocyte activation. Activation of nonclassic monocytes by HIV-1 may result directly in an increased risk of clot formation. In addition, in both controlled and uncontrolled HIV-1 infection, systemic translocation of microbial products such as LPS may activate and increase the expression of the procoagulant TF on all circulating monocyte subsets, perhaps contributing to the increased cardiovascular risks seen even in HIV-1 infection controlled with antiretroviral therapy.2,54
The pattern of altered monocyte homeostasis in HIV-1 disease may be mechanistically relevant to increased cardiovascular risk. In contrast to uninfected patients with known cardiovascular risk factors, patients with ACS display a profile of TF expression and increased proportions of intermediate and nonclassic monocytes similar to that of HIV-1–infected patients with uncontrolled viremia. The upstream drivers of inflammation and monocyte activation in HIV-1 disease and ACS may be distinguishable, but the resulting alterations in monocyte subpopulations and TF expression are very similar. The cross-sectional nature of the present study prevents us from making strong mechanistic conclusions about the involvement of monocyte subsets in ACS, but future longitudinal studies measuring monocyte subset activation and inflammatory biomarkers in patients after myocardial infarction and percutaneous coronary intervention or after therapeutic interventions that reduce immune activation, viral replication, or microbial translocation may provide insights into the drivers of monocyte activation in ACS and HIV-1 disease.
A limitation of this study is related to the heterogeneous nature of our patient and control populations in that we cannot completely exclude a role for differences in other CVD risk factors on monocyte phenotypes. We tried to account for differences in age, sex, race, and smoking status by using multiple linear regression models and, after controlling for these variables, HIV-1 infection appeared to be associated with significant abnormalities of monocyte activation and coagulation phenotype.
The results of the present study have important implications for understanding the mechanisms that lead to ACS. Despite advances in our understanding of atherosclerosis, the mechanisms that lead to conversion from stable CAD to ACS remain elusive. This study is the first to compare directly the inflammatory state associated with ACS with that of HIV-1 disease. Despite differences in the pathogenesis of HIV-1 and ACS, these 2 diseases lead to a common profile of prothrombotic monocyte activation. Further studies are required to distinguish the upstream initiators of monocyte activation in these 2 settings.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Bad Boys of Cleveland/Cleveland Immunopathogenesis Consortium for helpful comments and discussions related to this project.
This work was funded by grants from the National Institutes of Health (AI-07164, AI-67039, AI-68636, and 1K99HL108743-01A1), the Fasenmyer Foundation, and the Center for AIDS Research at Case Western Reserve University (AI 36219). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
National Institutes of Health
Authorship
Contribution: N.T.F., C.S., A.L., and J.M. performed the experiments; D.A.Z., D.I.S., M.A.C., B.R., and M.M.L. obtained the patient samples; B.R. provided statistical support; and all authors contributed to the experimental design, data analysis, and writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicholas Funderburg, 2109 Adelbert Rd, 1048B Biomedical Research Bldg, Cleveland, OH, 44106; e-mail: NTF1@case.edu.