Abstract
Posttransplantation human herpesvirus-8 (HHV8)/Kaposi sarcoma herpesvirus (KSHV) primary infection and/or reactivations are associated with uncommon and sometimes fatal, neoplastic, and non-neoplastic diseases. HHV8-related clinical manifestations notably range from Kaposi sarcoma (KS) to either primary effusion lymphoma or multicentric Castleman disease B-cell malignancies, and from polyclonal HHV8-positive plasmacytic lymphoproliferative disorders to bone marrow failure and peripheral cytopenias, associated or not with hemophagocytic syndromes, and to acute hepatitis syndromes. We reviewed the patient series reported in the literature and summarized clinical management aspects, in terms of diagnosis, follow-up, and treatment. We described typical clinical presentations and histopathologic diagnostic features of these diseases, and we discussed the role of HHV8-specific serologic, molecular, and immunologic assays, particularly focusing on recent data from HHV8-specific T-cell monitoring in posttransplantation KS patients. We finally discussed actual therapeutic options, namely, the reduction or discontinuation of immunosuppressive therapy or the switch from calcineurin inhibitors to mTOR inhibitors, as alternatives to antineoplastic chemotherapy, along with the use of antiherpesvirus agents as prophylactic or therapeutic measures, and treatment with rituximab in posttrans-plantation multicentric Castleman disease patients and non-neoplastic HHV8-associated syndromes.
Introduction
Herpesvirus-associated hematologic diseases often represent primary clinical challenges during the multidisciplinary follow-up of posttransplant patients, either with hematopoietic stem cell transplant (HSCT) or with solid organ transplant (SOT). In immunosuppressed organ recipients, latent human γ-herpesvirus, namely, EBV and human herpesvirus-8 (HHV8)/Kaposi sarcoma herpesvirus (KSHV) are typically responsible for several virus-driven neoplastic proliferations, as well as they may sometimes cause uncommon, but equally life-threatening, non-neoplastic infectious complications. During the past decade, large amounts of experimental evidence have come out on these topics, and some of them have yet begun to influence the clinical behavior, in terms of diagnosis and treatment. Chiefly, EBV-related complications have been extensively studied and discussed, so that now physicians can rely on accurate and updated clinical recommendations, in particular dealing with early diagnosis, monitoring and treatment of EBV+ posttransplantation lymphoproliferative diseases (PTLDs).1 With regards to HHV8, practical management of the virus-associated neoplastic lymphoproliferations has deeply been revised in HIV-positive subjects,2,3 whereas in SOT and HSCT settings, the diagnostic and therapeutic issues about the various HHV8-driven manifestations (either neoplastic or non-neoplastic) are only occasionally reported in the literature, but most of these diseases revealed to be rapidly lethal if not timely recognized and adequately treated. Indeed, posttransplantation HHV8-associated neoplastic and non-neoplastic pathologies, although rare, may notably range from (1) Kaposi sarcoma (KS) mucocutaneous and visceral angioproliferations, to either (2) intracavity primary effusion lymphoma (PEL) or (3) systemic multicentric Castleman disease (MCD) B-cell lymphoproliferations, and from (1) Castleman-like or otherwise called atypical HHV8-positive plasmacytic lymphoproliferations, which are usually nonclonal but often fatal, to (2) bone marrow and peripheral cytopenias, associated, or not, with hemophagocytic syndromes (HPSs), and to (3) acute hepatitis syndromes. These complications may offer some diagnostic challenges, both on molecular and immunologic grounds, and demand specific and “not trivial” therapeutic interventions. Thus, HHV8-related clinical issues should be kept in mind by the hematologists who are often called to guide difficult diagnostic processes and, then, suggest optimal therapeutic approaches for atypical viro-pathologic manifestations, not only in HSCT patients, but, even more frequently, as consultant specialists, in SOT recipients.
In this article, we review HHV8-driven malignancies and non-neoplastic manifestations, which may occur in immunosuppressed transplant patients, as rare but severe diseases, either after HHV8 primary infections or in association with viral reactivations, particularly focusing on clinical presentations, diagnostic and monitoring approaches, and available treatments for these often neglected posttransplantation complications.
Biologic aspects
Similarly to EBV,4 HHV8 is able to infect B cells and other cell types and persist lifelong in a latent form, although it sometimes reactivates its lytic replicative cycle to produce viremia, mainly when specific immune control is weak.5,6 In posttransplant patients, iatrogenic immunosuppression may frequently cause harmful HHV8 lytic reactivations and uncontrolled monoclonal/oligoclonal expansions of latently infected lymphoendothelial cells (the so-called KS spindle cells) or mature postgerminal center B cells.5-7 Either neoplastic or non-neoplastic HHV8-related posttransplant diseases can also be the result of primary infections in patients showing HHV8-seroconversion after receiving allografts from HHV8-seropositive donors.8 Notably, HHV8+ cells may be harbored in the transplanted organ and transmit the virus to the recipient,9,10 or, rather, KS tumors themselves may directly originate from donor-derived HHV8+ cells, seeded with the graft and then proliferating in the immunosuppressed recipient.11
Epidemiology and clinical presentations
Contrary to EBV, HHV8 infection has been revealed not to be ubiquitous in the worldwide population. Indeed, the geographic diffusion of HHV8 prevalence rather shows some typical endemic regions, with seroprevalence higher than 50% in some ethnic groups, particularly in sub-Saharan Africa as well as Latin America, Caribbean, Mediterranean, and Middle Eastern countries, where the incidences of either “endemic” or “classic” clinical forms of KS well mirror this particular geo-distribution of HHV8 infection.12 Thus, it is also conceivable that the risk to develop posttransplantation HHV8-related pathologies is primarily dependent on the HHV8 prevalence in the geographic area or ethnic origin of donor and recipient populations.13
Posttransplantation KS, PEL, and MCD
After some decades since the first report of posttransplantation KS (PT-KS) in a kidney recipient in 1969,14 and closely following the HHV8 discovery in 1994,15 early epidemiologic surveys by different transplantation centers reported variable PT-KS morbidity, ranging from 0.6% to 5.3%, and consistently varying in relation to HHV8 prevalence and to the allograft type.12,13,16-30 In particular, PT-KS was primarily observed in kidney recipients (Figure 1A), whereas lower PT-KS incidences have been recorded in patients receiving other SOTs, mainly liver and heart. PT-KS usually occurs rather early after transplantation (median interval, 30 months), usually as cutaneous or mucosal lesions, whereas visceral involvement occurs in 25%-50% of the cases. Some remarkable hematologic manifestations, mainly thrombocytopenia, anemia, and bone marrow progenitor abnormalities, are frequently associated with aggressive forms of PT-KS, which are characterized by a disseminated disease involving multiple visceral, mucosal, and cutaneous sites. PT-KS–associated mortality has been reported to range from 8% to 14%. Clinical staging of PT-KS has been proposed to assess aggressiveness of the disease and guide clinical management.31 CMV infection has been reported to reactivate HHV-8 and contribute to KS onset and/or recurrence.32,33 Recently, updating previous serologic analyses in 2 different series of HHV8-seropositive kidney transplant recipients, which reported similarly high PT-KS rates (23% and 28%, respectively),24,25 a large prospective study by Francès et al34 identified 24 cases of PT-KS (15%) among 161 HHV8-seropositive renal graft recipients, and described 2 cases of PT-KS (3.1%) among 64 seronegative patients who received HHV8+ kidney allografts. Similarly, preliminary data from our ongoing regional-multicentric prospective study actually show a marked trend toward a decreased incidence of PT-KS cases (15%) compared with the higher PT-KS incidence (37.5%) we observed in a retrospective series of HHV8-seropositive recipients, enrolled between 2000 and 2003 (M.L., unpublished data, 2008). In the first instance, this lower PT-KS incidence recorded by recent kidney transplant surveys probably results from a more appropriate clinical management, aiming to minimize immunosuppression levels during posttransplantation follow-up, and often including anti-HSV agents and sirolimus, which has recently emerged as an immunosuppressive drug with specific anti-KS activities.34
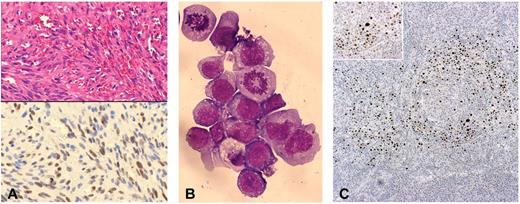
Histologic and immunocytochemical features of posttransplantation HHV8-related neoplastic diseases. (A) Top panel: KS. Hematoxylin and eosin–stained section of a skin lesion characterized by typical “spindle-like” neoplastic cells and surrounding “slit-like” vascular spaces (original magnification ×200). Bottom panel: On contiguous section from the same skin biopsy, LANA-1 immunostaining demonstrated HHV8 latent infection in the neoplastic spindle-like cells, within the KS lesion (original magnification ×200). (B) PEL: cytospin preparation from pleural effusion, showing a massive aggregate of neoplastic lymphoid cells with 2 evident mitotic figures. May-Grünwald-Giemsa stain typically revealed immunoblastic cells with dark basophilic cytoplasm and anaplastic cells with prominent scant light staining basophilic cytoplasm (original magnification ×600). (C) MCD: LANA-1 immunostaining in lymph node biopsy showing typical MCD-associated pattern, with positive plasmablastic B cells surrounding the follicular center (original magnifications ×100 and ×200).
Histologic and immunocytochemical features of posttransplantation HHV8-related neoplastic diseases. (A) Top panel: KS. Hematoxylin and eosin–stained section of a skin lesion characterized by typical “spindle-like” neoplastic cells and surrounding “slit-like” vascular spaces (original magnification ×200). Bottom panel: On contiguous section from the same skin biopsy, LANA-1 immunostaining demonstrated HHV8 latent infection in the neoplastic spindle-like cells, within the KS lesion (original magnification ×200). (B) PEL: cytospin preparation from pleural effusion, showing a massive aggregate of neoplastic lymphoid cells with 2 evident mitotic figures. May-Grünwald-Giemsa stain typically revealed immunoblastic cells with dark basophilic cytoplasm and anaplastic cells with prominent scant light staining basophilic cytoplasm (original magnification ×600). (C) MCD: LANA-1 immunostaining in lymph node biopsy showing typical MCD-associated pattern, with positive plasmablastic B cells surrounding the follicular center (original magnifications ×100 and ×200).
PT-KS occurrence clearly appears to be an uncommon event in HSCT patients, as also recently confirmed by the reporting of no PT-KS cases in an Italian cohort of 187 allogeneic bone marrow recipients.35 Whether myeloablative conditioning chemotherapy and total body irradiation may impair HHV8+ cell reservoirs, or, rather, the state of alloreactivity, as well as the abnormal inflammatory conditions associated with immune reconstitution, may exert a protective effect against the emergence of PT-KS, either directly or through cytokine-mediated mechanisms, still remains to be investigated. However, to date, 10 cases of PT-KS, after either allogeneic or autologous HSCT, have been described in the literature.32,36-44 Notably, PT-KS disease resulted lethal in 2 of the 8 allogeneic HSCT cases and in 1 of the 2 autologous HSCT patients, both also showing bone marrow hypoplasia. Skin-only localized disease was the usual clinical presentation in adult HSCT patients, whereas all 3 pediatric PT-KS cases showed visceral involvements and resulted in 2 fatalities (after either allogeneic or autologous HSCT).
Both HHV8-associated lymphoproliferative diseases (LPDs), more often occurring in AIDS patients as well as in elderly HIV-negative persons, can also affect the clinical course of SOT (although apparently not the course of HSCT) patients (Figure 1B-C), frequently with high HHV8 viral loads, readily detectable during active disease. Since the first report of a fatal posttransplantation PEL (PT-PEL) in 1998,45 these HHV8+ postgerminal center plasmablastic lymphomas have revealed to be as rare as life-threatening complications in SOT setting. To our knowledge, 12 cases of PT-PEL and 11 cases of PT-MCD have so far been observed. PT-PEL occurrence was reported in 7 renal and in 3 cardiac transplant recipients, in concomitance with PT-KS in 2 cases, and leading to patient's exitus in most cases, particularly after heart transplantation.45-52 Furthermore, we have recently observed 2 patients who rapidly died with pleural and peritoneal effusions early after liver transplant, and the autopsy revealed a PEL diagnosis in both cases (M.L., unpublished data, December 2005; M.L. and T. Lazzarotto, unpublished data, June 2011). Withdrawal of immunosuppressive therapy has been reported to be associated with a significant clinical response in 1 case of PT-PEL.46 PT-MCD was diagnosed in 6 and 4 patients after either kidney or liver transplantation, respectively, showing a more favorable outcome compared with PT-PEL cases.53-62 Of note, 1 liver recipient affected with systemic PT-MCD, associated with constitutional symptoms and high HHV8 viremia, was successfully treated with valgancyclovir and weaning of immunosuppressive therapy (cyclosporine withdrawal), without worsening of the graft functions.62 Moreover, we have recently used rituximab to treat a case of PT-MCD occurring in a renal transplant patient, 10 years after transplantation, achieving a prolonged disease remission, with no adverse effects (M.L., P.B. and U. Maggiore, unpublished data, February 2012).
Non-neoplastic complications
In the posttransplantation setting, in addition to the frank neoplastic diseases discussed in “Posttransplantation KS, PEL, and MCD,” HHV8 may also be the causative agent of unusual, severe non-neoplastic clinical manifestations, typically associated with high HHV8 viremia levels, and, so far, frequently associated with dismal outcome. Fever, skin maculopapular rash and other various systemic infectious-like signs and symptoms, in concomitance with either (1) polyclonal HHV8-positive plasmacytic/plasmacytoid lymphoproliferations, involving lymph nodes and visceral organs; (2) acute bone marrow failure, often with plasmacytosis and signs of HPS, hepatosplenomegaly, and severe pancytopenia; or (3) remarkable elevation of liver enzymes, are the recurrent clinical features of posttransplant HHV8-driven viremic syndromes.
Soon after the discovery of the etiologic link between HHV8 and neoplastic proliferations, Chang and Moore's group also reported the first 2 cases of posttransplant HHV8-positive nonmalignant lymphoproliferative disorders, characterized by marked plasmacytic and plasmacytoid features, mimicking polymorphic-type EBV+ PTLDs.63 One patient showed recurrent pleural effusions and MCD-like changes in lymph nodes, whereas the other presented with systemic lymphadenopathy and hepatosplenomegaly resulting from diffuse infiltration by polyclonal plasma cells and plasmocytoid B cells. Furthermore, another kidney recipient developed HHV8-positive EBV-negative polymorphic-type PTLD, in concomitance with PT-KS and hemolytic anemia.64 More recently, Marcelin et al33 reported a series of 4 donor-derived HHV8 primary infections in liver transplant recipients: whereas 2 patients remained asymptomatic, the other 2 early developed fever, skin rash, polyadenopathy, anemia, and HHV8+/EBV− CD20+ large B-cell proliferation, with high plasmacytoid differentiation, which diffusely infiltrated pulmonary, splenic, nodal, and gastric tissues. Both patients died with multiorgan failure soon after and the autopsy also revealed disseminated HHV8/ latent nuclear antigen (LANA)–positive KS-like spindle cells in both cases.33
In addition, early in the past decade, we described the first 2 cases of a lethal HHV8-induced nonmalignant illness, which was characterized by fever, splenomegaly, cytopenia, and bone marrow failure with interstitial plasmacytosis, associated with high HHV8 viral loads (100 000 viral copies/mL), the former occurring in a renal transplant patient during donor-derived HHV8 acute primary infection and the latter emerging after autologous HSCT resulting from chronic HHV8 reactivation.65 Similarly to the latter case description, another report has described the fatal occurrence of fever, cutaneous rash, and bone marrow suppression, associated with elevated HHV8 viral levels, in an autologous HSCT patient.66 Furthermore, we have described a renal transplant patient who developed severe peripheral pancytopenia in the presence of normo/hypercellular bone marrow with hemophagocytosis and moderate plasmacytosis, after HHV8 primary infection.67 In this study, the HHV8 latent nuclear antigen was also demonstrated in myeloid progenitor cells from the bone marrow, and we and others further demonstrated that HHV8 may exert a direct myelosuppressive effect in vitro.68,69 With regards to HHV8-induced HPS, 2 further cases were identified in kidney transplant patients.70,71 It is possible to hypothesize that these syndromes are also putatively associated with an underlying condition of polyclonal HHV8-driven B-cell expansions, possibly evidenced by the recurrent findings of plasmacytosis and HHV8 positivity in aplastic marrow samples. In line with this, Thaunat et al first described a severe HHV8 primary infection (transmitted through a kidney allograft) successfully treated with rituximab, which induced a rapid reduction of circulating B cells, correlating with a dramatic clinical improvement.72 In this clinical report, the patient presented with fever, mononucleosis-like symptoms and signs (as pharyngeal erythema, hepatosplenomegaly, weight loss, night sweats), pancytopenia, normal bone marrow with moderate plasmacytosis without hemophagocytosis, and high HHV8 viral loads detected in PBMCs, bone marrow, and pharyngeal specimens.72
To date, acute hepatitis syndromes, characterized by fever, cutaneous rash, and elevated liver enzymes, have also been reported in several posttransplant patients, either during HHV8 reactivation after an autologous HSCT73 or in concomitance with donor-derived primary HHV8 infections, after either liver SOT61 or allogeneic HSCT (M.L., unpublished data, November 2008). The disease has revealed to be self-limiting in both the HSCT patients we observed. At opposite, in the 2 postliver transplant cases reported by Pietrosi et al, clinical presentations were more aggressive and challenging, being also associated with pleural and peritoneal effusions, and lethally evolving into multiorgan failures, despite cidofovir antiviral therapy.61
Diagnosis and clinical investigations
In general, the diagnosis of HHV8-related diseases relies on the identification of clinical and histopathologic features, along with consistent HHV8 detection in patient's pathologic tissues (Table 1).5-7 A severe posttransplantation HHV8-related illness should be clinically suspected whenever occurring typical cutaneous manifestations (either classic KS-like nodules or maculopapular rashes), pleural or peritoneal effusions, localized or systemic lymphadenopathy, variably associated with peripheral cytopenias, or acute herpesvirus infection-like symptoms and signs (eg, fever, pharyngeal erythema, respiratory distress, hepatosplenomegaly, elevated liver enzymes, and lymph node enlargements), which may often resemble the presentation of EBV+ PTLDs. Although HHV8-related neoplasias (either PT-KS or PT-LPDs) should always be proven with biopsy confirmation of typical histologic features along with HHV8 immunohistochemical demonstration (Figure 1),5-7 the diagnosis of posttransplantation HHV8-related nonmalignant diseases is more challenging, as it is mainly based on the detection of high levels of HHV8 viremia in association with clinical pathologic manifestations, in absence of other causal pathogens, and possibly also sustained by the detection of HHV8 in the affected organs (eg, bone marrow, lymph node, or liver biopsies).
Serologic and molecular assays
At the time of transplantation, it appears clinically relevant to define the HHV8 serostatus in paired organ donors and recipients (at least for SOT centers operating within HHV8 endemic areas), to early stratify patients on the basis of potential HHV8-related risks, which typically result from either viral reactivations in pretransplantation HHV8-infected recipients or to donor-derived HHV8 primary infections (R+ and R−/D+ groups, respectively).74,75 This basic information could indeed allow to appropriately perform a specific virologic and immunologic monitoring for early diagnosis of HHV8-driven complications. However, because of the extreme rarity of PT-KS and other HHV8-related diseases in HSCT patients, any recommendation for a baseline HHV8 screening in such transplant setting does not appear to be justifiable in terms of cost-effectiveness. To routinely perform pretransplantation HHV8 screening in the SOT setting, serologic assays for the identification of anti-HHV8 antibodies, either directed to latent LANA antigen or to lytic K8.1 glycoprotein, have appeared to be more accurate than quantitative HHV8 PCR analyses on either serum or PBMC samples.34,75,76 Considering the variable sensitivity and specificity of HHV8-specific serologic tests reported in the literature, at our center we choose to perform baseline HHV8 screening with both LANA immunofluorescent assay (latent IFA) and K8.1 enzyme-linked immunosorbent assay (lytic ELISA), and, to date, these assays have shown a high degree of concordance (> 90%). However, the disappointing lack of a gold standard in the serologic assays for anti-HHV8 antibodies still represents a major obstacle to routine implementation of screening protocols on organ donors/recipients.74-76
In addition to serology, the donor screening was performed by the search for HHV-8 DNA in the transplanted kidney grafts from 3 of 4 transplant recipients,9,10 although HHV-8 DNA sequences resulted invariably undetectable in liver grafts from 4 HHV8-seropositive donors.33 These few available and controversial data do not recommend routine PCR testing for the presence of HHV8 DNA in grafts. The quantitation of HHV8 DNA levels by PCR-based methods in HHV-8 seropositive persons appears to be useful to predict the occurrence of HHV-8 related non-neoplastic diseases, especially those after HHV-8 primary infections, which are associated with high viral loads. Conversely, PCR monitoring for HHV8 viremia in HHV8 seropositive renal recipients to predict PT-KS has been reported to show a sensitivity of only 23.8%.34 Moreover, PCR for HHV-8 load in PT-KS patients was found to be associated with disease progression, time from diagnosis, and disease staging in 17 of 43 renal recipients with PT-KS (40%),77 suggesting that PCR monitoring may be useful for the follow-up of only a proportion of transplant patients with PT-KS.75 Based on the available data from the literature and our own experience, it is clear that possibly more than a half of PT-KS patients are negative for detectable HHV8 viremia, raising the need for an additional marker of disease activity, which might be offered by HHV8-specific immunologic monitoring (see “Immunologic monitoring”). In addition, HHV8 quantitative PCR assays may also have a role in supporting diagnosis and management of non-neoplastic syndromes and PT-LPDs (particularly MCD) because elevated HHV8 viremia can usually be detected at disease onset and thereafter monitored as a disease-associated marker.78
Immunologic monitoring
With regards to PT-KS, it has well been documented that the disease clinical course tightly depends on the levels of immunosuppression. Tumor lesions may impressively regress after discontinuation or reduction of immunosuppressive treatments, whereas PT-KS disease may recur in concomitance with the restart of immunosuppression.5-7,75,79 Recently, the switch of the immunosuppressive regimen from calcineurin inhibitors (CNIs) to sirolimus (rapamycin), a mTOR inhibitor showing broad antineoplastic activities in vitro, has also been reported to induce frequent regression of PT-KS lesions.80,81 We and others have demonstrated that anti-HHV8 cytotoxic T-cell responses, as assessed by HHV8-specific immunoassays, were almost completely absent in posttransplantation recipients at the time of PT-KS diagnosis, whereas at the time of PT-KS remission, after the decrease of immunosuppressive therapy, they resurfaced at levels similar to those shown by HHV8-positive recipients who have never developed PT-KS (Figure 2).82,83 Similarly, HHV8-specific T-cell dynamics were also described in a iatrogenically immunocompromised autoimmune patient who developed remitting/recurring KS lesions.84 Moreover, clinical remissions, achieved by switching from CNIs to sirolimus in 2 PT-KS liver recipients, were also associated with recovery and maintenance of IFNγ-producing T-cell responses, both CD8+ and CD4+, either effector or central memory, against both lytic (K8.1) and latent (ORF73/LANA) HHV8 antigens.83 Conversely, a renal transplant patient who failed to achieve complete PT-KS regression after switch to sirolimus showed a lack of recovery of HHV8-specific memory T cells.83 In Figure 3, we describe clinical and immunologic data of 2 exemplificative unreported cases of posttransplant patients, who achieved PT-KS remission after switching from CNIs to sirolimus-based immunosuppressive therapy. In patients with PT-LPDs and HHV8-related nonmalignant diseases, frequencies and functional dynamics of HHV8-specific T cells are still lacking. It is generally accepted that the HHV8-specific immune control is not dramatically suppressed in both MCD and PEL occurring in HIV-positive or elderly patients,85,86 compared with AIDS-KS or iatrogenic KS onset.82-84,87 However, in our clinical practice, we have recently observed 1 renal transplant patient with MCD and 1 liver recipient with PEL, who have both shown almost undetectable HHV8-specific T-cell responses at the time of disease diagnosis. The MCD patient has also shown a recovery of a remarkable frequency of HHV8-specific IFNγ-producing T cells, later on, during a durable remission obtained after reduction of immune suppression, followed by rituximab (Figure 2; M.L., unpublished data, December 2005; M.L., P.B., and U. Maggiore, unpublished data, February 2012).
HHV8-specific T-cell frequencies in posttransplant patients. Specific IFNγ-enzyme-linked immunosorbent spot assay (IFNγ-ELISPOT) monitoring, either for lytic (orfK8.1; ▴) or for latent (orf73/LANA; ○) HHV8-derived antigens, was applied to assess HHV8-specific T-cell frequencies in our series of 15 PT-KS patients, either at PT-KS diagnosis or after remission, and in 2 PT-LPD patients (PEL or MCD; gray symbols), as well as in 5 HHV8-seropostive posttransplantation asymptomatic carriers (PT-AC). The Mann-Whitney nonparametric test was used to compare groups.
HHV8-specific T-cell frequencies in posttransplant patients. Specific IFNγ-enzyme-linked immunosorbent spot assay (IFNγ-ELISPOT) monitoring, either for lytic (orfK8.1; ▴) or for latent (orf73/LANA; ○) HHV8-derived antigens, was applied to assess HHV8-specific T-cell frequencies in our series of 15 PT-KS patients, either at PT-KS diagnosis or after remission, and in 2 PT-LPD patients (PEL or MCD; gray symbols), as well as in 5 HHV8-seropostive posttransplantation asymptomatic carriers (PT-AC). The Mann-Whitney nonparametric test was used to compare groups.
HHV8-specific T-cell profiling in 2 PT-KS patients who achieved remission after switch to sirolimus. IFNγ-enzyme–linked immunosorbent spot assay (IFNγ-ELISPOT; top panels) and cytokine secretion assay (CSA; bottom panels) were used to perform HHV8-specific immunologic monitoring in 2 renal recipients (patient A, left panels; patient B, right panels), who developed skin PT-KS after 9 and 18 months from transplantation, respectively. Both lytic (orfK8.1) and latent (orf73/LANA) HHV8-derived antigens were used as specific T-cell stimulations in both assays. After switching from tacrolimus- to sirolimus-based immunosuppressive regimens, both patients rapidly achieved complete PT-KS regressions and concurrently showed the recovery of HHV8-specific cytotoxic T cells. Such responses, which were absent at PT-KS diagnosis, were readily detectable during all post-remission follow-up, as demonstrated by IFNγ-ELISPOT long-term immunologic monitoring. Furthermore, in both cases, we applied CSA assays to perform a phenotypic and functional characterization of HHV8-specific T cells, either at the time of PT-KS onset (or before, in patient A) or after remission. Here we have also shown that frequencies of different HHV8-specific T-cell subsets, as defined by surface markers (CD4+ and CD8+) and cytokine productions (IFNγ, IL-17, IL-4, IL-10, and IL-2), may also well correlate with the disease course, possibly revealing a “nonprotective” Th2 prevalence at time of PT-KS diagnosis and a “protective” Th1 prevalence after remission. HHV8-specific T-cell subsets are expressed as percentages of total CD4+ or total CD8+ T cells. Nonspecific memory T-cell profiling data are also reported over the related CSA assays. PDN indicates prednisone; SRL, sirolimus; IFNγ-SFCs, interferon-γ–producing spot-forming cells; PHA, phytohemagglutinin; CEF, CMV, EBV, influenza virus-derived antigens; CM, central memory; EM, effector memory; and EMRA, CD45RA+ effector memory.
HHV8-specific T-cell profiling in 2 PT-KS patients who achieved remission after switch to sirolimus. IFNγ-enzyme–linked immunosorbent spot assay (IFNγ-ELISPOT; top panels) and cytokine secretion assay (CSA; bottom panels) were used to perform HHV8-specific immunologic monitoring in 2 renal recipients (patient A, left panels; patient B, right panels), who developed skin PT-KS after 9 and 18 months from transplantation, respectively. Both lytic (orfK8.1) and latent (orf73/LANA) HHV8-derived antigens were used as specific T-cell stimulations in both assays. After switching from tacrolimus- to sirolimus-based immunosuppressive regimens, both patients rapidly achieved complete PT-KS regressions and concurrently showed the recovery of HHV8-specific cytotoxic T cells. Such responses, which were absent at PT-KS diagnosis, were readily detectable during all post-remission follow-up, as demonstrated by IFNγ-ELISPOT long-term immunologic monitoring. Furthermore, in both cases, we applied CSA assays to perform a phenotypic and functional characterization of HHV8-specific T cells, either at the time of PT-KS onset (or before, in patient A) or after remission. Here we have also shown that frequencies of different HHV8-specific T-cell subsets, as defined by surface markers (CD4+ and CD8+) and cytokine productions (IFNγ, IL-17, IL-4, IL-10, and IL-2), may also well correlate with the disease course, possibly revealing a “nonprotective” Th2 prevalence at time of PT-KS diagnosis and a “protective” Th1 prevalence after remission. HHV8-specific T-cell subsets are expressed as percentages of total CD4+ or total CD8+ T cells. Nonspecific memory T-cell profiling data are also reported over the related CSA assays. PDN indicates prednisone; SRL, sirolimus; IFNγ-SFCs, interferon-γ–producing spot-forming cells; PHA, phytohemagglutinin; CEF, CMV, EBV, influenza virus-derived antigens; CM, central memory; EM, effector memory; and EMRA, CD45RA+ effector memory.
In addition, testing of virus-specific T cells has been suggested to enhance the predictive value of PCR detection of EBV viral loads in transplant patients at risk for PTLDs.1,88,89 In the context of HHV8-associated posttransplantation diseases, further investigations on prospective cohorts are required to support any firm recommendations for the use of immunologic monitoring. However, we suggest that clinicians start exploiting a HHV8-specific T-cell monitoring in their daily practice (where already available) as a specific tool to complement HHV8 DNA quantifications, at least in the PT-KS setting (Table 1). Indeed, HHV8-specific IFNγ-ELISPOT may be readily useful to identify patients with an actual risk to develop PT-KS, either during asymptomatic follow-up or during postremission phase. Basically, in the clinical care decision making for HHV8+ posttransplant patients (Figure 4), the absence of HHV8-specific T-cell responses should suggest to attempt a preemptive reduction of immunosuppressive therapy, to possibly allow the recovery of the protective immunity and prevent PT-KS outgrowth or relapse.
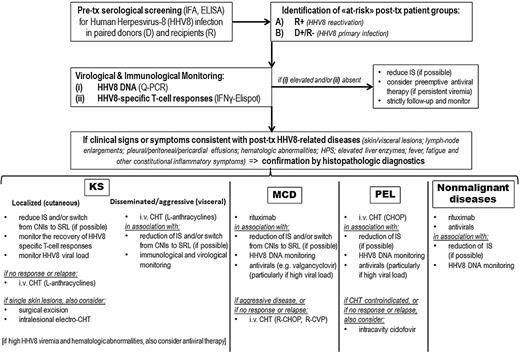
Monitoring and treatment algorithm for clinical management of HHV8-related complications in SOT patients. Q-PCR indicates quantitative polymerase chain reaction; IFNγ-ELISPOT, interferon-γ enzyme-linked immunospot; IS, immunosuppression; SRL, sirolimus; CHT, chemotherapy; and L, liposomal.
Monitoring and treatment algorithm for clinical management of HHV8-related complications in SOT patients. Q-PCR indicates quantitative polymerase chain reaction; IFNγ-ELISPOT, interferon-γ enzyme-linked immunospot; IS, immunosuppression; SRL, sirolimus; CHT, chemotherapy; and L, liposomal.
Treatment options
As in many rare and relatively newly recognized diseases, standard therapeutic approaches in HHV8-related complications have yet to be acquired, and the therapeutic evidence mainly relies on small case series, sporadic clinical reports, and personal experience (level of evidence: III, according to the US Preventive Services Task Force system; Table 1). In clinical management of SOT patients, our personal algorithm for diagnosis, monitoring, and treatment of HHV8-associated diseases is outlined in Figure 4. In our opinion, either cautious decrease or complete withdrawal of immunosuppressive therapy should ideally represent the first timely provision in the clinical management of all HHV8-related neoplastic and non-neoplastic diseases (III). Indeed, even partial restore of a normal immunity, and, putatively, of protective HHV8-specific T cells, may turn to be clinically relevant in all these contexts. However, the risk of allograft rejection often hinders the clinical feasibility of such strategy. Anti-HSV agents that have been demonstrated effective against HHV8 (eg, cidofovir, foscarnet, ganciclovir, or valgancyclovir)90,91 may offer a valuable treatment to reduce HHV8 viremia and possibly contribute to control diseases presenting with high levels of HHV8 replication (typically PT-LPDs)62,92 (III). In addition, prophylactic or preemptive treatments with specific antivirals could be useful to lower the incidence of posttransplantation HHV8-related complications. Prophylactic courses with ganciclovir have been suggested to decrease the number of primary clinical HHV8 infections in seronegative patients receiving HHV8+ allografts (II-2),34 whereas in patients with asymptomatic but persistently high HHV8 viremia, the preemptive role of antiviral therapy should be further investigated (III). However, cytotoxic chemotherapy, as well as surgical excision and radiation therapy, are still the most common treatments for PT-KS and other HHV8-related malignancies (II-3).7,75,79 More recently, therapeutic potentials of anti-CD20 monoclonal antibody (rituximab) have started to be revealed not only for MCD3 but also for non-neoplastic pathologies associated with HHV8-driven plasmacytic proliferations (III).72
PT-KS
Modifications of the immunosuppressive therapy have largely appeared to be useful in the therapeutic management of PT-KS patients because long-term tumor remissions can commonly be achieved by the sole reduction (or discontinuation) of the immunosuppression (II-3).7,75,79 In addition, the switch of immunosuppressive regimen from CNIs to mTOR inhibitors (mainly sirolimus) has been reported to induce dramatic KS regressions,80,81 together with the recovery of HHV8-specific T cells (II-3).83 However, switching to sirolimus may be ineffective or transiently effective in patients with severe PT-KS, particularly if not timely and abruptly performed, as suggested by the report of Lebbè et al in a series of 14 patients, showing 5 refractory cases and 3 disease progressions after partial response.93
According to our clinical practice, we tend to use a stratified approach, using single-agent liposomal anthracycline infusions (20 mg/m2),94 in association with modifications of immunosuppression, as front-line therapy for disseminated aggressive PT-KS. In localized skin KS, the switch from CNI- to sirolimus-based immunosuppressive regimens is the first option, while reserving chemotherapy as salvage treatment for refractory or relapsing diseases, despite the reduction of the immunosuppressive levels. In addition, we also consider surgical excision, and, to a lesser extent, electro-chemotherapy,95,96 for limited cutaneous PT-KS lesions, refractory to the changes of immunosuppressive regimen. Radiotherapy has largely been abandoned at our center because of persistently associated local side effects (eg, edema, ulcerative lesions; III).
Anti-HSV agents are considered probably useless for treating PT-KS, also because neoplastic KS spindle cells typically harbor a latent HHV8 infection.5-7 Notwithstanding this acceptable notion, in patients presenting with aggressive disseminated PT-KS, associated with either high HHV8 viremia or hematologic abnormalities and systemic inflammatory symptoms, we usually suggest to promptly start an antiviral course (possibly with cidofovir or foscarnet), at least with the aim to resolve HPS or refractory thrombocytopenia (III). However, it remains unclear whether and to which extent HHV8 replication may contribute to the spreading of KS disease,5,6 or, rather, if the increased HHV8 viremia during PT-KS may underlie an ongoing subclinical MCD/PTLD-like process, as already suggested in the AIDS-KS setting.3,78
PT-MCD and PT-PEL
Clinical efficacy of immunosuppression modulation has scarce evidence in the therapeutic management of PT-LPDs (III).60,62 However, a pediatric patient with PT-MCD has shown tumor remission after switching (and tapering) immunosuppressive therapy from tacrolimus to sirolimus.60 Although sirolimus has also been demonstrated to be effective against PEL cell lines, PT-PEL patients may not benefit from sirolimus therapy, as so far suggested by the 2 cases of PT-PEL occurring under sirolimus.48 However, based on the scarce available data, a switch to sirolimus should not be discouraged in PT-PEL.
Cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP)–based chemotherapy still remains a pivotal therapeutic option for the treatment of PT-LPDs, particularly for PT-PEL patients, who appear to be most refractory to modifications of immunosuppressive therapy and intravenous antivirals, as well as typically not eligible for anti-CD20 treatment (II-3). The cyclophosphamide, vincristine, prednisone (CVP) regimen is also suggested for PT-MCD treatment, as some experts treat MCD more akin to a low-grade indolent lymphoma (II-3).
Concerning HIV-positive patients affected with MCD, an emerging consensus for the use of anti-CD20 monoclonal antibody (rituximab), alone or in combination with chemotherapy, has recently been achieved, based on the impressive remission rates in early clinical trials.3 In keeping with this, we treated a case of MCD in a renal transplant patient with rituximab monotherapy (375 mg/m2 infusions for 4 weeks), and we observed a durable complete remission, without significant adverse effects (M.L., P.B. and U. Maggiore, unpublished data, February 2012). We suggest that rituximab should be included in the treatment of PT-MCD, either alone or in combination with chemotherapy (R-CHOP; II-3). A note of caution is, however, mandatory, as it is still unknown whether the use of rituximab to treat HHV8-driven lymphoproliferations in the posttransplant setting may either cause PT-KS exacerbations or increase the risk to develop PT-KS, as it has been described in many MCD HIV-positive patients, experiencing KS flares after rituximab therapy.3
In addition to rituximab, a novel monoclonal antibody targeting IL-6, namely, tocilizumab, has been labeled for the treatment of refractory rheumatoid arthritis. As high levels of IL-6 and viral IL-6 are detectable in association with active HHV8 replication, tocilizumab seems to be an attractive option for the management of either PT-LPDs or nonmalignant HHV8-related syndromes, as, indeed, already successfully described in nontransplantation MCD patients, at least to control inflammatory symptoms (III).97
In clinical practice, anti-HSV agents may be rationally used, in association with chemotherapy, to treat posttransplant patients affected with either MCD or PEL, as both are typically linked to high HHV8 viral loads (III). Whether, when, and for how long a specific antiviral drug should be added to the treatment of PT-LPDs still remains to be investigated. Of note, valgancyclovir (450 mg twice a day), together with an immunosuppression weaning, has recently been reported to be an effective and safe treatment for a PT-MCD case with high HHV8 viremia.62 Furthermore, we described the efficacy of intracavity infusions of cidofovir to control PEL effusions in several HIV-negative elderly patients, and thus we believe that such therapeutic strategy could worthily be tested also in posttransplantation settings (III).92 In addition, our preclinical experiments have recently demonstrated that liposomal formulations of cidofovir98,99 might be able to further increase its remarkable proapoptotic effect against PEL (G.R., M.L., B. Ruozi, D. Belletti, and M. A. Vandelli, unpublished data, March 2012).
Symptomatic infections and other non-neoplastic diseases
So far, the outcome of these HHV8-associated viremic syndromes has remained very poor, despite the extensive use of different anti-HSV drugs, often in association with reduction of immunosuppressive therapy. Indeed, antiviral treatments alone (ie, cidofovir and ganciclovir) failed to control either the severe primary HHV8 infections in liver transplant patients,33,61 or the aplastic syndrome after HHV8 reactivation in a renal recipient.65 However, in our experience, foscarnet therapy alone for 2 weeks was associated with the complete resolution of a primary HHV8 infection syndrome, characterized by fever, severe pancytopenia and HPS, occurring after renal transplantation.67 Larger case series are required to better define the putative role of anti-HSV agents in the therapeutic management of HHV8-related non-neoplastic complications. Thus, to date, antivirals should not be excluded from rational therapeutic options, in particular when high levels of HHV8 viremia have been detected (III).
In association with antivirals, we now propose to use rituximab as first-line treatment for severe clinical manifestations related to posttransplantation HHV8 primary infections (as directly supported by the report of Thaunat et al72 in a renal recipient), as well as for acute and chronic inflammatory syndromes related to HHV8 reactivations (III). The empirical use of rituximab in symptomatic HHV8 infections, in line with the successful treatment of primary EBV infections in patients with X-linked lymphoproliferative disease,100 is rationally based on the eradication of proliferating HHV8-infected mature B cells (CD20+), aiming to decrease the viral burden and the reactive inflammatory conditions in the patient. A delayed introduction of rituximab in this clinical context may result in a fatal outcome, which cannot be prevented by the sole use of antivirals.61,65
Future perspectives
In our opinion, immunologic data already available on PT-KS disease may well suggest the opportunity to harness such a net inverse correlation between PT-KS outgrowth and protective HHV8-specific T cells for clinical purposes. On one hand, during the follow-up of posttransplantation HHV8+ patients, we should start to take advantage of available HHV8-specific T-cell profiling tools, whenever possible, and then to try to prospectively validate immunomonitoring protocols oriented to optimize immunosuppressive therapy, to prevent KS proliferations. The final goal would eventually be to define specific immunologic tests, able to affordably assess KS risk and provide early prediction of tumor emergence or relapse. On the other hand, by considering the recently updated efficacy and safety of EBV-specific cytotoxic T-lymphocytes against PTLDs and EBV+ tumors,1,101 HHV8-specific autologous cytotoxic T-lymphocyte infusion in PT-KS patients could also improve the control of the disease, without affecting graft tolerance. However, this therapeutic achievement appears to be primarily dependent on the possible identification of optimal HHV8-derived antigens, able to induce efficient expansion of specific cytotoxic T-cell populations on long-term in vitro cultures. With regard to other posttransplant HHV8-related neoplastic and non-neoplastic diseases, we think that the role of HHV8-specific T-cell immunity could be worth investigating, aiming to disclose new relevant pathogenic profiles. Further clinical observations are required to update and refine the actual body of knowledge in such rare diseases.
In conclusion, in this article, we have described our behaviors in the clinical management of HHV8-associated posttransplantation diseases, inevitably based on the few evidences reported in the literature and on our own personal experience, in the past 10 years. As a final personal view, we guess that a proportion of both HHV8-related posttransplantation lymphomas and nonmalignant diseases could actually be missed, as the diagnosis may often be difficult, or anecdotal cases may remain undescribed. Thus, we encourage clinicians to actively and timely search for HHV8 when herpesvirus-associated posttransplantation complications are suspected, as well as not to miss reporting their experiences in this setting. The management of these clinically relevant complications represents a critical multidisciplinary issue in the modern posttransplantation medicine, and hematologists should have an active apart in the diagnostic and therapeutic decision process, whenever HHV8-related signs and symptoms do occur in iatrogenically immunocompromised patients.
Acknowledgments
The authors thank Gruppo Italiano Trapianto di Midollo Osseo (GITMO), the Nord Italian Transplant program (NITp), and Centro Riferimento Trapianti dell'Emilia-Romagna (CRT-ER) for active collaboration; Dr Denise Whitby for providing recombinant HHV8 proteins (LANA and K8.1); and Prof Tullio Artusi, Dr Goretta Bonacorsi, and Giulio Rossi for the histology of HHV8/KSHV positive lesions.
This paper is dedicated to retired Prof Giuseppe Torelli, our mentor.
This study was supported by Associazione Italiana per la Ricerca sul Cancro, Milan, Italy (IG 10811, M.L.), Programma di ricerca Regione-Università Emilia Romagna (2007-2009, M.L.), Ministero dell'Istruzione, Università e della Ricerca (PRIN2009, M.L.), and Società Italiana Ematologia Sperimentale (fellowship, L.P.).
Authorship
Contribution: G.R. and P.B. acquired experimental data; M.L. L.P., and F.F. provided clinical management and interpreted data; and all authors wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mario Luppi, Dip di Scienze Mediche e Chirurgiche Materno-Infantili e dell'Adulto, Università di Modena e Reggio Emilia, UO-C Ematologia, AOU Policlinico di Modena, Via Del Pozzo 71, Modena 41124, Italy; e-mail: mario.luppi@unimore.it.
References
Author notes
G.R., M.L., P.B., F.F., and L.P. contributed equally to this study.