Abstract
Approximately 5-10% of diffuse large B-cell lymphomas (DLBCL) harbor a 8q24/MYC rearrangement (MYC+). We determined the prognostic significance of MYC rearrangement in patients with relapsed/refractory DLBCL prospectively treated by R-ICE or R-DHAP followed by high-dose therapy and autologous stem cell transplantation. Twenty-eight (17%) of the 161 patients analyzed presented a MYC+ rearrangement, targeted as either simple hit (25%) or complex hits (n=75%) including MYC/BCL2, MYC/BCL6, and MYC/BCL2/BCL6. Results were statistically highly concordant in matched primary and relapsed biopsies (n = 45). Compared to the MYC− DLBCL patients, the MYC+ DLBCL patients presented with a more elevated lactico-deshydrogenase level (P = .0006) and a more advanced age adjusted international prognostic index (P = .0039). The 4-year PFS and OS were significantly lower in the MYC+ DLBCL patients than those in the MYC− DLBCL patients, with rates of 18% vs 42% (P = .0322), and of 29% vs 62% (P = .0113), respectively. Type of treatment, R-DHAP or R-ICE, had no impact on survivals, with 4-year PFS rates of 17% vs 19% and 4-year OS rates of 26% vs 31%. In conclusion, MYC rearrangement is an early event in DLBCL. MYC+ DLBCL patients have a significant inferior prognosis than MYC− DLBCL patients. Their outcome was not influenced by the proposed salvage therapy.
Introduction
Diffuse large B-cell lymphomas (DLBCLs) are recognized as a heterogeneous group of aggressive lymphomas, with numerous clinical, morphological, immunohistochemical and molecular subtypes, as demonstrated by the last World Health Organization (WHO) classification, which defines no less than 13 subentities.1 Among the DLBCLs, between 4% and 14% harbor a MYC rearrangement (MYC+ DLBCL) as evaluated by FISH.2-6 These cases differ from the defined “borderline” cases, which are considered unclassifiable B-cell lymphomas having features that are intermediate between DLBCLs and Burkitt lymphomas7,8 and presenting with a mixture of medium- to large-sized cells, a high proliferation rate, and an 8q24/MYC translocation in 35% to 50% of cases.7,8 In contrast to the Burkitt lymphomas, MYC aberrations in DLBCLs are usually associated with multiple cytogenetic abnormalities and other genetic lesions, such as concurrent BCL2 and/or BCL6 translocations, so-called “double-hit” or “triple-hit” lymphomas.5,9-13
The clinical importance of the presence of an MYC aberration in DLBCLs has been recently suggested in series of patients analyzed in first-line therapy. Patients with MYC+ DLBCLs have been reported in one series6 to present a disease similar to DLBCL but without an MYC aberration (MYC− DLBCL) showing no differences in median age, lactico-dehydrogenase, International Prognostic Index (IPI), or performance status. The only difference noted was a higher proliferative rate, as determined by Ki67 staining in excess of 80%, in patients harboring a MYC aberrations.6 In other series, patients with MYC+ DLBCLs present with a more aggressive disease including particular clinical features such as poor performance status and BM involvement,5 or more advanced stage, higher IPI, and a higher age-adjusted IPI.2 With respect to survival, all series agree in reporting that these patients with MYC+ DLBCLs have poorer outcomes than patients with MYC− DLBCLs,5,6 regardless of the treatment regimen included, such as cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP), and CHOP plus etoposide (CHOEP).4,5 The presence of MYC aberrations retains its negative prognostic significance even in patients treated with rituximab and anthracycline-based immunochemotherapy.2,3,6 However, one recent study reported that a regimen based on dose-adjusted etoposide, vincristine and doxorubicin for 96 hours with bolus of cyclophosphamide and oral prednisone, plus rituximals (DA-EPOCH-R) in first-line may improve the outcome of these patients.14 Importantly, the prognostic impact of the presence of MYC aberrations appears to be independent from other factors such as IPI, and BM involvement.6 Furthermore, the negative impact of MYC aberrations supersedes the favorable prognosis of germinal center (GC) DLBCL, with a significantly worse event-free and overall survivals (OS) compared with either the non-GC phenotype or MYC− DLBCL.4,6
The purpose of this study was to screen for MYC aberrations in a selected population of patients with relapsed/refractory DLBCLs to determine the frequency of this occurrence and whether there were any defining phenotypic or clinical features in the MYC+ group and to assess the prognostic impact of MYC aberrations in these relapsed/refractory DLBCL patients treated prospectively with R-DHAP (rituximab, dexamethasone, aracytine, cisplatin) versus R-ICE (rituximab, ifosfamide, etoposide, carboplatin) that was followed by high-dose therapy plus autologous stem cell transplantation (HDT/ASCT).
Methods
The patients studied in the present biologic analyses were a subset of the 477 patients analyzed in the CORAL study,15 which enrolled patients aged 18 to 65 years of age who presented a relapsed/refractory CD20+ DLBCL to compare the efficacy of R-ICE and R-DHAP followed by HDT/ASCT (part 1) and to test maintenance with or without rituximab (part 2). The study was registered under European Union Drug Regulating Authorities Clinical Trials (EudraCT; no. 2004-002103-32 and www.clinicaltrials.gov NCT 00137995) and was conducted in accordance with good clinical practice rules. All patients gave written informed consent to participate and to provide tissue material for biologic studies.
FISH analysis
Histologic material was available for a total of 161 patients at diagnosis (n = 121 cases) and/or at relapse (n = 87 cases) for FISH analysis. FISH analysis was performed on tissue microarray (TMA) or full slides of paraffin-embedded 2- to 3-μm tissue sections using the break-apart probes for c-MYC/8q24 (Abbott). Further analyses were conducted using break-apart probes for BCL2/18q21 and BCL6/3q27 (Abbott). Samples were analyzed with an AxioImager.M1 epifluorescence microscope (Carl Zeiss). Images were captured with a 63× or 100× oil objective and analyzed with the Isis software (MetaSystems). The hybridization signal scoring was performed according to Haralambieva et al,16 with a normal cutoff value of 10%.
Morphology, IHC, and cell of origin (COO) algorithms
A panel of 5 hematopathologists (J.B., P.G., H.-U.V., C.S., S.C.) conducted a central review to confirm the diagnosis of CD20+ DLBCL.1,17 None of the 161 cases were intermediate cases between DLBCL and Burkitt lymphoma: all were diagnosed as DLBCL. The same panel of hematopathologists centrally evaluated the immunostainings and the FISH results. Immunostainings against CD10 (clone 56C6, dilution 1/50; Novocastra), BLC2 (bcl-2 124; DAKO A/S), IRF4/MUM1 (clone Mum1p, dilution 1/20; DAKO A/S), BCL6 (clone P1F6, dilution 1/10; DAKO A/S), and FOXP1 (clone JC12, dilution 1/50; A. H. Banham) were performed using 3-μm sections either from either full slides or from TMAs containing 2 or 3 representative 0.6-mm cores of routinely formaldehyde fixed-parafin embedded tissue from each cases. The results of each immunostaining trial were considered positive when > 30% of the lymphoma cells were stained. The tissue quality was morphologically evaluated on H&E staining. All evaluable cases were given a secondary classification according to the COO algorithms previously described by Hans et al,18 Muris et al,19 and Nyman et al.20
Microarray procedures and analyses
A subset of 37 patients was selected with both the MYC FISH analysis and the gene expression profiling (GEP) analysis realized. A total of 47 samples (20 primary biopsies, 17 relapse biopsies, and 5 matched cases) were then included. The microarray procedures are previously described.17 Briefly, total RNA quantity and initial quality were estimated with a NanoDrop ND-1000 spectrophotometer, and RNA quality was further assessed by electrophoresis (Agilent 2100 Bioanalyzer; Agilent Technologies). The Agilent Whole Human Genome microarray (G4112F) and a gene-voting method were used to determine the COO based on the genes' discriminating GCB/ABC signatures previously reported by Alizadeh et al21 to define a GCB/ABC predictor similar to that described our previous work.17 The samples were then classified based on this predictor. The microarray data were submitted to the Gene Expression Omnibus (GEO; GSE26812).
Statistical analysis
As previously reported,17 no statistical variations in each biologic parameter analyzed by IHC obtained at diagnosis and at relapse were detected among the matched pairs (data not shown). This finding allowed us to analyze all data in a similar manner, irrespective of whether they were generated from diagnostic or relapse biopsies. All survival analyses were performed on an intention-to-treat basis. Patient characteristics and complete remission rates were compared by the χ2 and Fisher exact tests. Progression-free survival (PFS) was defined as the time from study entry until disease progression or death. OS was defined as the time from the start of treatment until death. Survival functions were estimated using the Kaplan-Meier method and compared with the log-rank test.22 Differences between the results of comparative tests were considered significant at a 2-sided P < .05. Because the CORAL trial was not stratified by biologic data, we controlled for the effects of prognostic factors on outcome because of sampling fluctuations in the treatment groups with a multivariate analysis of survival in a Cox model.23 All statistical analyses were performed using SAS 9.13 (SAS Institute) and S-Plus 6.2 (MathSoft) software.
Results
Overall, 161 of the 477 patients included in the CORAL trial were included based on a successful FISH analysis at defining the presence or absence of an MYC rearrangement using paraffin-embedded-tissue (TMA or full slides). One hundred twenty-one cases were collected at diagnosis (primary biopsy), and 84 cases were collected at relapse (relapse biopsy), including 45 matched cases with the biopsies obtained at both diagnosis and relapse.
In total, 28 (17%) of the 161 cases disclosed an MYC rearrangement. Twenty-one of the MYC+ cases (75%) had one or more concurrent translocations, implicating either BCL2 in t(14;18), or BCL6 in t(3;14), so-called “double-hits,“ or BCL2 in t14;18) and BCL6 in t(3;14), so-called “triple-hits.” The MYC rearrangements were mostly translocations (Table 1). In 2 cases, we observed multiple copies of MYC. Considering the 45 matched cases, the results for the primary and secondary biopsies were similar in 87% of the cases (Wilcoxon paired ranked test, P = .99). At diagnosis, 6 (6 of 45, 13%) cases were MYC+ cases, and 39 (39 of 45, 87%) were MYC− cases. At relapse 8 (8 of 45, 18%) cases were MYC+ cases, and 37 (39 of 45, 82%) were MYC− cases.
Clinical characteristics
MYC+ DLBCL patients were predominantly male (71%) with a median age of 55 years (range, 44-65 years). Compared with MYC− DLBCL patients, the MYC+ DLBCL patients presented with more advanced disease, showing a high (aaIPI) in 54% of the patients (P = .0039), and an elevated LDH level in 77% of them (P = .0006; Table 2). There was no difference in the frequency of extranodal site involvement. The number of patients with early relapse, defined as relapse < 12 months from the end of first-line treatment, was identical in both groups (MYC+ cases, 61% vs MYC− cases, 50%, P = .3196).
IHC and cell of origin
MYC+ DLBCL cases expressed significantly more CD10 than MYC− DLBCL cases (60% vs 34%, respectively, P = .0137) as shown in Table 3. Immunohistochemical expression of BCL6, MUM/IRF4, and FOXP1 in MYC+ tumor cells was observed in 77%, 33%, and 81% of the cases, respectively, without significant differences compared with the MYC− tumor cells.
The COO phenotype assignments by IHC based on the Hans algorithm, the Muris algorithm, and the Nyman algorithm were available for 152 cases. Based on the Hans algorithm, MYC+ DLBCL cases were classified as GC in 63% of the cases, whereas 46% were classified as GC in the MYC− DLBCL cases. Thirty-seven cases were assigned by GEP. Based on the GEP predictor for the GCB/ABC genotype, 3 MYC+ DLBCL cases exhibited a GCB profile.
Impact on response and survival of MYC rearrangements in patients with relapsed/refractory DLBCL
At salvage therapy, 83 patients were treated with R-DHAP and 78 were treated with R-ICE. After the induction treatment (R-ICE or R-DHAP), the overall response rate (ORR) was lower in the cohort of patients with MYC+ DLBCL than in the cohort of patients with MYC− DLBCL (50% vs 69%, respectively, P = .0519; see Table 2).
The complete response rate (CR) after induction treatment (R-ICE or R-DHAP) was significantly lower in patients with MYC+ DLBCL than in those with MYC− DLBCL (25% vs 45%, P = .0497). After the induction treatment, fewer MYC+ DLBCL patients underwent HDT/ASCT than those MYC− DLBCL patients (43% vs 60%, P = .0928).
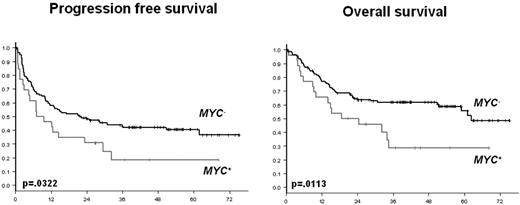
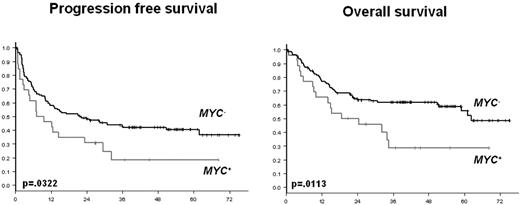
The 4-year PFS and OS were significantly lower in the MYC+ DLBCL patients than in the MYC− DLBCL patients (Figure 1), with rates of 18% versus 42% (P = .0322), and 29% versus 62% (P = .0113), respectively. The same result was observed for the MYC+ DLBCL patients who underwent the HDT/ASCT. These MYC+ DLBCL patients treated with HDT/ASCT had 4-year PFS and OS rates of 14% and 23%, respectively.
PFS and OS according to the presence (MYC+) or the absence (MYC−) of MYC aberration.
PFS and OS according to the presence (MYC+) or the absence (MYC−) of MYC aberration.
No difference was observed when we compared the group of cases with MYC rearrangements consisting of single chromosomal aberrations and the group of cases with MYC rearrangements presenting as complex aberrations with dual and triple translocations. The 4-year PFS (16% vs 17%, P = .8700) and OS (25% vs 33%, P = .7677) rates were similar in the single-hit and in the complex-hit groups.
Impact of R-ICE and R-DHAP treatment
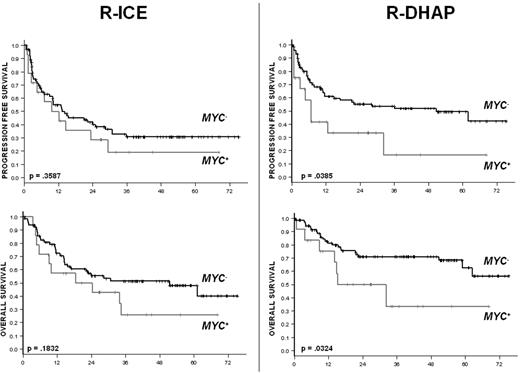
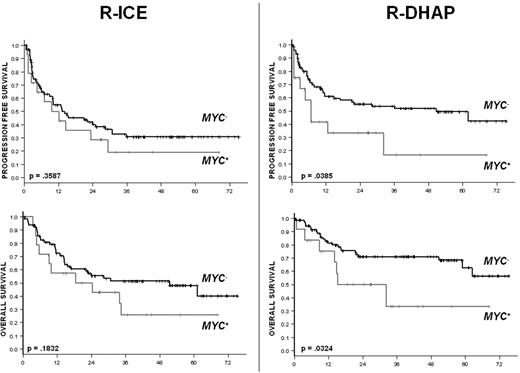
The type of treatment (R-ICE or R-DHAP) did not influence the outcomes of the MYC+ DLBCL patients. The corresponding ORR and CR rates were 50% and 25% in the MYC+ DLBCL patients, respectively, with no differences observed after R-ICE (ORR = 40%, CR 23%) or R-DHAP (ORR = 62%, CR = 26%; ORR P = .1607, CR P = .8268; Table 4). In patients with MYC− DLBCL, the CR rates were 35% and 54% with R-ICE and with R-DHAP, respectively (P = .0250).
With respect to survival, R-ICE and R-DHAP yielded similar results in the cohort of patients with MYC+ DLBCL. The 3-year PFS was at 19% in the R-ICE arm and 17% in the R-DHAP arm (Figure 2). The respective 3-year OS rates were 26% and 31% (Figure 2). In the cohort of patients with MYC− DLBCL, the 3-year PFS and OS rates were 31% and 51% in the R-ICE arm and 53% and 71% in the R-DHAP arm, respectively.
PFS and OS according to the treatment and the presence (MYC+) or absence (MYC−) of MYC aberration.
PFS and OS according to the treatment and the presence (MYC+) or absence (MYC−) of MYC aberration.
Impacts of the GCB and non-GCB phenotypes
When we classified the entire cohort of the 161 patients into subgroups based on the GCB versus non-GCB phenotype using the Hans algorithm, the GCB DLBCL patients treated with R-DHAP had a significantly higher complete remission rate than the non-GCB DLBCL patients (49% vs 31%, P = .0348), as previously reported17 (Table 4).
Multivariate analysis using a Cox proportional hazard model including the presence or absence of MYC aberrations, the type of induction treatment, R-ICE versus R-DHAP, and the GC versus non-GC phenotype based on the Hans algorithm confirmed that the only significant factor for both PFS and OS was the presence of a MYC aberration, with a relative risk (RR) of 1.8 for PFS (P = .0248), and an RR of 2 for OS (P = .0162).
Discussion
A limited number of studies evaluating the prognostic importance of MYC status have been reported to date but all were in the context of first-line treatment based on CHOP or CHOP-like regimens, with or without rituximab.2,4-6,11,13 This report is the first study to analyze the impact of MYC aberration in patients younger than 65 years of age with relapsed/refractory DLBCL.
Several characteristics of the clinical presentation of the disease and the patients with MYC+ DLBCL at relapse are interesting to highlight. First, the median ages MYC+ DLBCL and MYC− DLBCL patients are similar. Second, the times to relapse between the end of first-line treatment and the time of relapse treatment, which correspond to the randomization in the CORAL trial, are similar in both patient cohorts. Third, the clinical presentation of MYC+ DLBCL seems more aggressive than that of MYC− DLBCL, as reported previously in a series of patients undergoing first-line treatment.2,5,6 MYC+ DLBCL LDH levels were more often elevated, as were the IPI scores. However, we did not observe more patients with more than one extranodal site, representing 29% of the patients in our series.
All of the prior studies performed in first-line treatment using anthracycline-based regimens with or without rituximab found a strong prognostic impact of MYC gene rearrangement. In the context of relapsed/refractory DLBCL, the outcomes of the MYC+ DLBCL patients were also found to be worse than those of the MYC− DLBCL patients. This was true regardless of the type of induction treatments, that is, R-ICE or R-DHAP. Complete remission rates were low, accounting for ∼ 25% of the MYC+ DLBCL patients. Less than half of these responder MYC+ DLBCL patients followed the complete course of treatment and received the HDT/ASCT, compared with 60% in the MYC- DLBCL group.
With the caveat that this study examined a relatively small number of cases, the type of aberrations of MYC gene (single-, double-, or triple-hit) had no impact on these results. The role of the MYC aberration appeared to be the same whether the aberration was extra copies of the gene or a chromosomal translocation implicating MYC. We observed no differences when MYC aberrations were associated as complex hits with other aberrations including BCL2 and BCL6 gene rearrangements.
Taking the opportunity to analyze the tumors at both diagnosis and relapse in the 45 matched cases, MYC aberrations were found be present or absent in a similar ways in the biopsies at diagnosis and those at relapse. This suggests that the occurrence of MYC aberration is probably an early event in the pathogenesis of this tumor.
Regarding the phenotypes of the cases, an MYC aberration was strongly associated with the GCB phenotype, showing significant associations with CD10 expression and a GCB phenotype based on a GEP analysis. The MYC+ cases were more often classified as GCB based on the Hans algorithm. The outcome of the patients with GCB phenotypes based on the Hans algorithm who were treated with R-DHAP was better than the outcome of the patients with non-GCB phenotype, except when MYC gene was rearranged. MYC rearrangement was found to counteract the good prognosis associated with the GCB profile and R-DHAP treatment.
In conclusion, MYC rearrangements were found to be present in 17% of the patients with relapsed/refractory DLBCL, defining a small group of patients with a very bad prognosis independently from the known prognostic risk factors and regardless of the type of treatment proposed, even in the case of HDT/ASCT. Moreover, our results suggest that this genetic event is probably an early event in the pathogenesis of the tumor and is associated with a GCB phenotype. Finally, our data together with those of previous studies highlight the fundamental role of MYC in the prognosis of DLBCL, particularly GCB-like DLBCL. This finding suggests the importance of performing FISH analysis for MYC rearrangement in highly proliferative DLBCL and emphasizes the need for new treatments for patients with MYC+ DLBCL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge GELARC (Groupe d'Etude des Lymphomes de l'Adulte–Recherche Clinique) for the clinical management of this work; GELAP (Groupe d'Etude des Lymphomes de l'Adulte–Pathology), especially Jerome Cuvillier, for the management of the lymphoma cases; and Catherine Chevalier, Cecile Tuffier, and François Plassa for their excellent help in collecting the material and data as well as for their technical assistance.
This work was supported by the Program Hospitalier de Recherche Clinique 2009, INCa, Institut National du Cancer, France (grant PHRC AOM09271), and F. Hoffmann-La Roche Ltd. S.C. was sponsored by the SAKK (Schweizerische Arbeitgeminschaft für Klinische Krebsforchnung).
Authorship
Contribution: W.C. and H.-U.V. performed research, analyzed data and contributed to the writing of the manuscript; J.B. and N.M. designed and performed research, analyzed data, and wrote the manuscript; A.R., C.S., S.C., E.H., L.Y., D.B., J.S., P.G., and C.G. analyzed data and contributed to the writing of the manuscript; R.H. performed research, analyzed data, and wrote the manuscript; and C.T. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Catherine Thieblemont, Hôpital Saint-Louis, Service d'hémato-oncologie, Coquelicot 6, 1, Ave Claude Vellefaux, 75010 Paris, France; e-mail: catherine.thieblemont@sls.aphp.fr.