Abstract
Ligand-induced ectodomain shedding of glycoprotein VI (GPVI) is a metalloproteinase-dependent event. We examined whether shear force, in the absence of GPVI ligand, was sufficient to induce shedding of GPVI. Human-citrated platelet-rich plasma or washed platelets were subjected to increasing shear rates in a cone-plate viscometer, and levels of intact and cleaved GPVI were examined by Western blot and ELISA. Pathophysiologic shear rates (3000-10 000 seconds−1) induced platelet aggregation and metalloproteinase-dependent appearance of soluble GPVI ectodomain, and GPVI platelet remnant. Shedding of GPVI continued after transient exposure to shear. Blockade of αIIbβ3, GPIbα, or intracellular signaling inhibited shear-induced platelet aggregation but minimally affected shear-induced shedding of GPVI. Shear-induced GPVI shedding also occurred in platelet-rich plasma or washed platelets isolated from a von Willebrand disease type 3 patient with no detectable VWF, implying that shear-induced activation of platelet metalloproteinases can occur in the absence of GPVI and GPIbα ligands. Significantly elevated levels of sGPVI were observed in 10 patients with stable angina pectoris, with well-defined single vessel coronary artery disease and mean intracoronary shear estimates at 2935 seconds−1 (peak shear, 19 224 seconds−1). Loss of GPVI in platelets exposed to shear has potential implications for the stability of a forming thrombus at arterial shear rates.
Introduction
Platelet activation and accumulation at sites of vascular injury play a critical role in thrombus formation. This complex process is mainly initiated by 3 different but overlapping pathways: (1) exposure of subendothelial matrix proteins, including collagen and VWF, which activate the platelet adhesion-signaling receptors glycoprotein VI (GPVI) and GPIbα of the GPIb-IX-V complex, respectively; (2) exposure of tissue factor, which activates the coagulation cascade resulting in formation of active thrombin facilitating fibrin deposition as well as enhancing platelet activation; and (3) disturbed blood flow because of narrowing of the vascular lumen, which modulates the adhesive function of platelets and accelerates platelet activation and thrombus growth.1 Indeed, changes in blood flow rates and hydrodynamic force are now recognized to play a more critical role in thrombus formation, especially at sites of vascular occlusion, as indicated by the ability of elevated (pathologic) shear stress to induce stable platelet aggregate formation without the requirement for platelet activation and adhesion2 or for soluble agonists.3
Human platelets normally circulate in a resting state and are exposed to shear rates within a physiologic range (∼ 20-2000 seconds−1). Platelets may encounter shear rates beyond 10 000 seconds−1 under pathologic conditions, for example, in a stenosed atherosclerotic artery, and become activated and begin to aggregate.3-5 Shear-dependent platelet activation is initiated by binding of plasma VWF to platelets primarily through GPIbα, leading to platelet activation, secretion of ADP, and other agonists, and αIIbβ3-dependent aggregation.6,7 The inherited bleeding disorders typifying Bernard-Soulier syndrome caused by one or more mutations usually within the gene encoding GPIbα, and von Willebrand disease (VWD) type 3, where there is undetectable VWF protein and activity, illustrate the profound importance of VWF/GPIbα engagement under shear for platelet function and hemostasis.4 Shear-induced platelet adhesion on immobilized VWF also induces metalloproteinase-mediated, time-dependent shedding of GPIbα,8 suggesting that receptor shedding may provide a mechanism to allow negative regulation of GPIbα levels, limiting thrombus formation under high pathophysiologic flow conditions.
We were interested to examine whether GPVI shedding was also a consequence of shear-dependent platelet activation. We recently demonstrated spontaneous activation and aggregation in platelets, initiated via selective engagement of the GPIb-IX-V complex by a multivalent VWF A1 domain with a gain-of-function R543W mutation, found in type 2B VWD expressed on monkey kidney cells.9 This activation required active Src family kinases, Syk and PI3K, and resulted in rapid tyrosine phosphorylation of Syk, indicating that engagement of GPIbα by the A1 domain of VWF could generate signaling events comparable with events downstream of GPVI engagement by collagen.9 GPVI, GPIbα, and GPV are shed on engagement of GPVI by physiologic ligands in humans10-12 and in mice13-16 in a metalloproteinase-mediated process that requires activation of Src family kinases, Syk and PI3K, and yields a soluble approximately 55-kDa ectodomain fragment of GPVI (sGPVI) in plasma and approximately 10-kDa GPVI, approximately 16-kDa GPIbα, or approximately 5-kDa GPV cytoplasmic tail fragments that remain associated with platelets.12 Shedding of GPVI in response to ligands is probably mediated by members of the A Disintegrin And Metalloproteinase (ADAM) family of proteases.17,18 Whether exposure of platelets to elevated shear rates is sufficient to down-regulate GPVI expression and/or increase GPVI shedding has not yet been addressed; however, it may be of clinical relevance in thrombotic diseases where disturbed blood flow is important, for example, in aortic valve stenosis19 or in patients on coronary bypass.20 In this regard, elevated surface levels of GPVI on circulating platelets correlate with increased incidence of stroke and acute coronary syndrome,21 and elevated plasma levels of sGPVI have been reported in a stable angina pectoris (SAP) cohort.22
We investigated the ability of shear stress, in the absence of GPVI ligands, to induce metalloproteinase-dependent GPVI shedding and release of sGPVI into plasma. We found that GPVI shedding occurred within 1 minute of exposure to shear, continued after cessation of shear, and was independent of VWF binding to GPIbα, αIIbβ3 engagement, and platelet activation. Shear-induced GPVI shedding may be a novel protective mechanism for down-regulating platelet adhesiveness and reactivity, and increasing plasma sGPVI under atherothrombotic conditions.
Methods
Reagents
N-ethylmaleimide, GM6001 (a broad-range hydroxamic acid-based metalloproteinase inhibitor), Src family kinase inhibitors PP1 and PP2, and the PI3K inhibitors LY294002 and wortmannin, were from Calbiochem. Dimethyl-1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (DM-BAPTA) and apyrase were from Sigma-Aldrich. Protease inhibitor cocktail Complete was from Roche Diagnostic. GPVI agonist convulxin was a gift from Dr Kenneth Clemetson (Berne, Switzerland). Ristocetin was from Helena Laboratories. Synthetic peptides GRGDSP and VLQGHLC were from Auspep. VWF was purified as previously described.9 The hydroxamate inhibitor GI254023 has been previously described and characterized for preferential inhibition of ADAM10 over ADAM17.23
Antibodies
Affinity-purified mAbs AK2 and SZ2 against GPIbα, CRC54 against αIIbβ3, 7E3 Fab against αIIbβ3 (Eli Lilly) 12H1, 1G5, and 4B8 against GPVI,24 and anti-VWF mAb 6G125 have been previously described. Purified polyclonal rabbit IgGs against recombinant human GPVI cytoplasmic tail or a peptide with sequence matching sequences within the cytoplasmic tails of mature GPV and GPIbα were previously described.12 Polyclonal anti-ADAM10 prodomain was from Santa Cruz Biotechnology, and anti-ADAM10 cytoplasmic tail was from ProSci. Anti-Syk mAb was from Millipore. HRP- and Alexa Fluor-488–conjugated antibodies were from Chemicon and Invitrogen, respectively. 4B8 or 1G5 were directly conjugated to PE using Lightning-Link antibody labeling kit (Innova Biosciences) as instructed.
Preparation of platelet-rich plasma and washed platelets
Written, informed consent was obtained from all blood donors in accordance with the Declaration of Helsinki, and the study was approved by the Monash University Standing Committee for Research in Humans and the Human Ethics Committee of Concord Repatriation General Hospital, in accordance with the Helsinki Declaration of 1975, as revised in 1983. Blood was collected from healthy donors or an individual with type 3 VWD, and the platelet-rich plasma (PRP) or washed platelets were prepared as described previously.26
Exposure of platelets to shear
To determine the effects of exposure to elevated shear forces on platelet GP receptor levels, 350 μL of either PRP or washed platelets (5 × 108/mL in Tyrode buffer) were preincubated with 50 μL of either Tris-buffered saline, pH 7.0 (TS; positive control) or (final concentrations) 10 μg/mL AK2 or SZ2 mAbs, the metalloproteinase inhibitors 100μM GM6001, 0.5 to 2μM GI254023 or 10mM EDTA, calcium chelator 50μM DM-BAPTA, adenosine diphosphate (ADP) scavenger 1 U/mL apyrase, 1mM αIIbβ3-blocking peptide GRGDSP or a control peptide, inhibitors of Src kinase 10μM PP1 or PP2, or Syk 30 μg/mL piceatannol. Samples were then loaded on to a cone-plate viscometer (Carri-Med) and sheared for up to 10 minutes at 300 to 10 000 seconds−1 with a one-third degree cone as previously described.27 Shear-independent shedding was induced in some samples by treatment with 0.5 μg/mL convulxin, 10 μg/mL collagen, or 5mM N-ethyl-maleimide (NEM). In some experiments, aliquots of washed platelets or PRP were exposed to 3000- to 10 000-seconds−1 shear for 1 minute, incubated at room temperature for various times without shear or with an equivalent volume and concentration of unsheared platelets or PRP, and then 50 μL of 0.5M EDTA was added to PRP or platelet suspensions, aliquots (20 μL) of samples were collected, and the extent of platelet aggregation was determined by particle counting in a Sysmex counter (Coulter Electronics). Regardless of particle size, platelet aggregates are counted as one particle. Thus, a decrease in the particle count indicated an increase in aggregation. Unsheared PRP or platelets in Tyrode buffer were used as negative controls as appropriate, and particle counts were normalized to reflect percentage of maximal aggregation, with the positive control as 100% and unsheared sample as 0%.
Samples of PRP or washed platelets that had been exposed to shear were centrifuged at 10 000g and the platelet-poor plasma (PPP) or supernatant was isolated and sGPVI levels measured by ELISA.28 Platelet pellets from washed platelet suspensions were lysed on ice for 30 minutes in TS buffer containing 1% (weight/volume) Triton X-100 and Complete protease inhibitor; then levels of platelet receptors were analyzed by SDS 5% to 20% SDS-PAGE, and immunoblotting with 1 to 2 μg/mL antibodies against cytoplasmic domains of GPVI, GPV, or GPIbα or with CRC54 against the β3 subunit of αIIbβ3 (pellets).12 Some samples of supernatants were analyzed by Western blot with 1 μg/mL anti-GPVI mAb 12H1. Blots were visualized using HRP-conjugated secondary antibodies and enhanced chemiluminescence (GE Healthcare). Optical density of each band from the same scanned Western blot was quantitated with ImageJ Version 1.45 software (National Institutes of Health). To quantitate ADAM activity in platelet suspensions, some samples of washed platelets were resuspended in Tyrode buffer containing 10μM of the fluorogenic quenched ADAM9/10/17 substrate peptide (7-methoxycoumarin-4-yl)acetyl-PLAQAV-N-3-(2,4-dinitrophenyl)-L-2,3-diaminopropionyl-RSSSR-NH2 (R&D Systems)29 and exposed to shear in the presence or absence of 10mM EDTA, or 2μM GI254023. ADAMs activity was measured after 30 minutes in a 96-well white plate (OptiPlate; PerkinElmer Life and Analytical Sciences) at 37°C using a FLUOstar Optima plate reader (BMG Labtech) set at 320/405 nm.
Shear-induced shedding of GPVI was also assessed by flow cytometry. Samples (400 μL) of PRP, which had been mixed with 10 μg/mL 7E3 Fab to prevent aggregation, were exposed to 7500-second−1 shear for various times, with the reaction then quenched with 10mM EDTA. Aliquots (100 μL) of sheared PRP were either centrifuged at 10 000g for 2 minutes to isolate plasma for measurement of sGPVI by ELISA or incubated with saturating concentrations of PE-labeled 4B8 or a control PE-conjugated anti–mouse IgG, and levels of platelet-associated GPVI were measured by flow cytometry on a FACScalibur running CellQuest Pro Version 5.2.1 software (BD Biosciences). The platelet gate was defined by forward and side scatter characteristics that contained αIIbβ3-positive events in untreated PRP.
VWD type 3 patient
After informed consent was given, blood was obtained from a 55-year-old woman, with spontaneous bleeding/bruising who was classified as having VWD type 3 according to International Society of Thrombosis and Hemostasis Scientific Sub-Committee guidelines.30 The most recent laboratory assessments indicated that blood parameters were FVIII 8%, VWF antigen less than 1%, collagen binding assay less than 1%, and ristocetin cofactor activity 8%. Although she had received plasma-derived VWF/FVIII before surgical procedures or for treatment of bleeding in the past, she had not received any blood transfusions or treatment for her condition over the previous 6 months. Three weeks before blood collection, she had a platelet count of 217 × 109/L and was not receiving any medications.
To assess levels of active VWF in this patient, responses to ristocetin-induced aggregation of PRP isolated from the patient or a healthy donor were measured in the absence and presence of 10 μg/mL purified VWF.25 To assess levels of VWF protein in platelet lysates, aliquots of washed platelets from the patient and a healthy donor were lysed in SDS-containing sample loading buffer, and analyzed by SDS-PAGE, and Western blot using 1 μg/mL 6G1 and reprobed with 1 μg/mL anti-glycocalicin polyclonal antibody. Aliquots of PRP or washed platelets from the patient were then exposed to shear in a cone-plate viscometer and GPVI shedding assessed as described under “Exposure of platelets to shear.”
Quantitative coronary angiography and shear stress calculations
Clinical samples were obtained from 20 subjects representing a group of 10 subjects with stable but hemodynamically significant coronary stenoses, and 10 age-matched controls. The stable angina group were unselected patients with SAP who had single-vessel coronary artery disease and in whom lesion severity was determined by quantitative coronary angiography as previously described using standard commercial software (CAAS II system; Pie Medical Imaging).5,31 Controls were obtained from 6 patients undergoing coronary angiography and confirmed to be free of significant stenosis and 4 healthy controls without history or risk factors for coronary disease.
Geometric models obtained from 3-dimensional reconstructions of stenosed coronary arteries (performed using the Leonardo workstation, IC3D, Siemens and Cardio-op B system, Paieon Medical) were used to construct a grid for computational fluid dynamics in 6 patients with SAP as described.5,31 Shear rate was determined using Ansys Version 11.0 based on models that were converted to standard neutral format, and computational fluid dynamics analysis was performed. Steady laminar flow and constant Newtonian fluid properties were assumed.2,3 Mass flow rate was set at 0.9 g/s. The wall was assumed to be rigid, with no deformation and to be nonslip with zero velocity. Blood density was assumed to be 1060 kg/m3, blood viscosity was assumed to be 0.0035 Pa.s, and reference temperature was set at 37°C.
Results
Several laboratories have reported platelet agonist-mediated activation of platelet metalloproteinases leading to the proteolytic shedding of platelet receptor ectodomains.10,12,32,33 As platelets are sensitive to changes in shear forces, we determined whether shear-mediated platelet activation could also activate GPVI shedding in vitro. Citrated PRP from 19 healthy persons were either untreated or subjected to 10 000-second−1 shear rate for 5 minutes in a cone-plate viscometer. As reported by others,34,35 exposure of PRP to a 10 000-second−1 shear rate resulted in greater than 80% of platelets forming platelet aggregates as confirmed by platelet counts and by phase-contrast microscopy (data not shown). PPP was generated and assayed for sGPVI by ELISA. The level of sGPVI in untreated plasma from 19 healthy donors was (mean ± SD) 19.04 ± 7.45 ng/mL, consistent with levels previously reported in plasma from healthy donors.28 Exposure to shear rates of 10 000 seconds for 5 minutes significantly increased plasma sGPVI levels to 61.35 ± 25.67 ng/mL (P < .0001, paired, 2-tailed t test), indicating that elevated shear could induce sGPVI release in human plasma.
Using PRP from a single donor, more than 50% of total platelet GPVI was lost after 10 minutes exposure to a shear rate of 7500 seconds−1 (Figure 1A) with concomitant increase in sGPVI. The release of GPVI depended on amount of shear with higher sGPVI levels observed at the highest shear rate (Figure 1B) and increased with time (Figure 1C). Under the experimental conditions used, sGPVI release was only observed at pathologic shear rates (7500-10 000 seconds−1) but stable at physiologic shear rate levels less than 3000 seconds−1 (Figure 1B). Shear-induced GPVI shedding was significantly inhibited by a broad specificity metalloproteinase inhibitor, GM6001, and by the inhibitor GI254023 blocking ADAM10 but not ADAM17 under the conditions chosen23 (Figure 1D), indicating that shear-induced release of GPVI was metalloproteinase-mediated with ADAM10 but not ADAM17 playing an important role. Using a fluorogenic substrate peptide that can be cleaved by several ADAMs, including ADAM17 and ADAM10, after 30 minutes, platelet-associated ADAM activity was 3-fold higher in platelet suspensions that had been exposed to 10 000-second−1 shear for 1 minute than in untreated platelets. This activity could be blocked (> 80%) by inclusion of 2μM GI254023 (Figure 1E). Treatment with GI254023 also significantly inhibited the appearance of the approximately 10-kDa GPVI remnant fragment on human platelets treated with collagen under static conditions for 1 hour (Figure 1F), indicating that ADAM10 plays an important role in the cleavage of GPVI triggered by physiologic agonists.
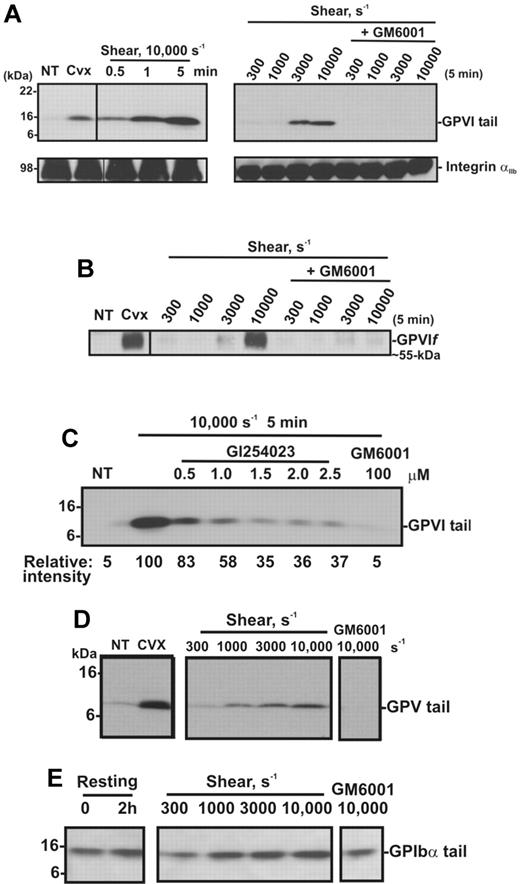
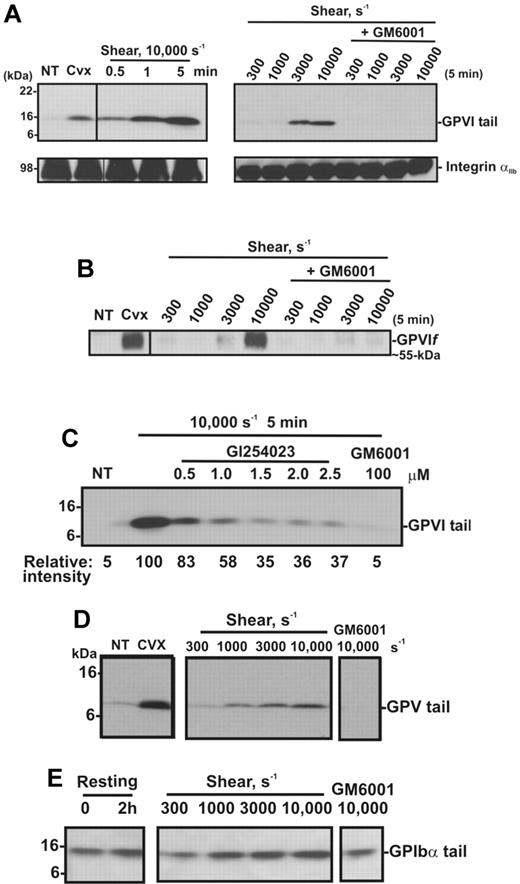
Shear induces loss of platelet GPVI and release of sGPVI in PRP. (A) PRP from a single donor was subjected to a shear rate of 7500 seconds−1 for up to 10 minutes in the presence of an inhibitor of αIIbβ3, or treated with 5mM NEM for 15 minutes. Aliquots of plasma and PRP were isolated for measurement of sGPVI by ELISA (light bars) and platelet surface levels of GPVI by flow cytometry (black bars), respectively. Data are expressed as percentage of total intact GPVI on resting platelets, or sGPVI released from platelets treated with 5mM NEM. (B) Citrated PRP from a single donor was left untreated or subjected to different shear rates for 5 minutes or (C) subjected to a shear rate of 10 000 seconds−1 for indicated times, or (D) subjected to 10 000-second−1 shear rate for 5 minutes in the presence of 100μM GM6001 or 2μM GI254023. Platelets were pelleted by centrifugation and assayed for sGPVI by ELISA in triplicate (sGPVI is shown as mean ± SD). Data are representative of at least 3 independent experiments using different single donors. (E) Human washed platelets in Tyrode buffer containing 10μM ADAM-cleavable quenched fluorogenic peptide were left untreated or exposed to 10 000-second−1 shear for 1 minute in the presence or absence of 2μM GI254023 or 10mM EDTA. Fluorescence activity (320/405 nm) at 30 minutes was quantified, and all values were corrected for background fluorescence measured in an untreated platelet sample containing substrate alone. Data are representative of at least 3 independent experiments using different single donors. (F) Human washed platelets (5 × 108/mL) were resuspended in Tyrode buffer and left untreated (NT) or treated with 5mM NEM as a positive control, or with 10 μg/mL collagen in the absence or presence of 2μM GI254023 (GI) for 1 hour at room temperature and then lysed and subjected to SDS-PAGE and Western blotted with anti-GPVI cytoplasmic tail antibody. (G) Human washed platelets (5 × 108/mL) were lysed and subjected to SDS-PAGE and Western blotted with anti-ADAM10 cytoplasmic tail antibody (ADAM10 CT) or anti-Syk (Syk). Vertical lines indicate a repositioned lane.
Shear induces loss of platelet GPVI and release of sGPVI in PRP. (A) PRP from a single donor was subjected to a shear rate of 7500 seconds−1 for up to 10 minutes in the presence of an inhibitor of αIIbβ3, or treated with 5mM NEM for 15 minutes. Aliquots of plasma and PRP were isolated for measurement of sGPVI by ELISA (light bars) and platelet surface levels of GPVI by flow cytometry (black bars), respectively. Data are expressed as percentage of total intact GPVI on resting platelets, or sGPVI released from platelets treated with 5mM NEM. (B) Citrated PRP from a single donor was left untreated or subjected to different shear rates for 5 minutes or (C) subjected to a shear rate of 10 000 seconds−1 for indicated times, or (D) subjected to 10 000-second−1 shear rate for 5 minutes in the presence of 100μM GM6001 or 2μM GI254023. Platelets were pelleted by centrifugation and assayed for sGPVI by ELISA in triplicate (sGPVI is shown as mean ± SD). Data are representative of at least 3 independent experiments using different single donors. (E) Human washed platelets in Tyrode buffer containing 10μM ADAM-cleavable quenched fluorogenic peptide were left untreated or exposed to 10 000-second−1 shear for 1 minute in the presence or absence of 2μM GI254023 or 10mM EDTA. Fluorescence activity (320/405 nm) at 30 minutes was quantified, and all values were corrected for background fluorescence measured in an untreated platelet sample containing substrate alone. Data are representative of at least 3 independent experiments using different single donors. (F) Human washed platelets (5 × 108/mL) were resuspended in Tyrode buffer and left untreated (NT) or treated with 5mM NEM as a positive control, or with 10 μg/mL collagen in the absence or presence of 2μM GI254023 (GI) for 1 hour at room temperature and then lysed and subjected to SDS-PAGE and Western blotted with anti-GPVI cytoplasmic tail antibody. (G) Human washed platelets (5 × 108/mL) were lysed and subjected to SDS-PAGE and Western blotted with anti-ADAM10 cytoplasmic tail antibody (ADAM10 CT) or anti-Syk (Syk). Vertical lines indicate a repositioned lane.
In nucleated cells, ADAM10 is synthesized and stored intracellularly as an inactive proprotein and on activation, proADAM10 is cleaved by convertases to yield mature ADAM10 that is enzymatically active and brought to the cell surface. ProADAM10 and mature ADAM10 migrate as approximately 98-kDa and approximately 72-kDa proteins, respectively, when analyzed by SDS-PAGE.36 To investigate the molecular weight of platelet ADAM10, platelet lysates were analyzed by SDS-PAGE and Western blot using an antibody against the ADAM10 cytoplasmic tail or anti-Syk mAb, to allow direct estimate of molecular mass. Only a single band that migrated to the same extent as platelet Syk was visible on membranes blotted with anti-ADAM10 cytoplasmic tail (Figure 1G), indicating that only mature ADAM10 was detectable in resting platelet lysates. By flow cytometry, an antibody against the extracellular portion of ADAM10, but not an antibody against the ADAM10 prodomain, detected ADAM10 on platelets (data not shown), indicating that only mature ADAM10 was present on the platelet surface.
To confirm the effect of shear on GPVI shedding, human washed platelets in Tyrode buffer were exposed either to shear (10 000 seconds−1) for up to 5 minutes or to increasing levels of shear for 5 minutes, in a cone-plate viscometer. Consistent with other reports,27,37,38 shear-induced platelet aggregation was induced on exposure of washed platelets to increasing levels of shear (300-10 000 seconds−1) with aggregate size being a function of shear and was dependent on both GPIb and integrin αIIbβ3, as pretreatment of platelets with either GPIbα- or αIIbβ3-blocking mAbs or GRGDSP peptide inhibited platelet aggregation induced by shear (data not shown). Platelet pellets were isolated from washed platelet suspensions that had been subjected to a shear rate of 10 000 seconds−1 for various times, or subjected to different shear rates and analyzed by SDS-PAGE and Western blot using anti-GPVI cytoplasmic tail antibody or CRC54 anti-αIIb mAb (platelet pellets) or anti-GPVI mAb 12H1 (supernatants). Both antibodies detect full-length GPVI, and either an approximately 55-kDa ectodomain fragment in supernatant fractions (12H1) or an approximately 10-kDa fragment that remains associated with the platelet pellet (anti-GPVI cytoplasmic tail antibody).12 Figure 2A shows that exposure of platelets to high shear for increasing amounts of time or to increasing levels of shear for 5 minutes induces GPVI shedding as reflected by the appearance of the GPVI tail fragment at approximately 10 kDa (absent in untreated platelets), accompanied by the appearance of an approximately 55-kDa shed soluble GPVI fragment in the supernatant (Figure 2B). These fragments are comparable with those induced by convulxin, a GPVI-binding snake venom protein, previously shown to trigger platelet activation and receptor shedding. The appearance of the shed fragments was more pronounced at a pathologic shear rate of 10 000 seconds−1 and completely blocked by the metalloproteinase inhibitor GM6001. Consistent with data shown in Figure 1, a role for ADAM10 was identified as the appearance of the 10-kDa GPVI tail fragment was ablated by up to 70% in the presence of the preferential ADAM10 inhibitor GI254023 (Figure 2C). Shedding of platelet receptors GPIbα and GPV, known to be cleaved by ADAM10 and/or ADAM1712 on exposure to increasing shear rates, was assessed by Western blot using antibodies raised against the cytoplasmic tail of GPV (Figure 2D) or GPIbα (Figure 2E). These antibodies recognize approximately 5-kDa and approximately 16-kDa membrane-associated fragments of GPV and GPIbα, respectively, in lysates of platelets treated with convulxin or NEM.12 Although a temporal increase in the membrane associated fragment of GPV was evident under these experimental conditions, the 16-kDa GPIbα fragment did not increase significantly above background levels observed in control unsheared, washed platelets over the same time period. Taken together, these data suggest that metalloproteinase-mediated shedding of GPVI and GPV from platelets is independent of platelet activation, as previously observed in response to ligand engagement, or treatment of platelets with calmodulin inhibitors or thiol-modifying reagents.10,12
Shear induces GPVI shedding in washed platelets. Washed platelets (5 × 108 platelets/mL) were left untreated (NT), or treated with either 0.5 μg/mL convulxin for 15 minutes, subjected to shear (10 000 seconds−1) for various times, or subjected to different shear rates for 5 minutes, with and without 100μM GM6001 (A-E) or up to 2.5μM GI254023 (C). (A) Platelet pellets were lysed and analyzed by SDS-PAGE and immunoblotting using anti-GPVI cytoplasmic tail antibody (top panel) or anti-αIIbβ3 mAb CRC54 (bottom panel). (B) Supernatants were blotted with anti-GPVI mAb 12H1. (C) Platelet pellets were lysed and analyzed by SDS-PAGE and immunoblotting using anti-GPVI cytoplasmic tail antibody. Densitometry measurements (relative intensities) of each GPVI tail fragment in blot C were compared relative to the densitometry of the fragment detected in the 10 000-second−1/5-minute sample. (D-E) Platelet pellets were lysed and analyzed by SDS-PAGE/Western blotting using (D) anti-GPV or (E) anti-GPIbα cytoplasmic tail antibodies.
Shear induces GPVI shedding in washed platelets. Washed platelets (5 × 108 platelets/mL) were left untreated (NT), or treated with either 0.5 μg/mL convulxin for 15 minutes, subjected to shear (10 000 seconds−1) for various times, or subjected to different shear rates for 5 minutes, with and without 100μM GM6001 (A-E) or up to 2.5μM GI254023 (C). (A) Platelet pellets were lysed and analyzed by SDS-PAGE and immunoblotting using anti-GPVI cytoplasmic tail antibody (top panel) or anti-αIIbβ3 mAb CRC54 (bottom panel). (B) Supernatants were blotted with anti-GPVI mAb 12H1. (C) Platelet pellets were lysed and analyzed by SDS-PAGE and immunoblotting using anti-GPVI cytoplasmic tail antibody. Densitometry measurements (relative intensities) of each GPVI tail fragment in blot C were compared relative to the densitometry of the fragment detected in the 10 000-second−1/5-minute sample. (D-E) Platelet pellets were lysed and analyzed by SDS-PAGE/Western blotting using (D) anti-GPV or (E) anti-GPIbα cytoplasmic tail antibodies.
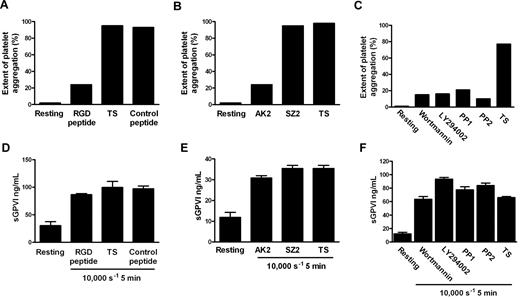
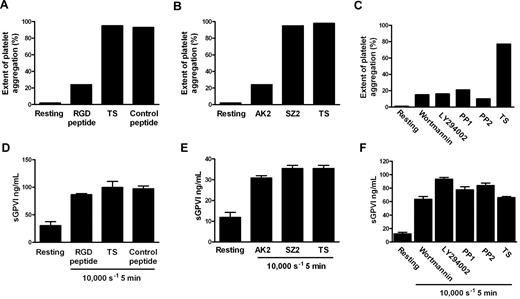
Although the precise mechanism by which shear stress triggers platelet aggregation remains unclear, elevated shear facilitates engagement of GPIb-IX-V with VWF in plasma, and shear-induced platelet aggregation requires VWF/GPIbα engagement, intracellular platelet signaling,39 and αIIbβ3 activation. To investigate whether shear-dependent shedding of GPVI also requires GPIbα-VWF interaction, Src family kinase and PI3K activity, and αIIbβ3 activation, citrated PRP was subjected to 10 000-seconds−1 shear for 5 minutes in a cone-plate viscometer in the absence or presence of blocking concentrations of either GRGDSP peptide or AK2 mAb. Control samples were treated with TS alone or either an irrelevant peptide of comparable molecular mass or mAb SZ2 against the sulfated tyrosine sequence of GPIbα, which does not block GPIbα/VWF interaction. Other samples were preincubated with 10μM PP1 or PP2 (Src family kinase inhibitors), or 100 nM wortmannin and 10μM LY294002 (PI3K inhibitors). Aliquots of samples were assessed for extent of platelet aggregation by particle counting, and the remainder of each sample was processed to generate PPP for analysis of sGPVI levels by ELISA. Data in Figure 3A through C show that exposure of citrated PRP to 10 000-seconds−1 shear for 5 minutes induced platelet aggregation that was αIIbβ3- and GPIbα-dependent and required both Src family kinase and PI3K activity as aggregation was blocked by inclusion of either GRGDSP peptide, AK2, or inhibitors of Src kinases or PI3K but not a control peptide or mAb SZ2, confirming that the concentrations of reagents used were appropriate and the reagents were functional. However, shear-induced shedding did not require platelet aggregation or signaling because inclusion of any of the kinase inhibitors, peptides, or mAbs had minimal effect on levels of sGPVI after exposure to shear (Figure 3D-F). Further, platelets treated with the snake venom protein mocarhagin, to specifically remove the VWF-binding N-terminal portion of GPIbα, demonstrated elevated sGPVI levels after exposure to high shear (data not shown). This lack of effect of αIIbβ3 inhibition on shear-induced shedding of GPVI is consistent with previous data showing that ligand-mediated shedding of GPVI also does not require active αIIbβ3 or platelet aggregation.10 These results also imply that shear-induced GPVI shedding was independent of VWF binding to GPIbα and, in contrast to ligand-induced shedding, was also independent of downstream signaling pathways.
Shear-induced GPVI shedding does not require platelet aggregation, intracellular signaling, or platelet activation. Citrated PRP was either untreated or preincubated with TS buffer alone or containing 1mM RGD peptide, 1mM control peptide, 10 μg/mL AK2, 10 μg/mL SZ2, 10μM PP1, 10μM PP2, 100 nM wortmannin, or 10μM LY294002 as indicated and then subjected to a shear rate of 10 000 seconds−1 for 5 minutes. Aggregation was measured in samples by obtaining platelet particle count (A-C), and then plasma was generated and assayed for sGPVI by ELISA in triplicate (D-F). Panels A and D, B and E, and C and F contain data from 3 different donors that are representative of 3 independent experiments.
Shear-induced GPVI shedding does not require platelet aggregation, intracellular signaling, or platelet activation. Citrated PRP was either untreated or preincubated with TS buffer alone or containing 1mM RGD peptide, 1mM control peptide, 10 μg/mL AK2, 10 μg/mL SZ2, 10μM PP1, 10μM PP2, 100 nM wortmannin, or 10μM LY294002 as indicated and then subjected to a shear rate of 10 000 seconds−1 for 5 minutes. Aggregation was measured in samples by obtaining platelet particle count (A-C), and then plasma was generated and assayed for sGPVI by ELISA in triplicate (D-F). Panels A and D, B and E, and C and F contain data from 3 different donors that are representative of 3 independent experiments.
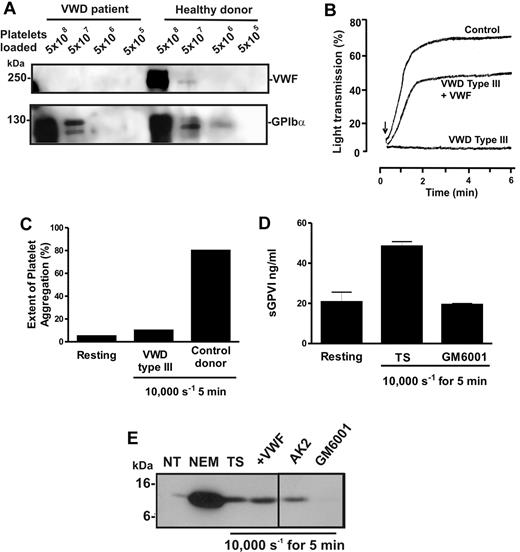
To confirm this unexpected observation, blood was isolated from a patient with VWD type 3, a condition characterized by congenital absence of VWF.30 Levels of VWF were assessed in preparations of washed lysed platelets from the patient or a healthy donor by analyzing equal numbers of platelets by SDS-PAGE and immunoblot using either 6G1 or anti-GPIbα (Figure 4A). Whereas platelets from the patient and the healthy donor displayed normal levels of GPIbα, VWF was undetectable in patient platelets. VWF-mediated ristocetin-induced aggregation was absent in patient-citrated PRP but rescued by addition of purified VWF (Figure 4B). Further, unlike PRP from healthy donors, patient PRP failed to aggregate in response to high shear (Figure 4C). However, exposure of patient citrated PRP to 10 000-seconds−1 shear resulted in elevated levels of sGPVI, which was blocked by GM6001 (Figure 4D). These data provide additional evidence that shear-induced GPVI shedding was metalloproteinase-mediated, did not require platelet aggregation, and occurred in the absence of VWF/GPIbα engagement. Treatment of VWD type 3 washed platelets with the thiol-modifying agent NEM activated GPVI shedding to produce the expected 10-kDa remnant fragment, indicating that the sheddase was present and functional (Figure 4E) and the cleavage was similar to ligand-induced GPVI proteolysis because a similar GPVI tail fragment was associated with sheared platelet pellets (Figure 4E). Inclusion of AK2 or purified VWF had minimal effect on GPVI cleavage; however, 100μM GM6001 blocked GPVI proteolysis, further confirming that shear-induced shedding of GPVI was metalloproteinase-mediated and occurred independently of VWF/GPIbα engagement.
Shear-induced shedding of GPVI occurs in the absence of VWF. Citrated PRP or washed platelets were prepared from blood isolated from a patient with VWD type 3 or a healthy donor. (A) To assess levels of VWF and GPIbα in each sample, equivalent concentrations of washed platelets were lysed in nonreducing sample loading buffer and subjected to SDS-PAGE and Western blot using 1 μg/mL anti-VWF mAb, 6G1 (top panel) or 1 μg/mL anti-glycocalicin antibody (bottom panel). (B) To assess VWF function, citrated PRP from the same patient or a healthy donor was subjected to ristocetin-induced platelet aggregation using light transmission aggregometry. Arrow indicates the time at which 1.5 mg/mL ristocetin was added. Absent ristocetin-induced aggregation in the patient sample was rescued by inclusion of 10 μg/mL purified VWF before addition of ristocetin. (C-D) Citrated PRP from the patient or a healthy donor was subjected to 10 000-second−1 shear for 5 minutes, and samples were then analyzed for (C) platelet aggregation by particle counting or (D) sGPVI levels by ELISA. (E) VWD type 3 washed platelets were either left untreated (NT) or treated for 15 minutes with 5mM NEM, or exposed to 10 000 seconds−1 shear for 5 minutes in the presence of TS buffer alone, or containing 10 μg/mL AK2, 10 μg/mL purified VWF, or 100μM GM6001. Samples were then lysed in nonreducing sample loading buffer and subjected to SDS-PAGE and Western blot using 1 μg/mL anti-GPVI cytoplasmic tail antibody. A vertical line indicates a repositioned lane.
Shear-induced shedding of GPVI occurs in the absence of VWF. Citrated PRP or washed platelets were prepared from blood isolated from a patient with VWD type 3 or a healthy donor. (A) To assess levels of VWF and GPIbα in each sample, equivalent concentrations of washed platelets were lysed in nonreducing sample loading buffer and subjected to SDS-PAGE and Western blot using 1 μg/mL anti-VWF mAb, 6G1 (top panel) or 1 μg/mL anti-glycocalicin antibody (bottom panel). (B) To assess VWF function, citrated PRP from the same patient or a healthy donor was subjected to ristocetin-induced platelet aggregation using light transmission aggregometry. Arrow indicates the time at which 1.5 mg/mL ristocetin was added. Absent ristocetin-induced aggregation in the patient sample was rescued by inclusion of 10 μg/mL purified VWF before addition of ristocetin. (C-D) Citrated PRP from the patient or a healthy donor was subjected to 10 000-second−1 shear for 5 minutes, and samples were then analyzed for (C) platelet aggregation by particle counting or (D) sGPVI levels by ELISA. (E) VWD type 3 washed platelets were either left untreated (NT) or treated for 15 minutes with 5mM NEM, or exposed to 10 000 seconds−1 shear for 5 minutes in the presence of TS buffer alone, or containing 10 μg/mL AK2, 10 μg/mL purified VWF, or 100μM GM6001. Samples were then lysed in nonreducing sample loading buffer and subjected to SDS-PAGE and Western blot using 1 μg/mL anti-GPVI cytoplasmic tail antibody. A vertical line indicates a repositioned lane.
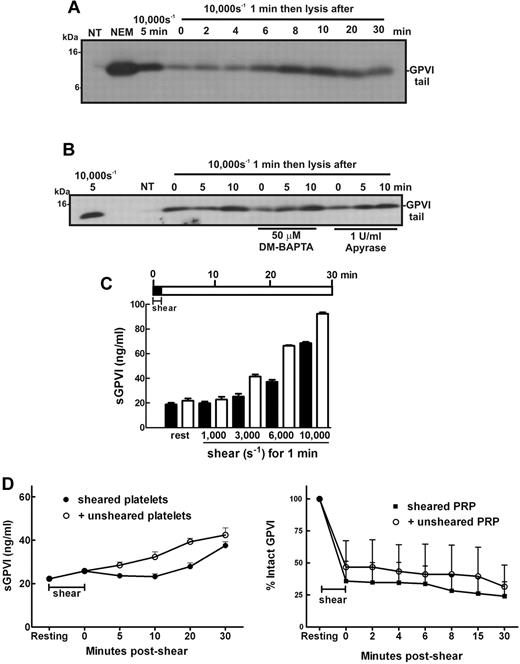
In the circulation, platelets are transiently exposed to a range of shear levels4,5,40 that could contribute to chronic low levels of platelet activation. To experimentally replicate conditions of transient high shear stress and examine how this type of stimulus affects platelet GPVI levels temporally, samples of washed platelets in Tyrode buffer alone, or containing DM-BAPTA to chelate intracellular calcium or apyrase to inhibit ADP released from platelets, were exposed to high levels of shear for 1 minute and then incubated at room temperature for up to 30 minutes before the incubation was halted by addition of EDTA. Levels of GPVI shedding were assessed by SDS-PAGE and immunoblot. Figure 5A shows that the remnant fragment of GPVI was detectable in samples exposed to 10 000-second−1 shear rate for 1 minute, indicating that this stimulus was sufficient to trigger shedding of GPVI and levels of the remnant fragment increased for up to 10 minutes after cessation of shear indicating GPVI continued to be proteolysed and that shear-induced GPVI sheddase activity did not require constant shear. The observed proteolysis did not require calcium flux and was independent of ADP release (Figure 5B). Interestingly, the level of shear required to activate a shedding response and release sGPVI was markedly reduced if the sheared PRP was incubated at room temperature for an additional 30 minutes after shear (Figure 5C). These data indicate that brief and transient shear exposure can trigger GPVI shedding, which, once initiated, can continue in the absence of shear.
Brief exposure to high shear triggers significant GPVI shedding over time. Washed platelets (5 × 108/mL) from a healthy donor were resuspended in (A) Tyrode buffer alone or (B) containing 50μM DM-BAPTA or 1 U/mL apyrase and then subjected to a shear rate of 10 000 seconds−1 for 1 minute. Samples were then left at room temperature for the indicated time before addition of EDTA followed by centrifugation and lysis of platelet pellets. Platelet lysates were analyzed by SDS-PAGE and Western blot using 1 μg/mL anti-GPVI cytoplasmic tail antibody. Data are representative of 3 independent experiments with different donors. (C) PRP from healthy donors was subjected to the indicated level of shear for 1 minute before addition of EDTA (dark bars) or incubated at room temperature for 30 minutes followed by addition of EDTA (light bars) as shown in the pictogram. All samples were then processed to isolate plasma and analyzed for sGPVI levels by ELISA. Data are representative of 4 independent experiments with different donors. (D) In the presence of an inhibitor of αIIbβ3, washed platelets in Tyrode buffer were exposed to 10 000-second−1 shear for 2 minutes and then mixed with equal amounts of buffer or unsheared platelets for up to 30 minutes (left panel), or PRP was subjected to 7500 seconds−1 for 1 minute and then mixed with equal amounts of buffer or unsheared PRP for up to 30 minutes (right panel). Aliquots of supernatant and PRP were isolated for measurement of sGPVI by ELISA (right panel) and platelet surface levels of GPVI by flow cytometry (left panel), respectively.
Brief exposure to high shear triggers significant GPVI shedding over time. Washed platelets (5 × 108/mL) from a healthy donor were resuspended in (A) Tyrode buffer alone or (B) containing 50μM DM-BAPTA or 1 U/mL apyrase and then subjected to a shear rate of 10 000 seconds−1 for 1 minute. Samples were then left at room temperature for the indicated time before addition of EDTA followed by centrifugation and lysis of platelet pellets. Platelet lysates were analyzed by SDS-PAGE and Western blot using 1 μg/mL anti-GPVI cytoplasmic tail antibody. Data are representative of 3 independent experiments with different donors. (C) PRP from healthy donors was subjected to the indicated level of shear for 1 minute before addition of EDTA (dark bars) or incubated at room temperature for 30 minutes followed by addition of EDTA (light bars) as shown in the pictogram. All samples were then processed to isolate plasma and analyzed for sGPVI levels by ELISA. Data are representative of 4 independent experiments with different donors. (D) In the presence of an inhibitor of αIIbβ3, washed platelets in Tyrode buffer were exposed to 10 000-second−1 shear for 2 minutes and then mixed with equal amounts of buffer or unsheared platelets for up to 30 minutes (left panel), or PRP was subjected to 7500 seconds−1 for 1 minute and then mixed with equal amounts of buffer or unsheared PRP for up to 30 minutes (right panel). Aliquots of supernatant and PRP were isolated for measurement of sGPVI by ELISA (right panel) and platelet surface levels of GPVI by flow cytometry (left panel), respectively.
We assessed whether ADAM10 on a shear-exposed platelet could cleave GPVI on an unsheared platelet, addressing the question of whether shear-induced GPVI shedding occurs in cis (where GPVI is cleaved by ADAM10 on the same platelet surface) or in trans (ADAM10 can cleave GPVI on a neighboring platelet). Shear-exposed washed platelets were mixed with either buffer or unsheared washed platelets for 30 minutes, and then levels of sGPVI were measured by ELISA (Figure 5D left panel) or sheared PRP was mixed with either autologous plasma or unsheared PRP and surface levels of GPVI were estimated by FACS (Figure 5D right panel). There was no evidence of accelerated shedding of GPVI in either experiment, suggesting that presentation of GPVI on unsheared platelets to ADAM10 on shear-exposed platelets was not sufficient to increase release of GPVI, implying that GPVI shear-induced shedding occurs predominantly in cis.
To gauge whether shear rates that occur in intracoronary vessels were sufficient to trigger shear-induced shedding of platelet receptors in vivo, sGPVI was assessed in 2 groups of 10 age-matched control subjects and SAP patients with stable single vessel coronary disease (age 63 ± 9 vs 63 ± 12 years, mean ± SD, P = 1.0) as defined by angiography. Circulating platelets in this group of patients would be briefly but chronically exposed to highly elevated intravascular shear rates. Plasma sGPVI was significantly elevated in patients presenting with SAP relative to controls (62.0 ± 7.5 vs 36.8 ± 5.5 ng/mL, P = .014, mean ± SEM; Figure 6). In 6 of the subjects from the SAP group, intracoronary shear was calculated by computational hemodynamics using methods previously published5 ; the average peak shear rate was determined to be 19 224 ± 10 780 seconds−1, and the average mean shear rate was 2935 ± 1243 seconds−1 (mean ± SEM; Table 1). These determinations confirmed that values of shear generated in our in vitro studies are physiologically relevant and are associated with elevated sGPVI levels in vivo.
sGPVI is elevated in patients with stable single vessel coronary disease. Ten patients with SAP and single vessel coronary disease (mean % diameter stenosis, 57.0% ± 2.9%) confirmed by quantitative coronary angiography and 10 age-matched controls were assessed for plasma sGPVI levels. sGPVI levels were significantly higher in the SAP group than in the control group (62.0 ± 7.5 vs 36.8 ± 5.5 ng/mL, mean ± SEM, P = .014, unpaired t test). *P < .05.
sGPVI is elevated in patients with stable single vessel coronary disease. Ten patients with SAP and single vessel coronary disease (mean % diameter stenosis, 57.0% ± 2.9%) confirmed by quantitative coronary angiography and 10 age-matched controls were assessed for plasma sGPVI levels. sGPVI levels were significantly higher in the SAP group than in the control group (62.0 ± 7.5 vs 36.8 ± 5.5 ng/mL, mean ± SEM, P = .014, unpaired t test). *P < .05.
Discussion
In this study, exposure of platelets to high shear induced a metalloproteinase-dependent GPVI cleavage, producing an approximately 55-kDa soluble ectodomain fragment and an approximately 10-kDa platelet-associated tail fragment, indicating that the observed proteolysis generated fragments similar to, but more rapidly than, shedding induced by GPVI ligands, calmodulin inhibitors, thiol-modifying reagents, and anti-GPVI monoclonal antibodies.10,41 We also measured plasma sGPVI levels in a group of patients chronically exposed to elevated intracoronary shear rates that were 3-fold higher than sGPVI in healthy donors,42 consistent with other reports of elevated sGPVI in SAP,22 suggesting that elevated shear contributes to shedding of GPVI in vivo. Shear-induced GPVI shedding was significantly inhibited by an ADAM10-selective inhibitor GI254023,23 suggesting that ADAM10 may play an important role in this process (Figures 1D-E and 2C). This is in agreement with ADAM10 being the main metalloproteinase responsible for GPVI shedding on human platelets, based on studies with recombinant ADAM10 and synthetic peptides corresponding to the GPVI cleavage site,12 although ADAM10 may be redundant in mice.14
To study the consequences of exposure to elevated levels of shear, a cone-plate viscometer was used to apply uniform shear stress to platelets in vitro, replicating shear forces experienced by platelets circulating in vivo. The advantages of this system are the ability to apply a uniform shear rate to platelets suspended in plasma or buffer for various times at different shear rates, the ability to address the effects of different inhibitors or agonists, and importantly, allow analysis of shear as the only agonist initiating platelet activation and aggregation induced by the interaction of VWF with GPIbα,4 and in the absence of known GPVI ligands including collagen. Collagen is only exposed in the event of vascular injury or under pathologic conditions, mainly after atherosclerotic plaque rupture. It is possible that shear-induced shedding of GPVI is a negative regulatory process that decreases platelet reactivity to subendothelial proteins including collagen. Shear force has been shown to influence metalloproteinase activity on other cell types. On leukocytes, L-selectin mediates recruitment and rolling of leukocytes on inflamed endothelium. Exposure of leukocytes to shear increases clustering of L-selectin and ADAM17 into microdomains and increased L-selectin shedding.43 Prolonged exposure of saphenous vein endothelium to arterial shear stress induced metalloproteinase-dependent shedding of soluble intercellular adhesion molecule-1, which could modulate endothelial cell responses to shear.44 On platelets, shear-induced engagement of GPIbα by VWF resulted in both metalloproteinase-mediated shedding of GPIbα, which might limit thrombus formation under flow conditions,8 and calpain-mediated cleavage of PECAM-1.45
Shear-induced GPVI shedding was more pronounced at high pathologic shear conditions (10 000 seconds−1); however, shedding was detectable at lower shear rates of 3000 to 6000 seconds−1 if the enzymatic reaction was allowed to proceed for up to 30 minutes. This implies that GPVI shedding can be triggered by brief, transient exposure to pathologic shear, and is supported by our data demonstrating elevated plasma sGPVI in a cohort of patients with a significant coronary stenosis with demonstrably increased intracoronary shear. Correspondingly, relatively low levels of sGPVI have been reported in plasma from healthy controls (∼ 19.04 ± 7.45 ng/mL [this study] or 159 random community-based controls, ∼ 19.6 ± 8.1 ng/mL).42 In contrast, shear-induced GPIbα shedding is constitutive and prominent at shear rates less than 1000 seconds−1, and the extent of shedding is reduced with increasing shear rate.8 This could explain why there is detectable GPIbα tail but relatively low levels of GPVI tail fragment present on resting platelets isolated in the presence of metalloproteinase inhibitors,12 and the relatively high concentration of soluble GPIbα ectodomain (glycocalicin; ∼ 1-3 μg/mL) present in plasma from healthy persons.46 Elevated shear rates in stenosed blood vessels are conditions that are considered prothrombotic with heightened platelet reactivity. Recent work by others has used soluble dimeric GPVI-Fc fusion protein to attenuate adhesion of platelets on collagen and thrombus formation under shear conditions in vitro, and block platelet aggregation in a mouse vascular injury model.47 Shear-induced shedding of GPVI could represent an innate mechanism to reduce platelet receptor density and diminish platelet responsiveness to exposed collagen; however, conceivably, it could also locally block GPVI-collagen interactions in stenosed and/or atherothrombotic vessels. Significantly, the use of sGPVI as a therapeutic to reduce thrombosis without bleeding complications is currently being evaluated.48 Potentially, sGPVI measured in plasma could be a useful marker of pathologic conditions associated with high shear rate, including vascular atherosclerosis and thrombosis, prosthetic devices, graft junction, and vascular abnormalities.17,22,42
As an activator of metalloproteinase-dependent GPVI shedding, elevated shear had very few prerequisites. There was no requirement for engagement of GPIbα or platelet aggregation, and shear-induced shedding was independent of intracellular signaling pathways as pathway inhibitors that blocked platelet aggregation did not block release of sGPVI. Shedding of GPVI also did not require calcium flux or release of ADP and did not require platelet activation. In contrast, shear-induced loss of GPIbα was reported to be dependent on VWF concentration, PI3K activity, and functional αIIbβ3.8 GPVI ligands also induce activation-dependent shedding of GPVI that is inhibitable by PP1 and PP2 (Src family kinase inhibitors) or wortmannin and LY294002 (PI3K inhibitors). However, calmodulin inhibitors or thiol-modifying reagents induce activation-independent GPVI shedding. None of these triggers required active αIIbβ310; however, the time frame for treatment with GPVI ligands or calmodulin inhibitors to detect either sGPVI by ELISA or the 10-kDa GPVI remnant by Western blot is more than 10 minutes up to hours.
Significant shear-induced shedding of GPVI was detected after exposure of PRP to elevated shear for 1 minute (Figures 1 and 5), which may indicate that shear-induced GPVI shedding involves a mechanism where there is direct activation of the platelet ADAM by shear stress. Recently, we have shown that active Factor X can promote ADAM10-mediated shedding of GPVI independent of platelet activation.49 Shear may unfold GPVI into a conformer able to be cleaved by ADAM10, consistent with the “memory of shear” in which GPVI continues to be shed after the discontinuance of shear (Figures 1E and 5) and the presence of only mature ADAM10 on platelets (Figure 1G), an effect possibly elicited via shear-induced changes to actin and cytoskeletal networks,50 which are directly linked to GPIb-IX-V and coassociated GPVI.26 The finding that shear influences GPVI cleavage warrants further research into shear-dependent shedding of other ADAM substrates on platelets and other cell types in vivo.
In conclusion, pathologic shear stress was identified, for the first time, to induce GPVI shedding from washed platelets and PRP, and increased plasma sGPVI was linked with elevated intracoronary shear in patients with SAP. Shear-induced GPVI shedding did not require VWF interaction with GPIbα, αIIbβ3 integrin engagement, or platelet activation and was metalloproteinase-dependent, with ADAM10 playing an important role. Shear-induced GPVI shedding is an activation- and aggregation-independent mechanism that may down-regulate GPVI expression, potentially decreasing platelet reactivity to collagen under pathologic shear conditions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by IZKF Aachen of the RWTH Aachen (Germany), Science Foundation Ireland, and the National Health and Medical Research Council of Australia.
Authorship
Contribution: M.A.-T., C.W.T., J.Q., F.-T.M., J.R.H., J.J., J.F.A., A.S.C.Y., G.J.P., A.J., and E.E.G. performed the experiments; A.L. and A.K.D. provided essential reagents and intellectual input; J.R.H., S.P.J., L.K., and C.M.W. provided intellectual input; and M.A.-T., M.C.B., R.K.A., and E.E.G. conceived and designed the study and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elizabeth E. Gardiner, Australian Centre for Blood Diseases, Monash University, Alfred Medical Research and Education Precinct, 89 Commercial Road, Melbourne, VIC, 3004 Australia; e-mail: elizabeth.gardiner@monash.edu.
References
Author notes
M.A.-T., C.W.T., J.Q., R.K.A., and E.E.G. contributed equally to this study.