Abstract
Severe Plasmodium falciparum malaria evolves through the interplay among capillary sequestration of parasitized erythrocytes, deregulated inflammatory responses, and hemostasis dysfunction. After rupture, each parasitized erythrocyte releases not only infective merozoites, but also the digestive vacuole (DV), a membrane-bounded organelle containing the malaria pigment hemozoin. In the present study, we report that the intact organelle, but not isolated hemozoin, dually activates the alternative complement and the intrinsic clotting pathway. Procoagulant activity is destroyed by phospholipase C treatment, indicating a critical role of phospholipid head groups exposed at the DV surface. Intravenous injection of DVs caused alternative pathway complement consumption and provoked apathy and reduced nociceptive responses in rats. Ultrasonication destroyed complement-activating and procoagulant properties in vitro and rendered the DVs biologically inactive in vivo. Low-molecular-weight dextran sulfate blocked activation of both complement and coagulation and protected animals from the harmful effects of DV infusion. We surmise that in chronic malaria, complement activation by and opsonization of the DV may serve a useful function in directing hemozoin to phagocytic cells for safe disposal. However, when the waste disposal system of the host is overburdened, DVs may transform into a trigger of pathology and therefore represent a potential therapeutic target in severe malaria.
Introduction
Severe malaria evolves through the interplay among capillary sequestration of parasitized erythrocytes, a deregulated inflammatory response, and hemostasis dysfunction.1-5 The responsible molecular mechanisms remain the subject of debate, and the identification of the triggering events still constitutes a major open challenge in science. Seminal findings on complement activation in malaria were published in the 1970s. Complement turnover was shown to be triggered in human patients suffering from severe malaria,6 and experiments in monkeys demonstrated that complement consumption coincided with schizont rupture.7 Clinical data now show that substantial complement activation occurs in human patients8 and enhanced early complement activation in experimental human malaria has been demonstrated.9 Experiments in a murine model of cerebral malaria also suggest a pathogenic role of complement activation. C5 deficiency protects mice against cerebral malaria,10 in which dysregulation of the terminal complement sequence is apparently centrally involved11
Hemozoin, which is formed in the digestive vacuole (DV) of developing intra-erythrocytic parasites, has emerged as a possible trigger of inflammation. This assumption is primarily based on the fact that hematin, considered to represent the synthetic analog of hemozoin,12 provokes inflammatory responses in macrophages13,14 and activates the alternate complement pathway.15 Also suggestive is the finding that malarial pigment colocalizes with fibrin in tissues.16 However, when the erythrocytic schizonts rupture, the malarial pigment is still surrounded by the membrane of the DV and it is the organelle rather than free hemozoin that naturally gains contact with the host environment.17,18
We reported recently that when parasitized erythrocytes rupture in blood, the DVs are rapidly phagocytosed by polymorphonuclear granulocytes (PMNs).19 Phagocytosis only occurred in active serum and complement factor C3 was found attached to the surface of the organelle. It followed that the DV might directly activate complement, so experiments were performed to explore this possibility, leading to the novel findings reported herein. We show that the DV is endowed with the capacity to dually activate the alternative complement and the intrinsic clotting pathway. It is possible that efficient opsonization of the DV is initially beneficial because it enables the host to rapidly dispose of the waste product; however, transition to a pathologic state may occur as the disposal system is overrun. Unreigned activation of complement and coagulation may then contribute to events underlying the pathogenesis of severe malaria.
Methods
Serum
Banked human blood, group A Rh+ RBCs, and group A human serum were continuously supplied by the Transfusion Center of the University Medical Center in Mainz, Germany. Aliquots of human serum were stored at −20°C until use. Serum was heat inactivated for 30 minutes at 56°C for use in routine parasite cultures. Active serum was used as indicated.
Abs and reagents
Rabbit anti–human C3c Ab was from DakoCytomation and mAb clone 979/143 against C5b-9 neoantigen (purified IgG, 10 mg/mL) was from this laboratory.20 mAb 1E1 specific for MSP119 was a kind gift from A. Holder (Medical Research Council, London, United Kingdom).21,22 Alexa Fluor 594–conjugated goat anti–mouse IgG, donkey anti–rabbit IgG, Alexa Fluor 488–conjugated goat anti–mouse IgG, and 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester (BCECF-AM) were from Invitrogen. mAb against RBC band 3 protein, protease inhibitor L-trans-epoxy-succinyl-leucylamido- (4-guanidino)butane (E64), Hoechst 33342, and low-molecular-weight dextran sulfate (LMW-DXS; MW5000) were from Sigma-Aldrich.
Plasmodium falciparum culture and isolation of DVs and merozoites
P falciparum clone 3D7 was maintained as a synchronous continuous culture and DVs were isolated and banked in 50% glycerol at −20°C as described previously.19 DVs were enumerated using a hematocytometer (CELL-DYN Ruby; Abbott; Version 2.0ML software). Hemozoin was isolated as described previously,19 and heme was quantified by measuring absorbance at 405 nm as described previously.15
To isolate merozoites, cultures were tightly synchronized twice with a 4-hour lap using 5% sorbitol. Naturally liberated merozoites and DVs were isolated from the supernatants of enriched cultures. DVs were first selectively sedimented by centrifugation at 400g for 5 minutes, and the merozoites were then obtained by subsequent centrifugation at 2400g for 5 minutes in a tabletop centrifuge. Merozoites were washed 3 times with RPMI and stored frozen at −20°C.
Isolation of PEMSs
Enriched, late-stage parasitized RBC (pRBC) cultures were suspended to 0.2% hematocrit in culture medium containing 10% inactivated serum in the presence of 15μM E64 for approximately 8 hours as described previously.23 The culture was centrifuged at 250g for 5 minutes to remove intact cells, at 1600g for 5 minutes to remove intact late schizonts, and then at 2500g for 10 minutes to pellet the parasitophorous vacuole membrane-enclosed merozoite structures (PEMSs). The pellet was washed twice with RPMI and twice with PBS and used for the complement consumption experiments.
Complement consumption experiments
To measure consumption during pRBC rupture, enriched cultures of late-stage pRBCs were suspended in Veronal-buffered saline with 0.25mM Ca2+ and 0.8mM Mg2+ (Virion/Sirion) containing 20% active normal human serum (NHS) at 50% hematocrit. Cells were incubated at 37°C. At hourly intervals, small aliquots were collected and analyzed for hemolysis and complement consumption, which was detected by hemolytic assays using sensitized sheep erythrocytes and expressed as the percentage relative consumption as described previously.24 Background absorption derived from lysed, parasitized cells were determined in parallel controls using heat-inactivated serum and subtracted from absorption values of the consumption assay. Two-dimensional immunoelectrophoretic analysis of C3 conversion was performed as described previously.24 In 2 experiments, 10mM EGTA/2mM Mg2+ was added to prevent classic pathway activation, and complement turnover was assessed by C3 immunoelectrophoresis. Classic pathway of the serum used was in the normal range of 70-80 hemolytic units/mL.
For the measurement of consumption with isolated DVs, DVs were incubated with 10% or 20% NHS in the presence or absence of 10mM EGTA/2mM Mg2+. C3 conversion was analyzed in 20% NHS after 20 minutes, and consumption of hemolytic activity with either sensitized sheep erythrocytes or rabbit erythrocytes in 10% serum after 60 minutes at 37°C.
Staining procedures
Hoechst 33342 was used to stain DNA. BCECF-AM (10μM) was added to pRBC cultures or to isolated DVs for 30 minutes. Cells and DVs were then washed 3 times and fluorescence microscopy was performed immediately on air-dried, unfixed smears.
Staining of merozoites and DVs after schizont rupture
Tightly synchronized cultures containing 8%-10% late-stage pRBCs were enriched and diluted to 0.2% hematocrit in active serum without the addition of fresh RBCs to prevent reinvasion of merozoites. Cultures were closely monitored for hemolysis and, immediately after the majority of schizonts ruptured, were centrifuged at 150g for 5 minutes. The supernatants were centrifuged at 1900g for 10 minutes in a Sorvall RC2B centrifuge to sediment the merozoites and DVs. Pellets were resuspended in RPMI medium and centrifuged twice at 400g for 1 minute in a tabletop centrifuge to remove the RBCs. Merozoites and DVs were then sedimented at 2400g for 5 minutes. Pellets were resuspended in RPMI medium and thin smears were prepared on glass slides, air-dried, and fixed with methanol for 10 seconds or in 2% paraformaldehyde for 10 minutes at room temperature and stained with anti-C3c or anti–C5b-9 as described in the next paragraph.
Staining of isolated DVs
DVs were incubated with 10% active human serum for 1 hour at 37°C or with 10% inactive serum as a control. DVs were washed with PBS and thin smears were made on glass slides. After fixation, the slides were washed with PBS and then blocked in 3% BSA for 1 hour at room temperature. The slides were washed and incubated with rabbit polyclonal C3c Ab (1:250 dilution), monoclonal anti–human C5b-9 (1:250 dilution), monoclonal anti–human band 3 (1:1000 dilution), or monoclonal anti-MSP1 (1:100 dilution) in blocking buffer for 1 hour at room temperature. Nonimmune rabbit serum and isotype-matched mouse IgG were used as respective controls. After incubation, the slides were washed and then incubated with Alexa Fluor 594–conjugated goat anti–mouse IgG, donkey anti–rabbit IgG, or Alexa Fluor 488–conjugated goat anti–mouse IgG (Invitrogen), all at a 1:500 dilution for 1 hour at room temperature. Nuclei were stained with Hoechst 33342 for 5 minutes at room temperature. Samples were viewed in a fluorescence microscope (Axiovert 200M; Carl Zeiss; AxioVision Version 4.7 software) or an Axioskop 2 microscope (Carl Zeiss) at a 1000× magnification. Images were obtained using AxioVision software.
Western blotting
RBC ghosts were prepared by lysis of RBCs with ice-cold 5mM sodium phosphate buffer, pH 8.0. RBC ghosts, isolated merozoites, and DVs were subjected to 10% SDS-PAGE, transferred to nitrocellulose membranes, and probed with mouse anti-MSP1 (1:200) and mouse anti–band 3 Abs (1:5000). HRP-conjugated goat anti–rabbit Ab (New England Biolabs), goat anti–mouse or goat anti–human Abs (both Santa Cruz Biotechnology) were used at a dilution of 1:10 000 for detection, and bands were visualized by enhanced chemiluminescence (Roche).
Flow cytometry
DVs were incubated in 10% NHS for 1 hour at 37°C and washed 3 times with PBS. After incubation in 3% BSA/PBS for 30 minutes, DVs were stained for C5b-9 as described in “Staining of isolated DVs” and analyzed in a FACS Scanflow cytometer (BD Biosciences). Controls consisted of identical DV preparations treated with heat-inactivated serum.
Determination of clotting times in recalcified plasma
Citrated blood was centrifuged and the cell-free plasma was diluted with 1 volume of saline. DVs were incubated with plasma for 1 minute at 37°C, and reactions were initiated with the addition of 10mM CaCl2+. Clotting times were measured in a coagulometer (KC10A micro; Amelung).
Prothrombinase assay
Assessment of thrombin formation was performed in a 2-step amidolytic substrate assay.25 The DVs were preincubated in a solution containing 150mM NaCl, 50mM Tris (pH 7.4), and 0.1% BSA with 1nM factor Va, 6.2 pM factor Xa, and 2mM CaCl2 at 25°C for 5 minutes. The reaction was initiated by the addition of 1μM prothrombin and incubated for 5 minutes at 25°C. The reaction was stopped by the addition of EDTA to a 10mM final concentration. The DVs were removed by centrifugation, and thrombin formation was assessed at 405 nm immediately after the addition of the chromogenic substrate S-2238 (0.1mM; Chromogenix Instrumentation Laboratory) in a kinetic microplate spectrophotometer (Multiskan RC; Labsystems) with Genesis Life Version 3.0 full mode software.
Inhibition assays with LMW-DXS
LMW-DXS (MW5000) was used at the given concentrations in complement consumption and coagulation assays. For in vivo experiments, 6 mg of LMW-DXS in PBS was injected intraperitoneally 45 minutes before injection of 5 × 109 DVs.
Animal experiments
Male Sprague-Dawley outbred rats [Crl:CD(SP)] were purchased from Charles River Laboratories and introduced into experiments at a body weight of 60-70 g. IV injections (300-350 μL) were performed after catheterization (24 G) of the lateral tail vein. All experimental protocols were approved by the responsible authority (Landesuntersuchungsamt Koblenz, approval number 23 177-07/G 10-1-034) and conducted according to European Union guidelines for the care and use of laboratory animals. Three video clips showing the behavioral reactions of the animals can be found in supplemental Videos (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
The hot-plate test
The hot-plate test was adapted from the method of Woolfe and McDonald.26 In brief, a rat is introduced into an open-ended plexiglas cylinder (18-cm diameter) to confine the animal to the heated surface of the hot-plate apparatus. The plate is adjusted to 56°C. When the pain threshold is reached, the rat starts to react by licking the fore or hind paws. Normal reaction times are in the range of 10 seconds. Reaction times are recorded with a maximum cutoff of 30 seconds to avoid tissue damage.
Statistical analysis
The assumptions for normality and equal variance were verified with SigmaStat Version 3.1 software (SYSSTAT). Depending on the experiment, either repeated measures ANOVA or 1-way ANOVA was followed by the Bonferroni t test. All results represent means ± SD of at least 3 independent experiments, if not indicated otherwise. P < .05 was considered to be statistically significant.
Results
Complement activation occurs on the surface of DVs but not on merozoites
In vitro culture of P falciparum was undertaken conventionally and late-stage pRBCs were enriched (Figure 1A Giemsa stain). BCECF-AM, a membrane-diffusible acetylated carboxyfluorescein derivative, becomes entrapped intracellularly after its cleavage by cytoplasmic esterases that are absent in RBCs but present in nucleated cells and DVs. Entrapment occurs only when an intact biologic membrane is present to limit its egress from the respective compartment. We found that merozoites and DVs could both be stained and were then clearly distinguishable from each other (Figure 1B). As was to be expected, hemozoin characterized the DVs, whereas Hoechst 33342 stained merozoites but not the DVs (Figure 1C). DVs and merozoites could therefore be easily distinguished. More importantly, intactness of the DV membrane, simply probed with the fluorescent dye, would subsequently emerge as the decisive element underlying the singular biologic properties of the DVs reported herein.
Differential staining of merozoites and DVs. (A) Giemsa stain of late-stage pRBCs undergoing schizont rupture. (B) Vital stain of the same culture with BCECF-AM showing intense staining of DVs (large arrow) alongside staining of merozoites (small arrow). (C) Same culture stained with Hoechst 33342, which detects merozoites (small arrow) but spares the DVs (large arrow). (D) Giemsa stain of isolated DVs. Insert shows the recording of analysis in a hemocytometer with DVs presenting as particles with the size of platelets (PLT). (E) Staining of isolated DVs with BCECF. (F) No staining with Hoechst 33342. (G) Phase-contrast image of DVs to which RBCs were added. (H) Same smear fluorescently stained for RBC band 3 protein. (I) Giemsa stain of purified merozoite preparation used in Western blots. (J) Western blots of RBC membranes, merozoites, and DVs probed with Abs against RBC band 3 protein and MSP1 (K). DV preparations contained very scant contaminations with MSP1. Arrows depict the positions of the respective molecular weight markers. Scale bars indicate 10 μm.
Differential staining of merozoites and DVs. (A) Giemsa stain of late-stage pRBCs undergoing schizont rupture. (B) Vital stain of the same culture with BCECF-AM showing intense staining of DVs (large arrow) alongside staining of merozoites (small arrow). (C) Same culture stained with Hoechst 33342, which detects merozoites (small arrow) but spares the DVs (large arrow). (D) Giemsa stain of isolated DVs. Insert shows the recording of analysis in a hemocytometer with DVs presenting as particles with the size of platelets (PLT). (E) Staining of isolated DVs with BCECF. (F) No staining with Hoechst 33342. (G) Phase-contrast image of DVs to which RBCs were added. (H) Same smear fluorescently stained for RBC band 3 protein. (I) Giemsa stain of purified merozoite preparation used in Western blots. (J) Western blots of RBC membranes, merozoites, and DVs probed with Abs against RBC band 3 protein and MSP1 (K). DV preparations contained very scant contaminations with MSP1. Arrows depict the positions of the respective molecular weight markers. Scale bars indicate 10 μm.
Naturally liberated DVs were isolated from supernatants of hemolyzed pRBCs. As expected from a previous study,27 they presented as platelet-sized particles that could be enumerated in a hematocytometer (Figure 1D). Staining with BCECF-AM could still be undertaken after their isolation and storage at −20°C in glycerol (Figure 1E), attesting to the remarkable stability of their membranes. Staining for DNA was negative (Figure 1F). To exclude potential contaminations of the DV preparation with either erythrocyte or merozoite fragments, the presence of the RBC anion transporter (band 3) and the merozoite surface protein 1 (MSP1) was analyzed. The band 3 protein was not detectable by immunofluorescence on the DV surface (Figure 1G). Instead, staining could only be observed on erythrocytes that were artificially added as controls (Figure 1H). This finding was confirmed by immunoblot analysis demonstrating the lack of band 3 on DVs and purified merozoites (Figure 1I-J). The presence of MSP1 was examined by Western blots of purified merozoites and DVs. The latter carry the C-terminal 19-kDa fragment of MSP1 but not the full-length protein,28 which was readily detectable on merozoites but virtually absent in the DV preparations (Figure 1K).
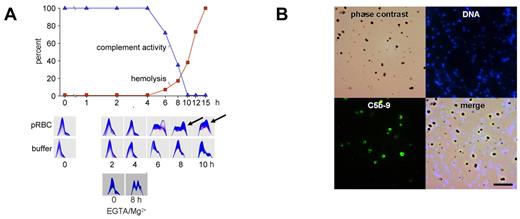
To determine whether complement activation would occur after schizont rupture, late-stage pRBCs were allowed to lyse in the presence of active, nonimmune serum, and complement activity, C3 turnover, and pRBC hemolysis were recorded (Figure 2A). No changes occurred before schizont rupture, but the onset of hemolysis was accompanied by C3 turnover and a decrease in complement activity. These findings are in agreement with the early observations of Glew et al that complement consumption coincided with schizont rupture in infected monkeys.7
Complement activation occurs to completion on the surface of DVs but not on merozoites. (A) Late-stage pRBCs were cultured and allowed to rupture in the presence of 10% active human serum. Complement activity and hemolysis were recorded over time and found to be inversely correlated. Two-dimensional immunoelectrophoresis confirmed that C3 turnover occurred at the onset of hemolysis concomitant to the fall in complement activity (6 hours). Buffer controls (in the absence of pRBCs) are shown in the second row of panels. The bottom 2 plates show C3-immunoelectrophoresis at 0 and 8 hours from an experiment conducted with pRBCs in the presence of 10mM EGTA/2mM Mg2+. First-dimension electrophoresis is shown left to right and second-dimension immunoelectrophoresis bottom to top. (B) Synchronized late-stage pRBCs were allowed to rupture in active human serum, whereafter unlysed cells were pelleted and merozoites and DVs were harvested from the supernatants and stained for DNA or C5b-9. Top left is phase-contrast microscopy; top right, Hoechst 33342 DNA stain; bottom left, complement C5b-9 complex; bottom right, merge. Note selective staining of DVs for C5b-9.
Complement activation occurs to completion on the surface of DVs but not on merozoites. (A) Late-stage pRBCs were cultured and allowed to rupture in the presence of 10% active human serum. Complement activity and hemolysis were recorded over time and found to be inversely correlated. Two-dimensional immunoelectrophoresis confirmed that C3 turnover occurred at the onset of hemolysis concomitant to the fall in complement activity (6 hours). Buffer controls (in the absence of pRBCs) are shown in the second row of panels. The bottom 2 plates show C3-immunoelectrophoresis at 0 and 8 hours from an experiment conducted with pRBCs in the presence of 10mM EGTA/2mM Mg2+. First-dimension electrophoresis is shown left to right and second-dimension immunoelectrophoresis bottom to top. (B) Synchronized late-stage pRBCs were allowed to rupture in active human serum, whereafter unlysed cells were pelleted and merozoites and DVs were harvested from the supernatants and stained for DNA or C5b-9. Top left is phase-contrast microscopy; top right, Hoechst 33342 DNA stain; bottom left, complement C5b-9 complex; bottom right, merge. Note selective staining of DVs for C5b-9.
To identify the activation pathway, schizont rupture was analyzed in the presence of 10mM EGTA/2mM Mg2+, which prevents classic pathway activation. As shown in Figure 2A, complement activation indeed occurred and could thus be attributed to the alternative pathway (Figure 2A bottom panels). At the same time, these experiments showed that Ca2+ was dispensable at the final stage of parasite development and was released from the RBCs. Because the alternative complement sequence is not triggered by soluble molecules, merozoites and DVs were examined for the presence of assembled C5b-9, the prime marker of membrane-associated complement activation.20,29 Positive stainings were observed on the DVs but never on merozoites (Figure 2B). The DV membrane was thus identified as the critical site of complement activation. To exclude any additional requirement of soluble activators, experiments were conducted with isolated, washed DVs. Figure 3A shows a dose-response experiment with matching data for complement consumption and C3 turnover in human serum. Decreases in total complement activity with concomitant C3 turnover were already provoked by low numbers (approximately 107/mL) of DVs. Activation occurred in the presence or absence of EGTA/Mg2+. The depicted panels are from an experiment with EGTA/Mg. When the fluid phase SC5b-9 was measured in the supernatants, a bell-shaped dose-response curve was observed that indicated a shift to the membrane-bound state as the number of DVs increased. A representative example is shown in Figure 3B. All DVs stained positively for C3 (Figure 3C) and C5b-9 (Figure 3D). The latter finding was also confirmed by FACS analysis, which demonstrated impressively that all DVs stained positive for the terminal complement complex (Figure 3D bottom panel).
Isolated DVs activate the alternative complement pathway. (A) Addition of isolated DVs to serum in the presence of EGTA/Mg dose-dependently provoked complement consumption (as assessed with rabbit erythrocytes) and C3 turnover. (B) Concentration of fluid-phase SC5b-9 in serum spiked with increasing numbers of isolated DVs. (C) Staining of C3 on isolated DVs after incubation with serum. Top row shows the controls, which were incubated in inactive serum; bottom row, cells incubated in active serum. (D) Detection of C5b-9 on DVs after incubation with active human serum. Corresponding flow cytometric analysis shows staining of all DVs in the sample. Representative results of 4 independent experiments are shown.
Isolated DVs activate the alternative complement pathway. (A) Addition of isolated DVs to serum in the presence of EGTA/Mg dose-dependently provoked complement consumption (as assessed with rabbit erythrocytes) and C3 turnover. (B) Concentration of fluid-phase SC5b-9 in serum spiked with increasing numbers of isolated DVs. (C) Staining of C3 on isolated DVs after incubation with serum. Top row shows the controls, which were incubated in inactive serum; bottom row, cells incubated in active serum. (D) Detection of C5b-9 on DVs after incubation with active human serum. Corresponding flow cytometric analysis shows staining of all DVs in the sample. Representative results of 4 independent experiments are shown.
Contact of the intact DV membrane with plasma is required for complement activation
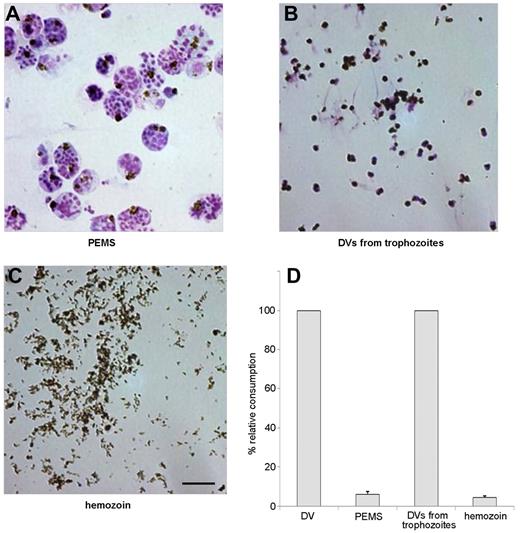
To discern whether direct contact of the DVs with plasma was required for complement activation, intact parasitophorous vacuoles and purified hemozoin were compared for their complement-activating capacity with DVs prepared from naturally ruptured pRBCs or from trophozoites. Parasitophorous vacuoles were prepared by permitting late-stage parasitized RBCs to lyse in the presence of the protease inhibitor E64.23 When the released PEMSs (Figure 4A) were tested, they were found to be devoid of complement-activating properties (Figure 4D). Control experiments showed that E64 itself did not suppress complement activation by isolated DVs. To determine whether activation required the presence of an intact DV membrane, DVs were isolated from hemolysis supernatants or from trophozoite-stage infected RBCs.27,30 The latter were prepared by saponin-lysis of late-stage infected RBCs and, as known from previous studies,27,30 such preparations contained some contaminating RBC-membrane debris (Figure 4B). Both preparations had similar complement-activating properties that were lost after sonication, and this finding was correlated with the detectability of C3 and C5b-9 exclusively on the intact DV surface but never on any surrounding membrane debris (data not shown). Centrifugation of the sonicates through Percoll led to retrieval of purified dispersed hemozoin crystals (Figure 4C), which were also devoid of complement-activating properties when tested at concentrations of 10-1000 μg of heme/mL (Figure 4D). Therefore, activation of complement required direct contact of intact DV membranes with serum and could not be mimicked by isolated hemozoin, which was also found to be devoid of procoagulant activity (data not shown). The fact that unfractionated, sonicated lysates had no activity reiterated that the activating effects could not have derived from protein or DNA contaminants in the DV preparations.
Preparations of PEMSs, intact DVs, and free hemozoin. (A) Merozoites and DVs encased in the parasitophorous vacuole from pRBCs after rupture in the presence of the protease inhibitor E64, which inhibits lysis of the parasitophorous vacuole membrane but not lysis of the erythrocyte membrane. (B) DVs isolated from trophozoites. (C) Hemozoin crystals isolated from sonicated DVs by centrifugation in Percoll. Scale bar indicates 5 μm. (D) Complement consumption tests were performed with materials in panels A through C using 10% NHS. DVs were used as a positive control and similar concentrations of heme were used throughout. Neither PEMSs nor isolated hemozoin had complement-activating capacity. One representative assay of 3 independent experiments is shown, with SD from triplicate determinations.
Preparations of PEMSs, intact DVs, and free hemozoin. (A) Merozoites and DVs encased in the parasitophorous vacuole from pRBCs after rupture in the presence of the protease inhibitor E64, which inhibits lysis of the parasitophorous vacuole membrane but not lysis of the erythrocyte membrane. (B) DVs isolated from trophozoites. (C) Hemozoin crystals isolated from sonicated DVs by centrifugation in Percoll. Scale bar indicates 5 μm. (D) Complement consumption tests were performed with materials in panels A through C using 10% NHS. DVs were used as a positive control and similar concentrations of heme were used throughout. Neither PEMSs nor isolated hemozoin had complement-activating capacity. One representative assay of 3 independent experiments is shown, with SD from triplicate determinations.
DVs activate the intrinsic clotting pathway
Several mechanisms involving the extrinsic clotting pathway have mainly been discussed as the cause of hemostasis dysfunction in malaria patients.3-5 However, intrinsic pathway activation also appears to be involved31 and late-stage pRBCs reportedly support the assembly of multimeric coagulation complexes.32 In the present study, we have demonstrated that the DV also has direct procoagulant activity.
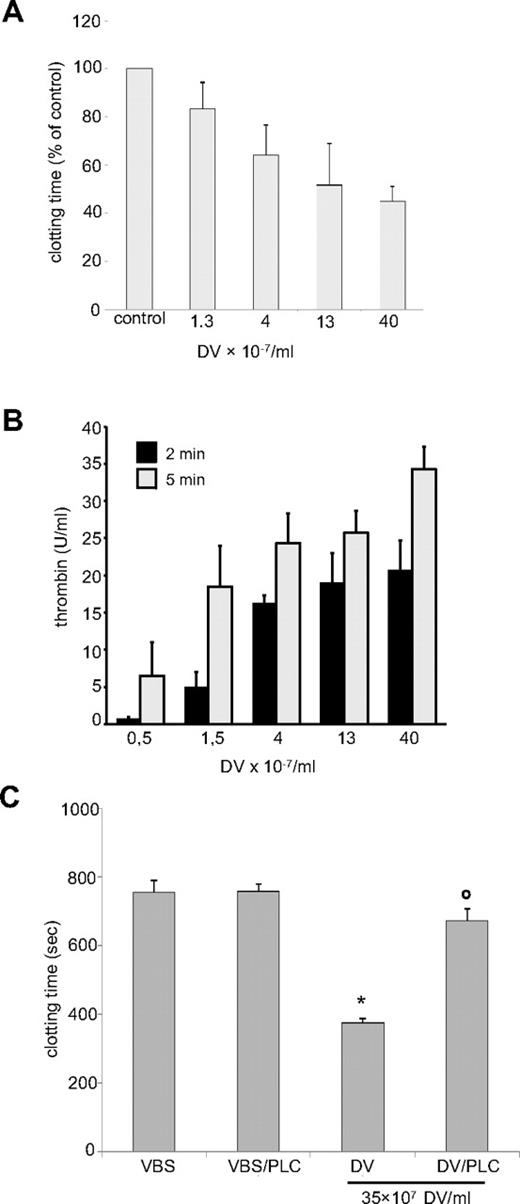
We tested the intrinsic clotting pathway routinely by determination of activated partial prothrombin time, in which an empiric mixture of activators is added to citrated plasma and clotting times after recalcification are read. Long clotting times (> 400-1000 seconds) were observed if the activators were omitted. When DVs were used instead of the activators, dose-dependent shortening of clotting times was observed (Figure 5A). These results were corroborated in prothrombinase assays using isolated factors FVa, FII, and FXa. In the conventional test, phospholipids are added to provide platforms for Ca2+-dependent assembly of the prothrombinase complex FVa/FXa. In the present study, the phospholipids were omitted and replaced by DVs, which dose-dependently provoked thrombin formation (Figure 5B). Therefore, the DVs could directly assemble the key convertase of the clotting pathway and, as with complement activation, this required only low numbers of DVs corresponding to less than 1% hematocrit. Prothrombinase assembly is generally mediated via Ca2+-bridged interactions with phospholipid head groups. When DVs were treated with phospholipase C, procoagulant activity was indeed destroyed (Figure 5C), whereas complement-activating capacity remained intact (not shown).
DVs directly activate the intrinsic clotting pathway. (A) Clotting time after recalcification of 50% plasma was accelerated significantly in the presence of DVs. Clotting times of the respective buffer controls were taken as the 100% reference in each experiment. Data are expressed as means ± SEM of 5 independent experiments. (B) Isolated DVs dose-dependently enhanced thrombin generation in the prothrombinase assay (n = 4 ± SEM). Controls without DVs did not induce any thrombin generation and are not shown. (C) Procoagulant activity of DVs is sensitive to phospholipase C treatment. Clotting times of 50% citrated plasma were determined after recalcification in the presence of Veronal-buffered saline, Veronal-buffered saline plus PLC, DVs, or DVs after PLC treatment. Clotting time was significantly accelerated in the presence of DVs (*P < .05 vs control). This effect was abolished after PLC treatment (oP < .05). Data are expressed as means ± SD of 3 independent experiments.
DVs directly activate the intrinsic clotting pathway. (A) Clotting time after recalcification of 50% plasma was accelerated significantly in the presence of DVs. Clotting times of the respective buffer controls were taken as the 100% reference in each experiment. Data are expressed as means ± SEM of 5 independent experiments. (B) Isolated DVs dose-dependently enhanced thrombin generation in the prothrombinase assay (n = 4 ± SEM). Controls without DVs did not induce any thrombin generation and are not shown. (C) Procoagulant activity of DVs is sensitive to phospholipase C treatment. Clotting times of 50% citrated plasma were determined after recalcification in the presence of Veronal-buffered saline, Veronal-buffered saline plus PLC, DVs, or DVs after PLC treatment. Clotting time was significantly accelerated in the presence of DVs (*P < .05 vs control). This effect was abolished after PLC treatment (oP < .05). Data are expressed as means ± SD of 3 independent experiments.
LMW-DXS suppresses the activation of complement and coagulation by the DV
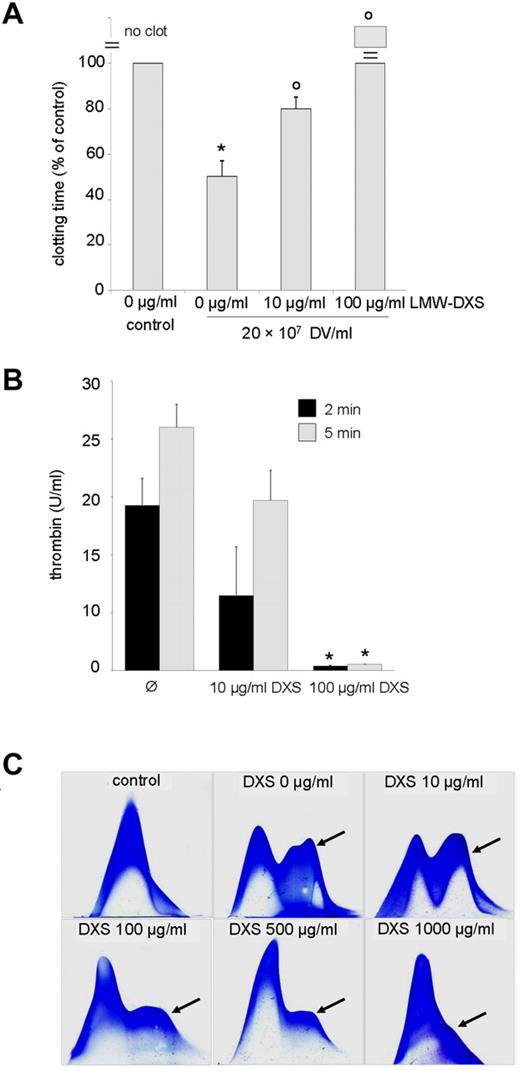
LMW-DXS blocks both the complement33 and coagulation cascade34,35 at micromolar concentrations that, in contrast to heparin, do not cause bleeding complications and are well tolerated in humans.36,37 If DV-induced activation of complement and clotting should indeed contribute to malaria pathogenesis, the existence of a well-established inhibitor could be of interest. Therefore, we tested the effect of LMW-DXS on DV effects. LMW-DXS at concentrations of 10-100 μg/mL effectively inhibited DV-dependent activation of the intrinsic clotting pathway (Figure 6A) and abrogated the assembly of prothrombinase complexes (Figure 6B). In agreement with a previous study,36 somewhat higher concentrations were required to block complement activation, but these effects were also apparent at a 100 μg/mL concentration (Figure 6C).
LMW-DXS abrogates procoagulant and complement triggering action of DVs. (A) Clotting times were determined after recalcification of 50% citrated plasma in the absence (control) or presence of DVs and LMW-DXS at the depicted concentrations. DVs provoked a significant reduction in clotting time (*P < .05 vs control), which was significantly reversed by 10 μg/mL of LMW-DXS (oP < .05). LMW-DXS (100 μg/mL) completely prevented clot formation despite the presence of DVs. Data are expressed as means ± SD of 3 independent experiments. (B) LMW-DXS abrogates prothrombinase assembly on DVs. Prothrombinase assays were performed in the presence of 5 × 107 DVs/mL and in the absence or presence of LMW-DXS at the given concentrations, and thrombin generation was determined after 2 and 5 minutes (n = 3 ± SD). DXS (100 μg/mL) abolished prothrombinase assembly (*P < .05 vs control). (C) Inhibition of DV-dependent C3 turnover by 100-1000 μg/mL of LMW-DXS. In the control (top left panel), 20% NHS was incubated for 30 minutes at 37°C. DVs/mL (108) were added to 20% NHS in the absence or presence of LMW-DXS at the depicted concentrations. Inhibition of C3 turnover (arrows) was observed at 100 μg/mL of LMW-DXS. Results shown are representative of 3 independent experiments.
LMW-DXS abrogates procoagulant and complement triggering action of DVs. (A) Clotting times were determined after recalcification of 50% citrated plasma in the absence (control) or presence of DVs and LMW-DXS at the depicted concentrations. DVs provoked a significant reduction in clotting time (*P < .05 vs control), which was significantly reversed by 10 μg/mL of LMW-DXS (oP < .05). LMW-DXS (100 μg/mL) completely prevented clot formation despite the presence of DVs. Data are expressed as means ± SD of 3 independent experiments. (B) LMW-DXS abrogates prothrombinase assembly on DVs. Prothrombinase assays were performed in the presence of 5 × 107 DVs/mL and in the absence or presence of LMW-DXS at the given concentrations, and thrombin generation was determined after 2 and 5 minutes (n = 3 ± SD). DXS (100 μg/mL) abolished prothrombinase assembly (*P < .05 vs control). (C) Inhibition of DV-dependent C3 turnover by 100-1000 μg/mL of LMW-DXS. In the control (top left panel), 20% NHS was incubated for 30 minutes at 37°C. DVs/mL (108) were added to 20% NHS in the absence or presence of LMW-DXS at the depicted concentrations. Inhibition of C3 turnover (arrows) was observed at 100 μg/mL of LMW-DXS. Results shown are representative of 3 independent experiments.
DVs activate the alternative complement pathway and produce clinical symptoms in rats
Animal experiments were performed to obtain an indication of whether the DVs also activated complement in vivo and might contribute to the development of clinical symptoms. These experiments were performed in rats because alternative complement pathway activity is extremely low in mice. Alternative pathway activity was determined using rabbit erythrocytes as targets. The first group of animals received 4-5 × 109 DVs injected into the tail vein. Nociceptive responses were determined with the hot-plate test and behavioral reactions were filmed. The acute onset of clinical symptoms was observed in all cases, commencing 1-2 minutes after injection. Animals became lethargic, exhibited diminished reactions to tactic and acoustic stimuli, and nociceptive responses were retarded (Figure 7A and supplemental Video 1). Alternative complement pathway activity was reduced by > 85% in all animals (n = 5). The second group of animals received the same batch and dose of DVs but that had been sonicated before application. Alternative complement pathway activity remained unchanged in the serum of these animals, which also developed no clinical symptoms (n = 3; Figure 7A and supplemental Video 2). The third group of animals received 6 mg of LMW-DXS IP, which led to a > 65% reduction in alternative pathway complement activity in plasma after 45-60 minutes. Remarkably, all animals (n = 4) were fully protected from the detrimental effects of DV infusion (Figure 7A and supplemental Video 3).
Effect of IV injection of isolated DVs in rats. (A) Reduction of nociceptive responses. The hot-plate test was used, in which rats were placed in jars warmed to 56°C and latency periods until licking of the paws were measured. Results are depicted as the quotient of reaction times measured after DV infusion to reaction times determined before infusion in each animal. One group of animals received a bolus injection of 5 × 109 DVs (n = 5). The second group received an injection of 5 × 109 sonicated DVs (n = 3). The third group received 6 mg of LMW-DXS IP 45 minutes before injection of 5 × 109 DVs (n = 4). All experiments were performed with the same banked DV pool. DVs provoked a significant increase in latency time (*P < .05 vs control), which was significantly decreased in the presence of LMW-DXS (oP < .05). Bars represent the mean values with SD. (B-C) Rapid cellular uptake of DVs by mononuclear cells. Paraffin-embedded sections of spleen (B) and lung (C) were stained with H&E. (B) Intracellular accumulation of DVs in the marginal zone (asterisk) containing abundant macrophages (left panel; magnification 252×). This pattern is illustrated in the right panel after polarization at lower magnification (63×). (C) Intravascular accumulation of PMNs (left panel, arrowhead; magnification 252×) containing intracellular DVs (right panel, arrowhead; magnification 630×).
Effect of IV injection of isolated DVs in rats. (A) Reduction of nociceptive responses. The hot-plate test was used, in which rats were placed in jars warmed to 56°C and latency periods until licking of the paws were measured. Results are depicted as the quotient of reaction times measured after DV infusion to reaction times determined before infusion in each animal. One group of animals received a bolus injection of 5 × 109 DVs (n = 5). The second group received an injection of 5 × 109 sonicated DVs (n = 3). The third group received 6 mg of LMW-DXS IP 45 minutes before injection of 5 × 109 DVs (n = 4). All experiments were performed with the same banked DV pool. DVs provoked a significant increase in latency time (*P < .05 vs control), which was significantly decreased in the presence of LMW-DXS (oP < .05). Bars represent the mean values with SD. (B-C) Rapid cellular uptake of DVs by mononuclear cells. Paraffin-embedded sections of spleen (B) and lung (C) were stained with H&E. (B) Intracellular accumulation of DVs in the marginal zone (asterisk) containing abundant macrophages (left panel; magnification 252×). This pattern is illustrated in the right panel after polarization at lower magnification (63×). (C) Intravascular accumulation of PMNs (left panel, arrowhead; magnification 252×) containing intracellular DVs (right panel, arrowhead; magnification 630×).
Rapid cellular uptake of DVs by mononuclear cells in rats after IV injection
To trace the fate of the DVs, rats were killed after 4-6 hours. DVs and PMNs were rarely observed in blood smears (not shown). Paraffin-embedded sections of spleen and lung were investigated microscopically. Staining with H&E revealed intracellular accumulation of DVs in the marginal zone of the spleen containing abundant macrophages (Figure 7B left panel). This pattern became very impressive after polarization at a lower magnification (Figure 7B right panel); likewise, intravascular accumulation of mononuclear cells with myriad intracellular DVs could be observed in the lungs (Figure 7C). These data demonstrated that free DVs are rapidly taken up by PMNs.
Discussion
Although complement activation and coagulation defects during P falciparum malaria have been recognized for decades, no single entity of parasite origin has yet been identified that might be involved directly in triggering these events. Rupture of each P falciparum parasitized erythrocyte is accompanied by release of one DV into the circulation. However, whereas the effects of DV uptake on macrophage function are under study, no significance has yet been attached to their presence in the circulation. The present study is the first to reveal the DV's capacity to dually activate complement and coagulation. In severe malaria, parasitemia levels of several percent develop corresponding to ≥ 108 cells/mL of blood, and capillary sequestration further heightens the local load of DVs. Activation of both complement and coagulation became detectable at concentrations of approximately 107 DVs/mL and displayed simple dose dependency with no prozone effects (Figure 3A and Figure 5A-B). Therefore, increases in the load of DVs as occurs at sites of pRBC sequestration would be expected to simply augment activation of both enzyme cascades. Provocation of clinical symptoms will naturally depend on myriad local factors of the microenvironment such as the presence or absence of regulatory factors such as tissue factor and thrombomodulin.1-5 Transactivation events occurring among the coagulation system, complement, platelets, and blood cells may then pave the way to devastating disease.
The present study originated from the observation that rupture of pRBCs in active serum led to C3 conversion and to binding of C3b and C5b-9 to the DVs, with conspicuous sparing of merozoites. The results were reproduced with isolated DVs, and attachment of C5b-9 indicated its presence in membrane-bound form. Direct contact between the DV membrane and serum was required for activation to take place, and PEMSs, in which DVs remained encased within the parasitophorous vacuole membrane, were without effect. DVs isolated via saponin lysis of late-stage infected RBCs also activated complement. These preparations contained contaminating membrane material that did not stain positively for C3 or C5b-9. The findings do not entirely exclude the possibility that other membranes or organelles may also have complement-activating properties. However, these remain to be identified. Quite remarkably, isolated hemozoin activated neither complement nor coagulation. This was somewhat unexpected because hematin, which is considered to represent the synthetic analog of hemozoin, activates the alternative complement pathway, albeit at very high concentrations.15 The possibility that the biologic properties of hematin and hemozoin may not be identical merits close attention in future studies.
Disruption of the DV membrane destroyed both complement-activating and procoagulant properties. Therefore, our findings led to the question of what particular membrane characteristics enabled the DV but not the merozoite to activate both cascades. Information on the composition and organization of the DV membrane is currently not available. Such analyses are impeded by the fact that entities other than hemozoin are encased within the DV, including lipid bodies of poorly defined composition.38 However, we found that procoagulant activity was selectively destroyed by phospholipase C treatment and must thus be borne by phospholipid head groups. This finding is not surprising because multimolecular assembly of clotting enzymes is generally promoted by Ca2+-bridged interactions with negatively charged phospholipid head groups. It is very likely that, like other activating surfaces, exposed phospholipid head groups play essential roles. Further studies are needed to identify the responsible moieties, but the key recognition remains that an intact membrane is required for the DV to unfold both its complement-activating and procoagulant properties. The latter are intrinsically borne and not dependent on any interaction with platelets. Clotting was triggered in platelet-free plasma, and thrombin could be generated directly by incubating purified DVs with FXa and prothrombin.
Why complement activation in nonimmune serum occurs exclusively on the DV remains to to be clarified. Glycophosphatidylinositol-anchored proteins, including complement inhibitors,39,40 have been shown to shuttle from the RBC to the parasitophorous vacuole membrane,41-44 and the possibility is being examined whether they are further recruited to the merozoite surface to shield the parasite from complement attack. Regardless, the DV might serve as a decoy for central host defense elements. Indeed, we have found that complement activation marks the DV for selective phagocytosis by neutrophil granulocytes, whereas merozoites are left free to reinvade cells. This situation was found to persist in the presence of serum from malaria patients.19 It is possible that alternative pathway activation plays a first role in mediating selective opsonization of DVs in nonimmune serum. As specific Abs appear, classic pathway activation may be triggered on both the DVs and merozoites. The density of activated complement components will consequently remain higher on the DV, thereby sustaining its preferential phagocytosis.
When DVs were injected into rat tail veins, complement consumption occurred within minutes and the animals became lethargic and behavioral responses were impaired. The clinical symptoms possibly derived from systemic activation of endothelial and phagocytic cells with the release of inflammatory molecules and mediators, which would be expected to occur after triggering of the clotting5 and alternative complement pathway.45 The effects of bolus DV infusions were transient, reminiscent of lipopolysaccharide injection, which also provokes systemic inflammatory responses and complement activation.46 Termination of the reaction to DVs might be explained by their rapid clearance by phagocytic cells. Indeed, PMNs were rarely seen in the bloodstream of the animals, and the massive uptake of DVs by tissue phagocytes was impressive. At the onset of malarial infections, the low load of DVs is perhaps first cleared by PMNs; then, as the clearance capacity is overrun, tissue macrophages may come into play. Malaria pigment is present in these cells in human patients,16,47 and our finding that isolated DVs reside in tissue macrophages within hours after IV application also agrees with this concept. Dysfunction of these cells occurring after DV uptake has been reported in several studies,14,48-50 and likely also contributes to the pathogenesis of disease.
A major challenge facing the DV-complement activation theory is the fact that high parasite loads develop in many patients, particularly in Africa and the Pacific, without provoking severe symptoms. Only 2 speculations for this have been advanced at this point: (1) the dynamics of DV release and removal may be important and the efficacy of phagocyte uptake of DVs is possibly subject to wide interindividual variations, and (2) genetic deficiencies in late complement components occur worldwide with varying and sometimes surprisingly high frequency.51 Heterozygotes are not prone to suffer from bacterial infections because serum complement activity is only lowered, but perhaps these individuals are protected against the effects of complement overactivation that lead to severe malaria.
If the major tenets of our hypothesis turn out to be correct, strategies to inhibit DV-dependent activation of complement and coagulation might have therapeutic potential. Activation of both cascades was found to be inhibited by LMW-DXS. In contrast to heparin, LMW-DXS does not cause bleeding complications and is well tolerated in humans. Indeed, the agent caused no side effects in rats but protected the animals from the harmful effects of very high doses of DV infusion. The agent was applied at the same concentrations that have been used previously in transplantation models.36 LMW-DXS has also been reported to suppress merozoite re-invasion52 and may therefore simultaneously fulfill dual beneficial functions.
It is intriguing that, having served its physiologic purpose in the lifecycle of the parasite, the DV should be endowed with the capacity to activate the 2 archaic enzyme cascade systems of the blood into which it is cast. Perhaps these events initially serve a protective function by enabling the infected host to rapidly remove alien waste material. However, pathologic consequences may evolve when the disposal machinery suffers overload, with these events then contributing to the evolution of severe malaria. Future studies should determine whether a novel determinant of parasite pathogenicity has been discovered that might also be targeted for therapy in patients suffering from what remains one of the most prevalent life-threatening diseases in the world.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Anthony Holder for the kind gift of anti-MSP1 Abs; Johannes Müthing for valuable advice on the detection of sialylated glycans; Walter Hitzler and Roland Conradi for continued supply of erythrocytes, banked human blood, and human sera; Antje Canisius for excellent technical assistance; and Monika Wiedmann for outstanding secretarial work.
This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 490 to S. Bhakdi; Sonderforschungsbereich 593 to K.L. and S. Baumeister; and CRC877 to K. Reiss); the Cluster of Excellence “Inflammation at Interfaces” (to K. Reiss); and the Thai Infectious Disease Network (to P.D., S. Bhakdi, and S.C.B).
Authorship
Contribution: P.D., S.D.H., A.F., A-L.Z., and S. Bhakdi performed the laboratory experiments; M.B., K. Reifenberg, and C.O. performed the animal experiments; M.T. and C.O. performed the immunohistochemistry work; S. Bhakdi and S.C.B. conceived of the project; P.D., S. Bhakdi, K.L., and K. Reiss designed the research; P.D., S. Baumeister, K.L., R.U., K. Reiss, and S. Bhakdi analyzed the data; and S. Bhakdi wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sucharit Bhakdi, Department of Medical Microbiology and Hygiene, University Medical Center, Johannes Gutenberg University, Hochhaus Augustusplatz, 55202 Mainz, Germany; e-mail: sbhakdi@uni-mainz.de.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal