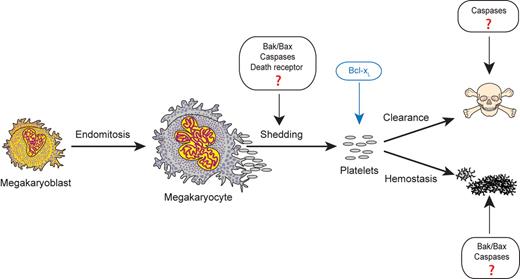
In this issue of Blood, White et al provide compelling evidence that the intrinsic apoptotic caspase cascade, while required for megakaryocyte and platelet death under pathophysiologic conditions, is dispensable for normal platelet formation and function.1
Apoptosis is an intrinsically wired program of cell suicide. Two general pathways have been described, one intrinsic and the other extrinsic. The intrinsic or “mitochondrial” pathway is controlled by the balance of prosurvival proteins (Bcl-2, Bcl-XL, Bcl-w, and Mcl-1) and prodeath proteins (the multidomain prodeath proteins Bak, Bax, and Bok; and the BH3-only domain proteins Bad, Bim, Bid, Bik, Noxa, Puma, Bmf, and Hrk/DP5). If not kept in check by the prosurvival proteins, Bak and Bax oligomerize and permeabilize the outer mitochondrial membrane. This leads to release of cytochrome c, which activates apoptotic protease-activating factor 1 (Apaf-1). Activated Apaf-1 recruits procaspase-9 to form an apoptosome. This initiates a cascade of events culminating in the activation of the downstream effector caspases -3 and -7. These caspases are ultimately responsible for dismantling the cell by cleaving a variety of substrates. The extrinsic pathway involves binding of death ligands to cell-surface receptors of the TNF receptor superfamily. This results in activation of caspase-8, which turns on caspase-3 independent of the intrinsic pathway.
It is well established that the intrinsic apoptotic pathway is active in platelets and modulates platelet survival.2,3 This is illustrated by the thrombocytopenia associated with clinical use of BH3 mimetic compounds, such as ABT-737 or ABT-263. It is also supported by gene knockout studies in mice showing cell-intrinsic reduction in platelet survival in BCL-XL knockout mice, and prolonged platelet survival in Bak-deficient mice.
What is less clear is whether apoptotic pathways play functional roles during normal platelet formation (see figure). Megakaryocyte maturation culminates in a cataclysmic cytoskeletal re-organization event that involves cytoplasmic fragmentation into branching proplatelet processes, platelet shedding, and nucleus extrusion. Similarities of this process to apoptotic morphology have led to the hypothesis that megakaryocytes may deliberately exploit apoptotic pathways to facilitate platelet production. Over the years a number of circumstantial pieces of evidence have accumulated supporting this hypothesis. De Botton et al showed activation of procapases -9 and -3 in mature and proplatelet-forming megakaryocytes.4 Kaluzhny et al demonstrated that overexpression of Bcl-XL reduces proplatelet formation in cultured murine megakaryocytes.5 Several groups have shown that the pan-caspase inhibitor zVAD-fmk reduces proplatelet formation of human CD34+ in vitro–differentiated megakaryocytes, human MEG-01 cells, and human trabecular bone marrow.4,6 Lastly, Morison et al reported a human variant of cytochrome c that has enhanced apoptotic activity and is associated with autosomal dominant thrombocytopenia due to premature megakaryocyte shedding of platelets in the intramedullary bone marrow space.7
Apoptotic mechanisms have also been implicated in platelet activation. This is in part due to similarities in phosphatidylserine (PS) membrane externalization during both apoptosis and platelet activation. This hypothesis has been supported by observations showing induction of the intrinsic apoptotic pathways during in vitro platelet activation.8
Apoptotic pathway components have been postulated to function during several stages of thrombopoiesisis and hemostasis, including platelet formation from megakaryocytes, platelet clearance, and platelet activation. However, the new data from White et al provide evidence that the intrinsic apoptotic initiator caspase-9 is dispensable for all of these steps under normal physiologic conditions. Figure adapted from White and Kile10 with permission.
Apoptotic pathway components have been postulated to function during several stages of thrombopoiesisis and hemostasis, including platelet formation from megakaryocytes, platelet clearance, and platelet activation. However, the new data from White et al provide evidence that the intrinsic apoptotic initiator caspase-9 is dispensable for all of these steps under normal physiologic conditions. Figure adapted from White and Kile10 with permission.
However, recent evidence has questioned the functional role of apoptosis pathways in normal platelet formation. Prior work by Kile and colleagues showed that platelet formation was unperturbed in Bak and Bax knockout mice under steady-state conditions.9 In the study reported here, the same group has now generated mice with deletion of caspase-9 within the hematopoietic compartment.1 Importantly, they demonstrate significantly impaired activation of the downstream effector caspases -3 and -7 in cultured megakaryocytes from these animals after exposure to ABT-737 or etoposide. This indicates that caspase-9 is essential for transmitting mitochondrial apoptosis signals to the downstream effector caspases in megakaryocytes, as it does in other cells. They also show that caspase-9 is required for the reduced platelet lifespan that occurs on treatment of the animals with ABT-737. However, despite the effect of caspase-9 loss under these perturbed conditions, the authors find no significant differences in steady-state platelet count, megakaryocyte number, proplatelet formation of cultured megakaryocytes, or thrombopoietin levels in these mice compared with controls under normal conditions. There is only a slight difference in megakaryocyte ploidy, which is of unclear significance. They also do not detect any differences in platelet activation as measured by platelet aggregometry or surface measurements of P-selectin, integrin GPIIb/IIIa, or PS levels after in vitro exposure to the agonists ADP, thrombin, or collagen-related peptide (CRP). In addition, the authors do not observe any significant differences in hemostasis in vivo using a variety of bleeding time assays.
These new findings put one more nail in the coffin of the apoptotic hypothesis of platelet birth and activation, at least for caspase-9–mediated processes. However, it is important to bear in mind that this study only examines intrinsic apoptosis pathways. Much less is known about the extrinsic apoptosis pathway in megakaryocytes and platelets, although there is evidence that it is present. Thus, further studies looking at potential compensatory roles of the extrinsic pathway, or possible caspase-9–independent mechanisms for intrinsic caspase activation in megakaryocytes, will be necessary before the apoptotic theory of physiologic thrombopoiesis can be pronounced dead.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■