Abstract
Pretargeted radioimmunotherapy (PRIT) using an anti-CD45 antibody (Ab)–streptavidin (SA) conjugate and DOTA-biotin labeled with β-emitting radionuclides has been explored as a strategy to decrease relapse and toxicity. α-emitting radionuclides exhibit high cytotoxicity coupled with a short path length, potentially increasing the therapeutic index and making them an attractive alternative to β-emitting radionuclides for patients with acute myeloid leukemia. Accordingly, we have used 213Bi in mice with human leukemia xenografts. Results demonstrated excellent localization of 213Bi-DOTA-biotin to tumors with minimal uptake into normal organs. After 10 minutes, 4.5% ± 1.1% of the injected dose of 213Bi was delivered per gram of tumor. α-imaging demonstrated uniform radionuclide distribution within tumor tissue 45 minutes after 213Bi-DOTA-biotin injection. Radiation absorbed doses were similar to those observed using a β-emitting radionuclide (90Y) in the same model. We conducted therapy experiments in a xenograft model using a single-dose of 213Bi-DOTA-biotin given 24 hours after anti-CD45 Ab-SA conjugate. Among mice treated with anti-CD45 Ab-SA conjugate followed by 800 μCi of 213Bi- or 90Y-DOTA-biotin, 80% and 20%, respectively, survived leukemia-free for more than 100 days with minimal toxicity. These data suggest that anti-CD45 PRIT using an α-emitting radionuclide may be highly effective and minimally toxic for treatment of acute myeloid leukemia.
Introduction
For more than a decade, antibodies (Abs) conjugated to a radionuclide emitting particulate radiation have been used in the management of leukemia in an effort to deliver targeted doses of radiation to bone marrow, spleen, and other sites of disease while sparing normal organs. This radioimmunotherapy (RIT) approach has been used to achieve significant remissions in patients with acute myeloid leukemia (AML), particularly when used at high doses of radioactivity in conjunction with myeloablation.1-10 One of the major limitations of this approach, however, has been the pharmacokinetic properties of the Ab protein. Abs accrete slowly in solid tumors and are eliminated slowly from the circulation. Use of radiolabeled Abs, therefore, results in prolonged exposure in radiosensitive tissues, particularly marrow, because of the extended time within the circulation. In addition, the extended time required for tumor localization of the Ab may result in loss of tumoricidal potency of the radionuclide because of ongoing isotopic decay. To address this shortcoming, the pretargeted (P)RIT system has been developed. This system differs from conventional RIT in that it uncouples the targeting agent from the radioisotope, which is administered in a separate step after facilitated clearance of non–tumor-bound targeting agent.11 Because the radioisotope can be delivered on a small molecule (< 1 kDa) that is rapidly excreted through the kidneys, normal organ exposure to circulating radiation is effectively reduced by this approach. It has been demonstrated that PRIT technology can further amplify the amount of radiation delivered to CD45+ tissues and, at the same time, diminish the radiation dose to nontargeted cells.12-15
A variety of radionuclides have been investigated for RIT of leukemias, where the types of emissions used have primarily focused on the use of β-particles (131I, 90Y, and 188Re). Over the past several years, interest has developed in targeting α-emitters to leukemia cells for RIT.8,16 As opposed to the relative nonspecific cytotoxicity of β-emitting constructs because of the crossfire effect, α-particle decay of radionuclides, such as 213Bi, 211At, and 225Ac, results in high-energy (6-8 MeV) delivery over a very short distance (50-80 μm). The short path length may provide a therapeutic advantage for targeting leukemic cells in the marrow and thus prevent the exposure of many normal hematopoietic stem cells to nonspecific irradiation. Therefore, the novel approach of PRIT combined with very short half-life of α-emitters may have the potential to further optimize the administration of radionuclide therapy and improve outcomes for leukemia patients.
To assess the merits of α- versus β-emitting CD45 PRIT for leukemia, we report here comparative biodistribution and therapy experiments using human leukemia xenografts implanted in athymic mice. We have demonstrated excellent localization to HEL leukemia tumor sites using both α- and β-emitting radionuclides with minimal uptake into normal organs because of elimination of nonspecific radiation exposure from blood-borne radiolabeled Ab after anti-CD45 Ab-SA pretargeting. The target-to-nontarget therapeutic ratios (based on radiation dose) obtained using PRIT with 213Bi were similar to those observed using 90Y. Using a novel α-camera, we have also shown that 213Bi-DOTA-biotin uniformly distributes within tumor tissue 45 minutes after injection. Lastly, data from comparative PRIT experiments suggest that anti-CD45 PRIT using an α-emitting radionuclide may allow for intensification of the targeted radiotherapy, with diminished toxicity, to sites of leukemic involvement to decrease the risk of relapse.
Methods
Cell lines, antibodies, and production of Ab-SA conjugates
All cell lines were obtained and maintained as described previously.12 The hybridoma cell lines expressing the murine anti–human IgG1 CD45 Ab BC8, and the isotype-matched human anti–bovine herpesvirus-1 Ab, used as nonspecific negative control, and all Ab-SA conjugates were produced as previously described.12
Radiolabeling
DOTA-biotin was synthesized and labeled with either 90Y (PerkinElmer) or 213Bi (isolated from 225Ac; Department of Energy) as previously described.12,17,18 Radiochemical purity was typically > 99% as determined by high performance liquid chromatography for each construct, and labeling efficiencies were > 90%.
Biotinylated clearing agent
A synthetic biotinylated CA containing 16 N-acetyl-galactosamine residues per dendrimeric molecule (Aletheon Corporation) was used to eliminate excess Ab-SA molecules from the circulation before the administration of radiolabeled biotin. The N-acetyl-galactosamine residues have a high affinity for hepatic asialoglycoprotein receptors and thus facilitate the rapid hepatic clearance of residual Ab-SA conjugates from the bloodstream and their endocytosis into liver cells.19
Mice
Female BALB/c mice, 6 to 12 weeks old, were purchased from Harlan Sprague Dawley. The animals were housed under protocols approved by the Fred Hutchinson Cancer Research Center (Seattle, WA) Institutional Animal Care and Use Committee. Results of all mouse studies are representative of at least 3 experiments.
Biodistribution studies
For murine biodistribution experiments, all mice were placed on a biotin-deficient diet (Animal Specialties) at least 5 days before injection of Ab-SA conjugates and radiobiotin. Mice were subsequently injected with 10 × 106 HEL cells subcutaneously in the flank. Five days later, mice were given 1.4 nmol unlabeled BC8 (300 μg) or bovine herpesvirus-1 (isotype-matched negative control) Ab-SA conjugate intravenously via tail vein. Mice were then administered 5.8 nmol (50 μg) of CA intravenously 22 hours after each Ab-SA followed by delivery of 1.2 nmol (1 μg) of 90Y-DOTA-biotin or 213Bi-DOTA-biotin (50 μCi) 2 hours after CA. At 10, 45, and 90 minutes after injection of 213Bi-DOTA-biotin and 1, 24, and 72 hours after 90Y-DOTA-biotin injection, tumors and normal organs were excised, weighed, and the percentage administered activities per gram of tumor or organ (% ID/g) were determined as previously described.12 In studies to reduce the nonspecific radionuclide uptake in the kidney, groups of 5 mice received 2,3-dimercapto-1-propanesulfonic acid (DMPS; Sigma-Aldrich) in the drinking water (1.2 mg/mL) 24 hours before 213Bi-DOTA-biotin.
Radiation dosimetry
Radiation absorbed doses were calculated for circulating blood and for each excised organ, tissue, and tumor based on wet tissue weights and time-activity plots that were constructed from the biodistribution data. To determine the total number of radioactive transformations (decay) in each tissue, we fit an exponential function to the time-activity data by least-squares regression analysis and then integrated the area under each curve from time of injection to infinity. The total number of decays were multiplied by the energy released from 90Y and 213Bi per decay. We accounted for the energy-absorbed fractions in that tissue and for all other neighboring tissues.20 This method is sensitive to the size, shape, and anatomic placement of organs and tissues in the mouse that can result in cross-organ doses from incorporated 90Y. For α-particles from 213Bi, a self-organ absorbed fraction of 1.0 was assumed and a cross-organ absorbed fraction of 0 due the very short range of α-particles.
α-camera imaging
The α-camera is a digital autoradiography technology using an optical registration of photons emitted from a scintillator dedicated to quantitative imaging of α-particles in tissues ex vivo.21 To perform α-camera experiments, tumors were placed in a cryomold containing a cryoprotective gel immediately after dissection. The molds were placed on a precooled aluminum block that was sunken in liquid nitrogen. The frozen tissues were placed in a HM 520 cryostat-microtome (Microm International GmbH) set to a temperature of −19°C. Two consecutive cryosections, used for histologic comparison, were cut and transferred to a glass slide for H&E staining and for immunohistochemistry. Sections (10-16 μm) were cut and transferred to the top surface of a piece of scintillation sheet for the imaging procedure. Blue photons that impinged on the scintillator were optically imaged with a charge-coupled device detector. The pixel intensities in acquired images were linear to the activity of the α-emitting radionuclide in the imaged tissue cryosection, allowing quantitative analysis of activity distribution at a spatial resolution approaching the microscopic level.21 The acquired and background-corrected α-images were used quantitatively by analyzing and comparing the intratumoral activity distribution of the α-emitter 213Bi at different micrometer scales. Circular regions of interest of different sizes were defined within the imaged tumors, and the mean (± SD) pixel intensities were compared for regions of interest of different size and spatial location. Cryosections were cut and air-dried overnight. H&E sections were fixed in 10% neutral buffered formalin for 30 minutes and then stained. Immunohistochemistry sections were fixed in 10% neutral buffered formalin for 3-5 days and antigen retrieval was performed in a 65°C water bath overnight in Target Retrieval solution (pH 6.0; Dako). Slides were rinsed 3 times in TBS-T wash buffer and all subsequent staining steps were performed at room temperature using the Dako Autostainer. Endogenous peroxide activity was blocked using 0.3% H2O2 for 8 minutes followed by protein blocking of 15% swine serum (Jackson ImmunoResearch Laboratories) in TBS containing 1% BSA for 10 minutes. CD45 LCA (M0701; Dako) was used at 7 μg/mL and detected with the Dako ARK kit per manufacturer's methods. The staining was visualized with 3,3'-diaminobenzide (DAB; Dako) for 8 minutes, and the sections were counter-stained with hematoxylin (Dako) for 2 minutes. Concentration-matched isotype control slides were run for each tissue sample (Jackson ImmunoResearch Laboratories).
Pretargeted radioimmunotherapy studies using human leukemia xenografts
To compare PRIT using either 90Y- or 213Bi-DOTA-biotin, groups of 10 mice were placed on biotin-deficient diet at least 5 days before study onset. Selected groups of mice were also treated with DMPS as described in “Biodistribution studies.” Mice were then injected subcutaneously with 10 × 1010 HEL cells and 2 days after tumor injection, mice received 1.4 nmol unlabeled BC8 (300 μg) or bovine herpesvirus-1 Ab-SA conjugate intravenously. Mice were subsequently given 5.8 nmol (50 μg) of CA intravenously 21 hours later. Three hours after CA administration, groups of mice received 1.2 nmol (1 μg) of DOTA-biotin labeled with increasing doses of either 90Y (112, 800, and 1200 μCi) or 213Bi (400 and 800 μCi). Mice were monitored as previously described12 and were killed if xenografts exceeded 10% of total body weight, caused obvious discomfort, or impaired ambulation. Tumor volumes (mm3) were calculated as previously described.12 Time-to-tumor growth and time-to-death were treated as time-to-event endpoints, and Cox regression was used to test for a dose-response effect. Mice were assigned levels of 1 to 5, and a factor treating these levels as a continuous linear variable was then included in a Cox regression model to test for a dose-response effect (ie, to ask whether the risk of failure was associated with a decrease in dose).
In a series of toxicity experiments, mice surviving more than 6 months after therapy were killed. Blood was obtained for complete blood counts. Serum was used to assay for hepatic function by determining levels of alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate transaminase (AST) and renal function by obtaining levels for blood urea nitrogen (BUN) and creatinine. Averages and SE values for hematology and chemistry data are reported for each group of mice studied.
Results
Biodistributions of radioactivity after PRIT using anti-CD45 Ab-SA conjugate followed by 90Y-DOTA-biotin or 213Bi-DOTA-biotin
The biodistributions of 90Y- and 213Bi-DOTA-biotin were evaluated after administration of an Ab-SA conjugate directed against human CD45+ leukemia xenografts given sequentially with a dendrimeric N-acetylgalactosamine-containing CA. These pretargeted biodistribution studies were performed using HEL leukemia-bearing mice injected with 1.4 nmol of anti-CD45 BC8 Ab-SA, followed 21 hours later by 5.8 nmol of CA and 3 hours later mice were administered either 1.2 nmol 90Y-DOTA-biotin or 213Bi-DOTA-biotin. Groups of 5 mice each were killed after 1, 24, and 72 hours after delivery of 90Y-DOTA-biotin and 10, 45, and 90 minutes after injection of 213Bi-DOTA-biotin. The uptake in tumors for mice treated with 90Y-DOTA-biotin was 7.8% ± 4.3% ID/g 1 hour after injection, peaking at 12.2% ± 4.7% ID/g 24 hours after injection (Figure 1A). The targeted concentrations of 90Y radioactivity remained at relatively high levels in tumors over time, measured at 4.7% ± 1.9% ID/g 72 hours after injection. The amount of radioactivity remained relatively high in the blood and normal organs 1 hour after delivery of 90Y-DOTA-biotin with 3.1% ± 0.2% ID/g remaining in the blood and 2.2% ± 0.2% ID/g in the kidneys. The tumor-to-normal organ ratios of 90Y radioactivity using pretargeted BC8 Ab-SA ranged from 3:1 (blood) to approximately 16:1 (muscle) after 24 hours.
Biodistributions of radioactivity in HEL xenograft-bearing athymic mice injected with 90Y-DOTA-biotin or 213Bi-DOTA-biotin after anti-CD45 BC8 Ab-SA conjugate and CA. HEL-xenograft-bearing mice were injected intravenously via the tail vein with 1.4 nmol of anti-CD45 BC8 Ab-SA conjugate followed 22 hours later with 5.8 nmol of CA and subsequently 2 hours later with 1.2 nmol of radiolabeled DOTA-biotin. Groups of 5 mice were killed at 3 time points after injection. The radioactivity in blood, tumor, and normal organs was measured by γ-counting, corrected for decay, and expressed as% ID/g of tissue. (A) 90Y-DOTA-biotin uptake at 1 (□), 24 (■), and 72 (▨) hours after injection. (B-C) 213Bi-DOTA-biotin uptake at 10 (□), 45 (■), and 90 (▨) minutes after injection with anti-CD45 Ab-SA without (B) or with DMPS (C). (D) 213Bi-DOTA-biotin uptake at 10 (□), 45 (■), and 90 (▨) minutes after injection after delivery of nonbinding control Ab-SA conjugate.
Biodistributions of radioactivity in HEL xenograft-bearing athymic mice injected with 90Y-DOTA-biotin or 213Bi-DOTA-biotin after anti-CD45 BC8 Ab-SA conjugate and CA. HEL-xenograft-bearing mice were injected intravenously via the tail vein with 1.4 nmol of anti-CD45 BC8 Ab-SA conjugate followed 22 hours later with 5.8 nmol of CA and subsequently 2 hours later with 1.2 nmol of radiolabeled DOTA-biotin. Groups of 5 mice were killed at 3 time points after injection. The radioactivity in blood, tumor, and normal organs was measured by γ-counting, corrected for decay, and expressed as% ID/g of tissue. (A) 90Y-DOTA-biotin uptake at 1 (□), 24 (■), and 72 (▨) hours after injection. (B-C) 213Bi-DOTA-biotin uptake at 10 (□), 45 (■), and 90 (▨) minutes after injection with anti-CD45 Ab-SA without (B) or with DMPS (C). (D) 213Bi-DOTA-biotin uptake at 10 (□), 45 (■), and 90 (▨) minutes after injection after delivery of nonbinding control Ab-SA conjugate.
In groups of mice where 213Bi was the radionuclide used, high uptake was demonstrated in tumors 10 minutes after injection at 5.9% ± 2.5% ID/g (Figure 1B). The tumor localization of 213Bi-DOTA-biotin remained steady with a measured uptake of 5.3% ± 0.8% ID/g after 45 minutes and 4.1% ± 3.4% ID/g and 90 minutes after injection. The use of pretargeted 213Bi-DOTA-biotin, however, also showed high uptake in the kidneys, measured at 5.4% ± 2.0% ID/g 10 minutes after injection, which remained relatively constant to the 90-minute time point (5.2% ± 0.3% ID/g). At all time points, control animals injected with nonspecific 213Bi-labeled nonbinding isotype-matched Ab-SA conjugate exhibited negligible tumor uptake of the radiolabel demonstrating the specificity of targeting in these experiments (Figure 1D).
Renal uptake and dosimetry
In an effort to reduce nonspecific uptake of radioactivity in the kidney, a metal chelator (DMPS) was administered in the drinking water for animals that subsequently received 213Bi-DOTA-biotin. In particular, DMPS has been shown to be effective in clearing the radioactive α-daughters of 225Ac, including 213Bi.22 Therefore, groups of 5 mice were treated with DMPS orally 24 hours before 213Bi-DOTA-biotin injection. DMPS-treated mice displayed significantly lower levels of radioactivity in the kidneys compared with the untreated controls at all time points (Figure 1C-D). For example, after 45 minutes, the renal uptake of 213Bi was 1.5 ± 0.2 for mice treated with DMPS, compared with 4.5 ± 0.9 for mice in the PRIT group that did not receive DMPS (P = .0006). Mice treated without DMPS displayed tumor-to-kidney ratios of radioactivity of approximately 1:1 at all time points after injection of 800 μCi of 213Bi-DOTA-biotin. In contrast, mice that were treated with DMPS and 800 μCi of 213Bi-DOTA-biotin demonstrated tumor-to-kidney ratios up to 3.5 by 45 minutes after injection.
Radiation absorbed doses for each organ per unit of administered activity were calculated based on the biodistribution data using standard Medical Internal Radiation Dose methods. The mean absorbed doses in all tissues other than kidney and tumor were similar either in the presence or absence of DMPS (data not shown because of space constraints). In DMPS-treated mice, however, the total absorbed dose of 213Bi per unit administered activity was found to be 0.7 cGy/μCi in the kidneys compared with 1.6 cGy/μCi for mice in the standard PRIT group without DMPS chelation. These data suggest a > 60% reduction in the renal absorbed dose of 213Bi for DPMS-treated mice. Although the total absorbed dose in tumor was slightly lower in the DMPS-treated mice (2.0 cGy/μCi) compared with the untreated group (2.6 cGy/μCi), the tumor-to-kidney dose ratio remained approximately 2 times greater in the mice that received the renal protective agent.
α-camera imaging and quantitative autoradiography
Because α-particle emitters used in internal radiotherapy may exhibit heterogeneous distributions in tumors, a novel α-camera was used to detect α-particles in tissues ex vivo and thus assess for nonuniform activity distribution causing a nonuniform dose distribution.21 We used this quantitative imaging technique to obtain data on the 213Bi activity distribution on a suborgan level in cryosections of human HEL xenograft tumors at various times after injection of radioactivity. Nonuniform intratumoral activity distributions were found for tumor-specific 213Bi-DOTA-biotin at the 10-minute time point; however, 45 minutes after injection, the distribution was more uniform. At this 45-minute time point, the α-camera disclosed that the radionuclide was well and deeply distributed within the tumor tissue. The blue areas seen in the periphery of Figure 2 represent areas with lower activity concentration, whereas areas of red higher activity were distributed throughout the central parts of the tumor. H&E staining verified a highly proliferative tumor cell population in the imaged area, and corresponding immunohistochemistry confirmed a vascular net well established within the same area. Based on these data combined with its short path length and high linear energy transfer (LET), we further investigated the biologic outcomes of PRIT using 213Bi in a series of in vivo therapeutic studies.
Quantitative α-camera autoradiography. The intratumoral distribution of 213Bi-DOTA-biotin after anti-CD45 PRIT was analyzed using a novel digital autoradiography bio-imaging system. This α-camera system is dedicated for the ex vivo detection of α-particles in tissue and uses a scintillation setup. The technique is quantitative and fully linear toward activity content in the imaged sections. At serial times after injection (10, 45, and 90 minutes), tumors were cryosectioned (thickness 12-16 μm). Three serial, immediately adjacent sections were obtained: (A) the first for α-imaging, (B) the second for H&E staining, and (C) the third for immunohistochemistry of blood vessels using CD34. This representative, digitally collected α-camera image was obtained 45 minutes after 213Bi-DOTA-biotin injection and was color-coded to express the different levels of activity concentration of 213Bi. Insets represent smaller sections of the image shown in the next panel (inset in panel A comprises entire picture in panel B; inset in panel B comprises entire picture in panel C).
Quantitative α-camera autoradiography. The intratumoral distribution of 213Bi-DOTA-biotin after anti-CD45 PRIT was analyzed using a novel digital autoradiography bio-imaging system. This α-camera system is dedicated for the ex vivo detection of α-particles in tissue and uses a scintillation setup. The technique is quantitative and fully linear toward activity content in the imaged sections. At serial times after injection (10, 45, and 90 minutes), tumors were cryosectioned (thickness 12-16 μm). Three serial, immediately adjacent sections were obtained: (A) the first for α-imaging, (B) the second for H&E staining, and (C) the third for immunohistochemistry of blood vessels using CD34. This representative, digitally collected α-camera image was obtained 45 minutes after 213Bi-DOTA-biotin injection and was color-coded to express the different levels of activity concentration of 213Bi. Insets represent smaller sections of the image shown in the next panel (inset in panel A comprises entire picture in panel B; inset in panel B comprises entire picture in panel C).
PRIT of human leukemia xenografts
In light of favorable tumor biodistributions shown by both γ-counting and α-camera methodologies, we performed therapy experiments to compare efficacy of 90Y and 213Bi in a minimal residual disease model of AML. Experimental groups of 10 mice were placed on a biotin-deficient diet and 2 days later were injected subcutaneously into the flank with 10 × 106 HEL cells. Two days after leukemia cell injection, the mice were treated with 1.4 nmol anti-CD45 BC8 Ab-SA, followed 21 hours later by 5.8 nmol CA, and then 3 hours later with either 800 μCi or 1200 μCi of 90Y-DOTA-biotin or 400 μCi or 800 μCi of 213Bi-DOTA-biotin. Comparison groups were untreated mice that had received subcutaneous injection of HEL cells alone and mice that were injected with 1200 μCi 90Y-DOTA-biotin or 800 μCi 213Bi-DOTA-biotin after treatment with a nonbinding isotype-matched control Ab-SA conjugate and subsequent CA. Untreated mice and PRIT control mice that received 1200 μCi 90Y-DOTA-biotin and control Ab-SA conjugate all had exponential growth of tumors requiring death by 14 days after injection of radiolabeled biotin (Figure 3). Mice that received BC8 Ab-SA conjugate and CA followed by 800 μCi of 90Y-DOTA-biotin had significant reduction in tumor growth compared with control groups. All mice in control groups experienced rapid tumor growth resulting in death of all mice before day 22 after treatment (Figure 3A). In the group that received 800 μCi of 90Y-DOTA-biotin, the first mouse did not require death until day 26 after therapeutic injection (Figure 3B). Mice from this treatment group displayed significantly improved survival (P < .0001) compared with control groups where 2 mice remained alive past 120 days after therapy. Eight of 10 mice in this treatment group died of progressive leukemia between 26 and 75 days after therapy, whereas 2 mice remained tumor-free and survived over 120 days (Figure 3B). Mice that received 1200 μCi of 90Y-DOTA-biotin exhibited an even more impressive therapeutic effect compared with control mice with 60% of mice in this group disease-free 120 days after therapy (P < .0001). Three mice that received 1200 μCi of 90Y-DOTA-biotin developed tumors and were killed by day 64 and 1 mouse in this treatment group early exhibited regimen-related toxicity with huddling behavior and extensive loss of body weight necessitating death 14 days after therapy (Figure 3).
Leukemia xenograft regression and survival after treatment with anti-CD45 PRIT using 90Y-DOTA-biotin. Athymic mice bearing HEL xenografts were injected intravenously via tail vein 2 days after tumor implantation with 1.4 nmol of anti-CD45 Ab-SA conjugate followed 22 hours later with 5.8 nmol of CA and then with 1.2 nmol of 90Y-DOTA-biotin (800 μCi and 1200 μCi) 3 hours later. Tumor-bearing control mice were either untreated or treated with a nonbinding control Ab-SA conjugate before 1200 μCi radiolabeled DOTA-biotin injection. (A) Tumor volume curves are truncated at the time of death because of excessive tumor growth in the first mouse in each group. (B) Mice were analyzed for survival as a function of time. †Deaths resulting from toxicity.
Leukemia xenograft regression and survival after treatment with anti-CD45 PRIT using 90Y-DOTA-biotin. Athymic mice bearing HEL xenografts were injected intravenously via tail vein 2 days after tumor implantation with 1.4 nmol of anti-CD45 Ab-SA conjugate followed 22 hours later with 5.8 nmol of CA and then with 1.2 nmol of 90Y-DOTA-biotin (800 μCi and 1200 μCi) 3 hours later. Tumor-bearing control mice were either untreated or treated with a nonbinding control Ab-SA conjugate before 1200 μCi radiolabeled DOTA-biotin injection. (A) Tumor volume curves are truncated at the time of death because of excessive tumor growth in the first mouse in each group. (B) Mice were analyzed for survival as a function of time. †Deaths resulting from toxicity.
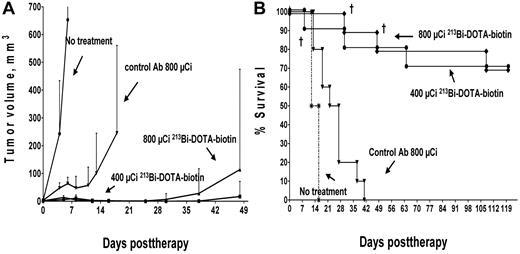
All 213Bi control groups exhibited anticipated growth of tumors requiring death by 16 days (untreated mice) or by 40 days (pretargeted isotype-matched negative control Ab-SA mice that received 800 μCi of 213Bi-DOTA-biotin; Figure 4), similar to the control mice receiving 90Y-DOTA biotin. We postulate that delayed tumor growth seen in the isotype-matched control mice injected with radiolabeled 213Bi-DOTA-biotin may have been the result of circulating 213Bi in the bloodstream, affecting the highly vascularized HEL xenografts. Overall, experimental mice pretargeted with anti-CD45 Ab-SA followed by either 400 or 800 μCi of 213Bi-DOTA-biotin experienced superior survival rates compared with similar mice treated with higher doses of 90Y-DOTA-biotin in our minimal residual leukemia model. Mice that received pretargeted anti-CD45 Ab-SA, CA, and 400 μCi of 213Bi-DOTA-biotin demonstrated a significant therapeutic benefit compared with the 800 μCi 213Bi-DOTA-biotin negative control mice (P = .002), as did anti-CD45 BC8 Ab-SA pretargeted mice that received 800 μCi of 213Bi-DOTA-biotin (Figure 4; P < .0001). One mouse pretargeted with anti-CD45 Ab-SA followed by 400 μCi of 213Bi-DOTA-biotin died by day 8 because of toxicity, whereas 2 other mice in this group were killed by day 64 because of progressive xenograft growth. In the group of mice that received anti-CD45 Ab-SA and 800 μCi of 213Bi-DOTA-biotin, 2 mice died of radiation-induced toxicity (one each on day 28 and day 49), and only one mouse developed overt leukemia leading to death on day 63. Both the 400 and 800 μCi of 213Bi-DOTA-biotin anti-CD45 pretargeted treatment groups had 70% long-term disease-free survival extending to > 120 days after therapy.
Leukemia xenograft regression and survival after treatment with anti-CD45 PRIT using 213Bi-DOTA-biotin. Athymic mice bearing HEL xenografts were injected intravenously via tail vein 2 days after tumor implantation with 1.4 nmol of anti-CD45 Ab-SA conjugate followed 22 hours later with 5.8 nmol of CA and subsequently with 1.2 nmol of 213Bi-DOTA-biotin (400 μCi and 800 μCi) 3 hours later. Tumor-bearing control mice were either untreated or treated with a nonbinding control Ab-SA conjugate before 800 μCi radiolabeled DOTA-biotin injection. (A) Tumor volume curves are truncated at the time of death because of excessive tumor growth in the first mouse in each group. (B) Mice were analyzed for survival as a function of time. †Deaths resulting from toxicity.
Leukemia xenograft regression and survival after treatment with anti-CD45 PRIT using 213Bi-DOTA-biotin. Athymic mice bearing HEL xenografts were injected intravenously via tail vein 2 days after tumor implantation with 1.4 nmol of anti-CD45 Ab-SA conjugate followed 22 hours later with 5.8 nmol of CA and subsequently with 1.2 nmol of 213Bi-DOTA-biotin (400 μCi and 800 μCi) 3 hours later. Tumor-bearing control mice were either untreated or treated with a nonbinding control Ab-SA conjugate before 800 μCi radiolabeled DOTA-biotin injection. (A) Tumor volume curves are truncated at the time of death because of excessive tumor growth in the first mouse in each group. (B) Mice were analyzed for survival as a function of time. †Deaths resulting from toxicity.
Direct comparison of anti-CD45 PRIT using 90Y- versus 213Bi-DOTA-biotin at equivalent absorbed dose
To further determine the therapeutic difference between the pretargeted α- and β-emitting radionuclides, equivalent absorbed doses to tissues were assessed (Table 1). These concentration ranges of radioactivity were determined based on equivalent absorbed doses over a specific organ volume.23 Thus, tissue dosimetry values for each radionuclide were normalized to an equivalent liver dose for comparative purposes. We estimated that the equivalent liver dose for mice bearing HEL tumors that received 800 μCi of 213Bi-DOTA-biotin would be a dose of 112 μCi 90Y-DOTA-biotin. Anti-CD45 Ab-SA PRIT was therefore subsequently performed using these respective doses in the model of minimal residual leukemia (Figure 5). Results from this experiment demonstrated no significant difference in survival detected between untreated mice and the group that received 112 μCi 90Y-DOTA-biotin; mice that received 112 μCi 90Y-DOTA-biotin survived 17 or fewer days, whereas mice that received no therapy survived a maximum of 14 days. There was a significant difference, however, between the group that was injected with 112 μCi 90Y-DOTA-biotin and the group that was injected with 800μCi 213Bi-DOTA-biotin (P < .0001). Mice receiving 800 μCi 213Bi-DOTA-biotin had delayed tumor growth compared with control animals, with only one mouse requiring death (because of tumor growth on day 30 after therapy) and with 7 mice achieving long-term disease-free survival (at least 120 days).
Leukemia xenograft regression and survival after PRIT with 213Bi- or 90Y-DOTA-biotin at equivalent absorbed doses. Athymic mice bearing HEL xenografts were injected intravenously via tail vein 2 days after tumor cell implantation with 1.4 nmol of anti-CD45 Ab-SA conjugate followed 22 hours later with 5.8 nmol of CA and subsequently with 1.2 nmol of radiolabeled DOTA-biotin 3 hours later to deliver an absorbed dose of 10 Gy from each radionuclide delivered to the liver (112 μCi 90Y-DOTA-biotin and 800 μCi 213Bi-DOTA-biotin). Control tumor-bearing control mice were untreated. (A) Tumor volume curves are not truncated. (B) Mice were also analyzed for survival as a function of time.
Leukemia xenograft regression and survival after PRIT with 213Bi- or 90Y-DOTA-biotin at equivalent absorbed doses. Athymic mice bearing HEL xenografts were injected intravenously via tail vein 2 days after tumor cell implantation with 1.4 nmol of anti-CD45 Ab-SA conjugate followed 22 hours later with 5.8 nmol of CA and subsequently with 1.2 nmol of radiolabeled DOTA-biotin 3 hours later to deliver an absorbed dose of 10 Gy from each radionuclide delivered to the liver (112 μCi 90Y-DOTA-biotin and 800 μCi 213Bi-DOTA-biotin). Control tumor-bearing control mice were untreated. (A) Tumor volume curves are not truncated. (B) Mice were also analyzed for survival as a function of time.
Assessment of delayed radiation-induced toxicity after anti-CD45 Ab-SA and 213Bi-DOTA-biotin PRIT
Long-term radiotoxicity in mice surviving 120 days after PRIT with anti-CD45 Ab-SA followed by 213Bi-DOTA-biotin at 400 μCi or 800 μCi was assessed by evaluating hepatic and renal functions in addition to hematologic recovery (Table 2). Of at least 4 mice surviving in each group for 120 days, all anti-CD45 PRIT animals displayed normal body weights despite a slight nonsignificant increase in hepatic transaminase levels detected in the 400 μCi or 800 μCi 213Bi-DOTA-biotin groups compared with a group of untreated age-matched control mice. ALT, AST, and ALP levels were 389 ± 530, 214 ± 170, and 91 ± 41 U/L in the mice that received 400 μCi 213Bi-DOTA-biotin, respectively, and 371 ± 480, 385 ± 290, and 119 ± 63 U/L in those that were treated with 800 μCi 213Bi-DOTA-biotin, respectively. In contrast, untreated mice displayed ALT, AST, and ALP levels of 122 ± 84, 215 ± 94, and 69.7 ± 21 U/L (P = not significant, for all tests). Blood counts in pretargeted mice remained similar to those in untreated age-matched control mice. Mice that received 800 μCi 213Bi-DOTA-biotin had a white blood count (1000/μL), platelet count (1000/μL), hemoglobin (g/dL), and neutrophil count (per μL) of 9.9 ± 2.0, 983 ± 400, 13.6 ± 0.7, and 3145 ± 1300, respectively, whereas mice that received 400 μCi 213Bi-DOTA-biotin had counts of 12 ± 2.0, 1152 ± 170, 15.0 ± 0.2, and 5835 ± 630, respectively. Age-matched control mice had a white blood count of 14.2 ± 1.9, platelet count of 1732 ± 149, Hgb of 14.4 ± 1.2, and neutrophil count of 5820 ± 355 (P = not significant, for all tests). No significant differences were seen in BUN or creatinine levels between mice that received 800 μCi 213Bi-DOTA-biotin (34 ± 10 and 0.57 ± 0.1, respectively), 400 μCi 213Bi-DOTA-biotin (28 ± 3.0 and 0.48 ± 0.04, respectively), or age-matched control mice (26 ± 8.4 and 0.48 ± 0.01, respectively). Necropsies revealed no evidence of leukemia in any 213Bi treated mice surviving beyond day 120, and histologic sections obtained from these mice did not show any evidence of disease or radiation-induced changes compared with age-matched controls (data not shown because of space constraints). We hypothesize that the minimal toxicity exhibited by anti-CD45 PRIT in this model may have been the result of the very short path length (50 μm) of the α-particles from 213Bi compared with β-emitting isotopes, such as 90Y (∼ 5000 μm).
Discussion
This report critically evaluates the merits of PRIT using an α-emitting radionuclide in a myeloid leukemia xenograft model. The results described in this manuscript demonstrated that anti-CD45 PRIT using 213Bi-DOTA-biotin provided rapid tumor localization and excellent biodistributions of radioactivity, with significant improvements in efficacy compared with a PRIT approach using a β-emitting radionuclide (90Y). In vivo images using a novel α-camera displayed rapid tumor localization and favorable 213Bi radionuclide distribution at a microdosimetric level.24 Importantly, the pretargeted approach provided an excellent therapeutic index (target-to-nontarget ratio) for each targeting radionuclide with tumor-to-blood ratios of up to 13:1 and tumor-to-normal organ ratios of as high as 24:1, thus ensuring that the toxic effects on normal tissues were balanced by the cytotoxic effects on tumor cells. Because the therapeutic index of an antineoplastic agent depends on the balance between toxic effects on normal tissues and cytotoxic effects on tumor cells, the pretargeted approach provided excellent tumor regressions and improved overall survival. The excellent tumor localization of the anti-CD45 Ab-SA conjugate using PRIT and 213Bi-DOTA-biotin translated into improved xenograft survival rates compared with animals receiving 90Y-DOTA-biotin.
Although these experiments were encouraging, we recognize that this mouse leukemia xenograft model differs significantly from the state of naturally occurring leukemias in patients. In this xenograft system, the human target CD45+ cells are confined to tumors, which are more similar to chloromas than to typical disseminated leukemia. In addition, all other tissues in the xenograft model lack expression of human CD45 and will not bind anti–human CD45 Ab. Therefore, toxicity profiles in the human may not be reliably mimicked in our xenograft system. To overcome this limitation, we previously tested anti-CD45 PRIT in a murine syngeneic system in which CD45 was present on normal hematolymphoid tissues. The results of these complementary studies suggested that PRIT supported an improvement in the therapeutic index over that currently achievable with conventional RIT methodologies.13
Although several clinical trials have provided encouraging results using β-emitting radionuclides (primarily 131I and 90Y) to treat malignancies, the relatively long path lengths of β-emissions can produce dose-limiting myelosuppression at conventional doses25,26 and nonhematologic dose-limiting toxicities at myeloablative doses when used as part of a hematopoietic cell transplant conditioning regimen.27-29 Although the longer path length of these β-emitting radionuclides may be best suited for diseases with enlarged tumor masses, such as lymphomas, it has been postulated that alternative radionuclides with shorter path lengths may be better suited for clinical scenarios of minimal residual disease or where circulating isolated tumor cells are present to maintain radiation-induced toxicities at a minimum.8,29 In particular, α-emitting radionuclides with their very short path lengths may be particularly advantageous for leukemias where malignant cells are dispersed and the goal is rapid and efficient cell kill with minimal toxicities inflicted on surrounding tissues.30-34 It has been estimated that up to twice as much energy is deposited outside a 200-μm tumor when using an Ab labeled with a β-emitting radionuclide, such as 90Y or 131I, compared with an Ab conjugated to an α-emitting agent.35 In addition, the relatively low LET characteristics of β-particles (∼ 0.2 keV/μm) may result in suboptimal killing of tumor cells and may ultimately contribute to the relapse of aggressive malignancies.
α-emitting radionuclides exhibit very high cytotoxicity, further making them attractive alternatives to the β-emitting radionuclides for RIT.8,32 The higher cytotoxicity delivered by an α-particle results from the large amount of energy that is emitted in a linear fashion within a few cell diameters (∼ 50-90 μm). Moreover, the high LET energy transfer of α-emitters (∼ 100 keV/μm) confers a high relative biologic effectiveness for cell killing compared with that for β-emitters.16,31 This high relative biologic effectiveness of α-particles results in double-strand DNA breaks that are commonly so great that cell repair mechanisms may not be effective.16 Not surprisingly, α-emitters have shown greater therapeutic efficacy in cell culture and animal studies than β-emitting radionuclides.36-38 Of particular relevance to our study, Friesen et al showed that a 213Bi-labeled anti-CD45 Ab, but not a 90Y-labeled anti-CD45 Ab, could overcome resistance of AML cells to β-irradiation and anthracyclines to achieve effective tumor cell killing.39 The 213Bi-labeled anti-CD45 Ab-induced DNA damage and apoptosis inflicted on AML cells could not be repaired, in contrast to DNA damage induced by either β- or γ-irradiation and doxorubicin. Taken together, these results suggest that α-emitting radionuclides may be superior to β-emitting radionuclides for treatment of leukemia.
Only a small number of α-emitting radionuclides have been considered suitable for in vivo applications, however, primarily because of availability and decay properties. The α-emitting radionuclides 211At (t½ = 7.2 hours), 212Bi (t½ = 60 minutes), and 213Bi (t½ = 45 minutes) have been favored because these radionuclides do not produce daughter radionuclides that also decay by α-emission.30,31 More recently, longer-lived α-emitting radionuclides, such as 225Ac (t½ = 10 days), 223Ra (t½ = 11.4 days), and 227Th (t½ = 18.7 days), have been investigated.40-44 These radionuclides do generate short-lived daughter therapeutic α-particles (221Fr, 217At, and 213Bi) from 225Ac inside a targeted leukemia cell, which largely account for the increased potency of 225Ac-atomic nanogenerators over 213Bi constructs.16 Importantly, however, studies in mice and cynomologous monkeys suggest that these α-daughters also have significant potential to inflict renal damage because of uptake of bismuth radioisotopes from the decay chain.45,46 A recent study by our group confirmed the efficacy of DMPS in animals treated with PRIT using an anti-CD20 Ab-SA conjugate followed by 213Bi-DOTA-biotin with a more than 50% reduction of the total radiation absorbed dose delivered to the kidneys.17 In this study, we demonstrate similar results when DMPS was administered to the animals' drinking water; serum BUN and creatinine levels from long-term surviving mice were not different from levels determined from age-matched control animals, suggesting the absence of functional radiation-induced nephritis. Moreover, after delivering a 10 Gy dose to the liver, the dose to the kidneys when 213Bi-DOTA-biotin was used was similar to the renal dose delivered by 90Y-DOTA-biotin (Table 1; 5.4 Gy and 5.7 Gy, respectively). This renal dose was considerably less than the 10 Gy delivered to the kidney using conventional α-particle RIT, which has been shown to result in an approximately 50% reduction in the glomerular filtration rate.47
In conclusion, our study confirms the encouraging results from pilot PRIT studies using short-lived α-emitters for leukemia therapy.38,48 Our study demonstrated the curative potential of the PRIT approach for mice with myeloid leukemia and the superiority of 213Bi compared with 90Y in our model. Although a number of challenges remain to achieve widespread applicability of this novel anti-CD45 PRIT approach using α-emitting radionuclides, further improvements targeting leukemic cells have the potential to increase the therapeutic index and form a basis for the treatment of a wide variety of malignant and life-threatening nonmalignant diseases, including hematologic as well as solid tumors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grants RO1 CA76287, K08 CA095448, PO1 CA44991, and R01 CA136639), the Leukemia & Lymphoma Society (SCOR grant), and the Frederick Kullman and Penny E. Petersen Memorial Funds (donations). O.W.P. is supported by an endowed Chair from James and Sherry Raisbeck. J.M.P. is a clinical scholar of the Damon Runyon Cancer Foundation. Histology and immunohistochemistry support was provided by FHCRC Experimental Histopathology Shared Resources.
National Institutes of Health
Authorship
Contribution: J.M.P. contributed to the conception, design, analysis, and interpretation of the research and wrote the manuscript; A.L.K. and S.I.P. contributed to the design of the research, performed research, and analyzed data; T.B., S.F., A.A., N.O., J.S., and J.O. performed research and collected data; D.K.H. contributed vital reagents; D.S.W., Y.L., and D.R.F. contributed to the conception and interpretation of research; A.K.G. and D.J.G. contributed to the interpretation of data; F.R.A. contributed to the conception and interpretation of research and revised the manuscript; and O.W.P. contributed to the conception, design, analysis, and interpretation of the research and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John M. Pagel, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, M/S D5-380, Seattle, WA 98109; e-mail: jpagel@fhcrc.org.