Abstract
Mammalian target of rapamycin (mTOR) is a downstream serine/threonine kinase of the PI3K/Akt pathway that integrates signals from the tumor microenvironment to regulate multiple cellular processes. Rapamycin and its analogs have not shown significant activity in multiple myeloma (MM), likely because of the lack of inhibition of TORC2. In the present study, we investigated the baseline activity of the PI3K/Akt/mTOR pathway TORC1/2 in MM cell lines with different genetic abnormalities. TORC1/2 knock-down led to significant inhibition of the proliferation of MM cells, even in the presence of BM stromal cells. We also tested INK128, a dual TORC1/2 inhibitor, as a new therapeutic agent against these MM cell lines. We showed that dual TORC1/2 inhibition is much more active than TORC1 inhibition alone (rapamycin), even in the presence of cytokines or stromal cells. In vitro and in vivo studies showed that p-4EBP1 and p-Akt inhibition could be predictive markers of TORC2 inhibition in MM cell lines. Dual TORC1/2 inhibition showed better inhibition of adhesion to BM microenvironmental cells and inhibition of homing in vivo. These studies form the basis for further clinical testing of TORC1/2 inhibitors in MM.
Introduction
Mammalian target of rapamycin (mTOR) kinase exists in at least 2 multiple protein complexes, TORC1 and TORC2. TORC1 is composed of the mTOR catalytic subunit and 3 associated proteins, Raptor, PRAS40, and mLST8/GβL, whereas TORC2 also contains mTOR and mLST8/GβL, but instead of Raptor and PRAS40, contains the proteins Rictor, mSin1, and Protor. TORC1 is activated by growth factor stimulation via the canonical PI3K/Akt/mTOR pathway and is partially sensitive to rapamycin.1 TORC1 also connects nutrient, stress, and hormone signaling via TSC and AMPK signaling components.2-4 Multiple myeloma (MM) is the second most prevalent hematologic malignancy and remains incurable, with a median survival of 3-5 years. Although no mutations are present in PI3K/AKT genes, this pathway is activated in the majority of patients with MM through either external signaling from insulin growth factor 1 (IGF-1), receptor tyrosine kinases (RTKs), or other dysregulations, including genetic mutations in other pathways or epigenetics that lead to activation of this pathway.5-13 MM is frequently associated with dysregulation and/or mutations of signaling genes such as RAS, PTEN, FGF, c-Myc, and CDKN pathways.6-13 These pathways often signal via TORC1/2 to regulate protein translation and cytoskeletal dynamics that are collectively required for cell division, growth, motility, and survival.6-13 Recent reports have shown that Deptor is an mTOR-interacting protein that is highly overexpressed in MM with cyclin D1/D3 (t:11;14 and t:6;14) or c-MAF/MAFB translocations (t:14;16), which are present in 30% of MM patients.12 In these cells, high DEPTOR expression is necessary to maintain PI3K and Akt activation by relieving feedback inhibition from TORC1, and a reduction in DEPTOR levels leads to apoptosis.12
The fact that TORC1 is one of the rate-limiting signal nodes in cellular protein translational controls makes it a prime target to address one of the cell-intrinsic molecular hallmarks of MM, and, therefore, it has been extensively studied in this context. However, the dynamic interaction between tumor cells and the BM microenvironment is crucial in myeloma pathogenesis, resistance to treatment leading to relapse of patients, and in this interaction TORC2 plays an essential role.5,14 Rapamycin analogs such as RAD001 and CCI-779 are TORC1 inhibitors and even at high concentrations do not completely inhibit TORC2 in most cells.5,15-19 Therefore, MM cells treated with rapamycin or its analogs usually display incomplete inhibition of the signaling cascades downstream of both TORC1/2 complexes. This eventually leads to increased phosphorylation of Akt due to loss of the feedback inhibitory circuit mediated by S6K, which can lead to enhanced survival and chemoresistance.15-22 In the present study, we investigated the baseline activity of members of the PI3K/Akt/mTOR pathway in MM cell lines with different baseline genetic abnormalities that reflect the genetic subgroups of MM, and then characterized INK128, a novel dual TORC1/2 inhibitor.
Methods
For a more detailed description of the methods used, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cells
The MM cell lines MM1S (a kind gift from Dr Kenneth Anderson, Dana-Farber Cancer Institute, Boston, MA), MM1R, OPM1, OPM2, H929, RPMI8226, U266 and U266LR7 (a kind gift from Prof Jesús F. San Miguel, University of Salamanca, Salamanca, Spain). Cell lines and BM cells obtained from MM patients were cultured in RPMI 1640 medium with l-glutamine and supplemented with antibiotics (penicillin at 100 U/mL and streptomycin at 100 μg/mL) and 10% FBS for cell lines or 20% FBS for patient cells. Approval for these studies was obtained from the Dana-Farber Cancer Institute Institutional Review Board. Informed consent was obtained from all patients and healthy volunteers in accordance with the Declaration of Helsinki protocol.
Reagents
INK128 was provided by Intellikine. For in vitro studies, INK128 was prepared as a 10mM stock solution in DMSO and diluted to a cell culture medium with the final DMSO concentration of 0.01%. For in vivo studies in mice, INK128 was formulated in 5% 1-methyl-2-pyrrolidinone, 15% polyvinylpyrrolidone K30, and 85% water, and was administered by oral gavage once daily. Rapamycin was purchased from Selleck. Annexin V–FITC was from BD Biosciences.
Growth-inhibition assay
DNA synthesis
DNA synthesis was measured with the bromodeoxyuridine (BrdU) uptake assay (Roche Biochemicals), following the manufacturer's instructions.
Flow cytometry
Whole BM cells were treated with red lysis buffer (Boston Bioproducts) and subsequently incubated with INK128 for 24 hours at 37°C. To discriminate between myeloma plasma cells and apoptotic residual BM cells, we performed a multiparametric technique based on the combination of annexin V–FITC and 4-color mAbs against myeloma-associated antigens (CD38, CD56, CD138, and CD45).
For in vivo studies, we injected 3 × 106 OPM2 cells into the tail veins of SCID mice. Vehicle or INK128 was administered by oral gavage at 1 mg/kg/d for 10 days. After treatment, we isolated BM cells from mice femurs (n = 24 mice; 6 per group), and incubated the cells with 5 μL of anti–CD138-PerCP/Cy5.5 human not murine Ab. A total of 100 000 cells were acquired on a FACSCalibur flow cytometer (BD Biosciences) and analyzed.
Cell-cycle and apoptosis assays
Cell-cycle analysis was profiled by flow cytometry using propidium iodide staining (5μg/mL; Sigma-Aldrich) after 24 hours of culture with INK128 or rapamycin. Apoptosis was quantitated using annexin V–FITC (BD Biosciences) staining and flow cytometric analysis according to the manufacturer's protocol.
Immunoblotting
Cells were harvested and lysed using lysis buffer (Cell Signaling Technology), reconstituted, and whole-cell lysates were subjected to SDS-PAGE and transferred to polyvinylidene fluoride membrane (Bio-Rad). Anti-mTOR, anti–phospho(p)-mTOR Ser2481, anti-PTEN, anti-Raptor, anti-Rictor, anti–p-rS6 Ser235/236, anti–p-4EBP1 Thr37/46, anti-Akt, anti–p-Akt Ser473, anti–pGSKα/β, anti–caspase-3, anti–caspase-8, anti–caspase-9, anti-PARP, anti-actin, and anti-LC3 Abs were from Cell Signaling Technology; anti-Deptor Ab was from Millipore; and anti–α-tubulin Ab was from Santa Cruz Biotechnology. The HRP-conjugated secondary Abs were from Cell Signaling Technology.
In vitro Akt kinase assays
The in vitro Akt kinase assay (Cell Signaling Technology) was performed according to the manufacturer's procedure. Western blot analysis for Akt and p-Akt were performed after immunoprecipitation.
Adhesion assay
We used an in vitro adhesion assay coated with fibronectin according to the manufacturer's recommendations (EMD Biosciences). Briefly, 2 × 105 MM.1S cells were incubated in a 96-well plate of adhesion with increasing concentrations of rapamycin or INK128 (0, 10, 25, 50, and 100nM). After 2 hours at 37°C, nonadherent cells were washed from the wells, adherent cells were labeled with calcein-AM, and the intensity of fluorescence was measured at Ex/Em = 485/520 nm.
For the HUVEC and stromal cell adhesion assay, stromal cells were obtained as described previously,25,26 and a confluent monolayer was generated by plating 10 × 105 BM stromal cells (BMSCs) in a 96-well plate for 48 hours. MM.1S cells, prelabeled with calcein-AM, were treated with rapamycin or INK128 and plated in coculture with stromal cells for 2 hours at 37°C. Nonadherent cells were washed from the wells and fluorescence intensity was assessed.
siRNA transfections
The delivery of siRNA into MM1S and OPM2 cells was performed using Lipofectamine 2000 reagent (Invitrogen). Cells were transfected with siRNAs at a final concentration of 50nM. The siRNAs used in this study were: Scrambled ON-TARGETplus nontargeting pool (Dharmacon #D 001810-10), Raptor ON-TARGETplus SMART-pool siRNAs (Dharmacon #D-52900811-12), and Rictor ON-TARGETplus SMART-pool siRNAs (Dharmacon #D-52900815-16).
Immunohistochemistry
BM biopsies from 16 patients with MM were fixed in Zanker formalin, embedded in paraffin blocks, and sectioned. Sections were stained for phospho-GSK3α/β and phospho-rS6 Ser235/236 (Cell Signaling Technology). IgG control Abs were used for negative controls. Normal BM was used to examine expression in MM compared with healthy controls. Scoring of all slides was performed by a hematopathologist at the Dana-Farber Cancer Institute.
Nanofluidic proteomic immunoassay
Nanofluidic proteomic immunoassay experiments were performed on MM cells treated or not with INK128 or rapamycin using a CB1000 instrument (Cell Biosciences), as described previously.27
In vivo flow cytometry
In vivo flow cytometry is a new technology that allows real-time continuous monitoring of fluorescent cells in the circulation of live animals without the need to draw blood samples.25,26 OPM2 cells were treated with rapamycin and INK128 and then labeled with calcein. Nontreated cells were labeled with calcein-red/orange. Equal numbers (3 × 106 cells) of labeled, treated, and nontreated cells were mixed in a final volume of 150 μL of medium and injected into the tail veins of SCID mice. Mice were anesthetized and lasers were focused on an appropriate arteriole in the ear. The calcein-labeled circulating cells were excited by 473- and 561-nm lasers, and signals were detected by photomultiplier tubes through 528/19- and 610/60-nm bandpass filters and analyzed with Matlab Version R2009a software. Cell counts were obtained at least every 5 minutes for 40 minutes.
Statistical analysis
The statistical significance of differences in drug-treated versus control cultures was determined using the Student t test. Drug synergism was analyzed by isobologram analysis using the CalcuSyn Version 2.0 software program (Biosoft), as described previously.28 Combination index < 1 indicates synergism, combination index = 1 indicates an additive effect, and combination index > 1 indicates antagonism.
Results
MM is characterized by constitutive activation of the PI3K/Akt/mTOR pathway
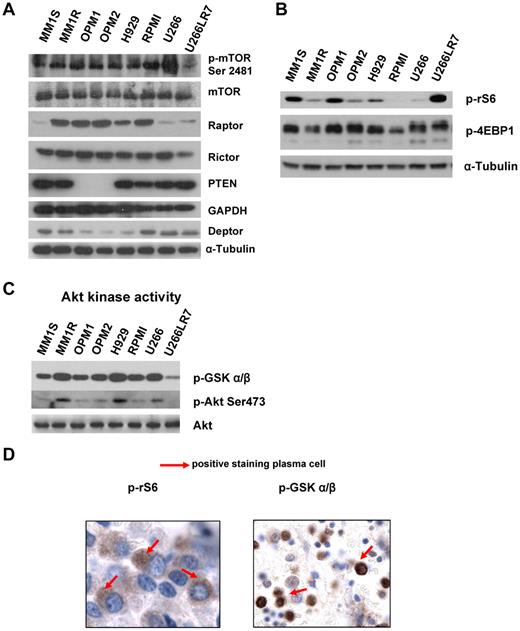
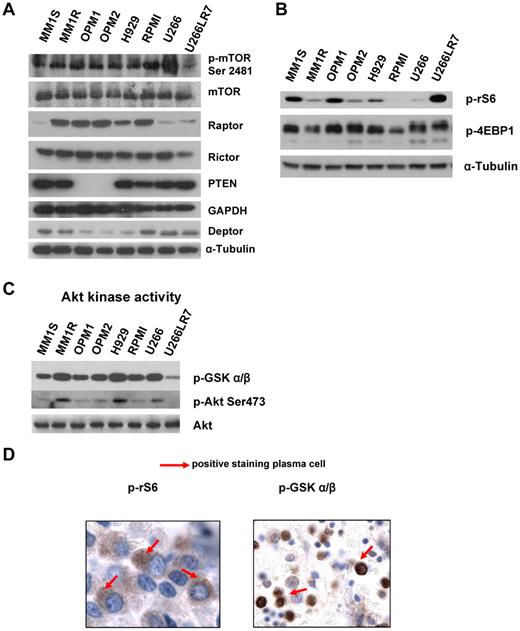
To assess whether mTOR was activated in MM, we initially examined the relative level of activity in a panel of 8 MM representative cell lines: MM1S and MM1R with t(14;16); OPM1, OPM2, and H929 with t(4;14); and RPMI8226 t(16;22) and U266 and U266LR7 with t(11;14). Figure 1A and B shows the protein expression levels of various mTOR complexes (TORC1/2) and their downstream effectors.
MM cells are characterized by constitutive activation of the PI3K/Akt/mTOR pathway. (A) Immunoblotting for p-mTOR at Ser2481, mTOR, Raptor, Rictor, Deptor, PTEN. and α-tubulin in MM cell lines. (B) Immunoblotting for p-rS6 Ser235/236, p-4EBP1 Thr37/46, and α-tubulin in MM cell lines. (C) In vitro Akt kinase assay and immunoblotting for p-Akt Ser473 and total Akt. For the Akt kinase assay, whole-cell lysates were immunoprecipitated with anti-Akt Ab, and then the immunoprecipitate was washed and subjected to the in vitro kinase assay according to the manufacturer's protocol. Western blot analysis was performed with anti–p-GSK3α/β. (D) BM biopsies from MM patients (n = 25) were fixed in Zanker formalin, embedded in paraffin blocks, and sectioned. Sections were then stained for p-rS6 Ser235/236 and p-GSK3α/β.
MM cells are characterized by constitutive activation of the PI3K/Akt/mTOR pathway. (A) Immunoblotting for p-mTOR at Ser2481, mTOR, Raptor, Rictor, Deptor, PTEN. and α-tubulin in MM cell lines. (B) Immunoblotting for p-rS6 Ser235/236, p-4EBP1 Thr37/46, and α-tubulin in MM cell lines. (C) In vitro Akt kinase assay and immunoblotting for p-Akt Ser473 and total Akt. For the Akt kinase assay, whole-cell lysates were immunoprecipitated with anti-Akt Ab, and then the immunoprecipitate was washed and subjected to the in vitro kinase assay according to the manufacturer's protocol. Western blot analysis was performed with anti–p-GSK3α/β. (D) BM biopsies from MM patients (n = 25) were fixed in Zanker formalin, embedded in paraffin blocks, and sectioned. Sections were then stained for p-rS6 Ser235/236 and p-GSK3α/β.
As shown in Figure 1A, we found 3 subgroups of MM cell lines. MM1S, U266, and U266LR7 have low levels of Raptor and high levels of PTEN and Deptor, which is consistent with prior findings on the role of Deptor in MM.12 OPM1 and OPM2 express both Rictor and Raptor and have low levels of Deptor, but interestingly show low/absent levels of PTEN, indicating activation of this pathway through PTEN mutation.8,17 MM1R, H929, and RPMI8226 showed similar expression of Raptor and Rictor. In addition, we showed that p-mTOR Ser2448, which is auto-phosphorylated by mTOR, is highly expressed in all cell lines, indicating that this pathway is activated in all cell lines tested.
Regardless of the different translocations present in the 8 cell lines, we detected expression of Deptor in all of them. Interestingly, the cell lines with t(4;14), including OPM1, OPM2, and H929, showed low levels of Deptor. We also noted that decreased Deptor levels were correlated with the undetectable status of PTEN in OPM1 and OPM2.
We also examined downstream targets of TORC1, and all 8 cell lines expressed both p-rS6 Ser235/236 and p-4EBP1 Thr37/46 (Figure 1B). To determine the downstream targets of TORC2, we examined Akt kinase activity in 8 MM cell lines. Lysates were prepared from 8 cell lines and Akt was immunoprecipitated and subjected to in vitro kinase assays using GSK-3α/β as a substrate. Activated p-Akt was confirmed by Western blot analysis in all cell lines tested (Figure 1C).
To determine whether mTOR activity was elevated in primary MM patients, we examined 16 different BM samples of patients with MM. Of the 16 MM samples tested, all (100%) were at least 1+ positive for pGSK-3α/β, and 9 of 16 (50%) showed positive staining of 2-3+ for pGSK. In addition, 9 of 16 were at least 1+ positive for prS6 Ser235/236, and 5 of 16 (30%) showed 2-3+ positive staining for p-rS6. The immunohistochemistry is shown in a representative sample in Figure 1D.
siRNA-mediated inhibition of Rictor and Raptor in MM cell lines
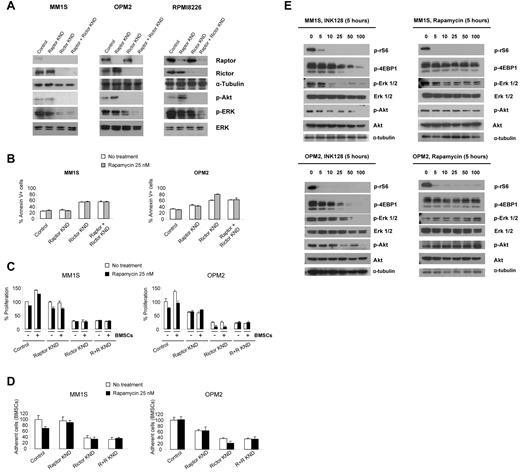
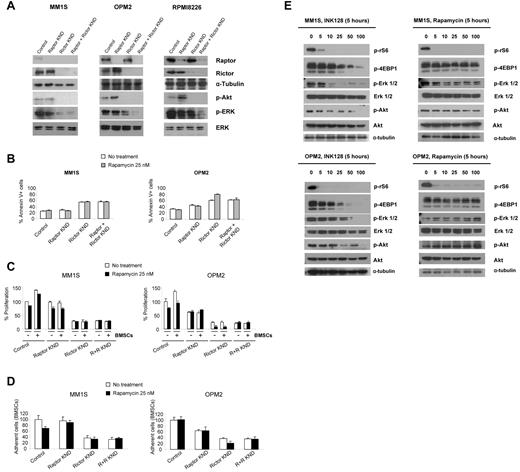
To determine whether reductions in Raptor or Rictor expression had effects on activated survival pathways in MM, we transfected MM1S (with baseline active Rictor and low Raptor) and OPM2 (with baseline active Rictor and Raptor) cells with siRNA targeting Raptor, Rictor, or a control siRNA containing a nontargeting sequence. As shown in Figure 2A, successful knockdowns were achieved in MM1S, OPM2, and RPMI8226 cells with siRNA targeting Raptor, Rictor, or both. In OPM2 and RPMI8226 cells, Raptor knockdown increased the levels of p-Akt but siRNA targeting Rictor reduced p-Akt levels in MM1S, OPM2, and RPMI8226 cells. The combination of Raptor and Rictor knockdowns decreased the phosphorylation of Erk in all 3 cell lines.
siRNA-mediated inhibition of Rictor and Raptor in MM cell lines. MM1S and OPM2 cells were transfected with scramble probe, Raptor, Rictor, or both siRNAs. (A) Whole-cell lysates were subjected to Western blot analysis using anti-Raptor, anti-Rictor, anti–p-Akt, anti–p-Erk, anti-Erk, and anti–α-tubulin Abs. (B) Cells were treated with rapamycin 25nM for 48 hours and apoptosis was assessed with annexin V. (C) MM1S and OPM2 knockdown cells were cultured with rapamycin 25nM for 48 hours in the presence or absence of BMSCs. Cell proliferation was assessed using the BrdU uptake assay. (D) Adhesion assay with MM1S and OPM2 knockdown cells to BMSCs in the presence or absence of rapamycin 25nM. All data represent means ± SD of quadruplicate experiments. (E) MM1S and OPM2 cells were cultured with control medium, rapamycin (5-100nM), or INK128 (5-100nM) for 5 hours. Whole lysates were subjected to Western blot analysis using p-rS6 Ser235/236, p-4EBP1 Thr 37/46, and α-tubulin.
siRNA-mediated inhibition of Rictor and Raptor in MM cell lines. MM1S and OPM2 cells were transfected with scramble probe, Raptor, Rictor, or both siRNAs. (A) Whole-cell lysates were subjected to Western blot analysis using anti-Raptor, anti-Rictor, anti–p-Akt, anti–p-Erk, anti-Erk, and anti–α-tubulin Abs. (B) Cells were treated with rapamycin 25nM for 48 hours and apoptosis was assessed with annexin V. (C) MM1S and OPM2 knockdown cells were cultured with rapamycin 25nM for 48 hours in the presence or absence of BMSCs. Cell proliferation was assessed using the BrdU uptake assay. (D) Adhesion assay with MM1S and OPM2 knockdown cells to BMSCs in the presence or absence of rapamycin 25nM. All data represent means ± SD of quadruplicate experiments. (E) MM1S and OPM2 cells were cultured with control medium, rapamycin (5-100nM), or INK128 (5-100nM) for 5 hours. Whole lysates were subjected to Western blot analysis using p-rS6 Ser235/236, p-4EBP1 Thr 37/46, and α-tubulin.
We next determined whether knockdown of Raptor or Rictor expression affected cell apoptosis in these cell lines. As shown in Figure 2B, MM1S and OPM2 Rictor knockdown cells showed a marked increase in the percentage of annexin V+ cells compared with control cells: from 25% to 54% in MM1S cells (top panel) and from 32% to 60% in OPM2 cells (bottom panel). The effect of Raptor knockdown in apoptosis was lower than Rictor knock-down: from 32% to 45% in OPM2 cells (Figure 2B). The combination of Raptor and Rictor knockdown did not increase the effect of Rictor knockdown alone (Figure 2B). Moreover, we found that treatment with rapamycin (a TORC1 inhibitor) increased the percentage of annexin V+ cells in OPM2 Rictor knockdown cells, whereas no changes were detected in MM1S cells. These findings suggest that Rictor suppression can significantly induce apoptosis and increase the effect of rapamycin in MM cells lines expressing Raptor. INK128 did not induce apoptosis or cytotoxicity in Rictor and Raptor dual knockdown in MM1S and OPM2 cells (supplemental Figure 1).
To further characterize the role of TORC2 in MM progression, we analyzed the effect of Raptor or/and Rictor knockdown on MM proliferation alone or in contact with BMSCs.
Using the BrdU uptake assay, we found that the proliferation of MM1S and OPM2 cells deficient for Rictor was markedly reduced (Figure 2C). Knockdown of Raptor also reduced the proliferation in OPM2 cells compared with control, however, to a lesser extent than Rictor knockdown (Figure 2C right panel). The presence of the BM microenvironment confers protection to myeloma cells through their adhesion or through the production of several cytokines, such as IL-6 or IGF-I.29 To determine whether down-regulation of Raptor and/or Rictor was able to overcome the protective effect of the BM microenvironment, Raptor and Rictor knockdown MM1S and OPM2 cells were cocultured with BMSCs and treated with rapamycin for 48 hours. Despite the proliferative advantage to MM cells conferred by BMSCs, Rictor knockdown cells completely abrogated the effect of the BMSCs in MM1S and OPM2 (Figure 2C). In contrast, Raptor knockdown was not able to overcome the BMSC effect (Figure 2C right panel). As we showed previously for apoptosis, Rictor knockdown also sensitized OPM2 to the rapamycin effect (Figure 2C).
To gain insight into the mechanism by which Rictor knock-down reduced MM proliferation in the presence of BMSCs, we analyzed the role of Raptor and Rictor knockdown on adhesion of MM1S and OPM2 cell lines to BMSCs in vitro. We found that Rictor knockdown induced significant inhibition of adhesion to BMSCs in both cell lines, whereas Raptor knockdown had less of an effect compared with Rictor knockdown in OPM2 (Figure 2D). These studies show that MM cells with Rictor knockdown become sensitive to rapamycin. These results show that inhibition of the mTOR2 protein Rictor leads to growth inhibition, apoptosis, and disruption between MM cells and BMSCs, suggesting that selective targeting of both TORC1 and TORC2 may represent a novel and promising therapeutic strategy for MM treatment.
We next investigated whether INK128, a novel, orally active, potent, and selective small molecule active-site mTOR kinase (TORC1/2) inhibitor, was able to mimic the effect of Rictor knockdown compared with rapamycin, which only targets TORC1. INK128 effectively suppressed p-rS6 and decreased the levels of p-4E-BP1 in MM1S and OPM2 cells (Figure 2E). In contrast, in both cell lines, rapamycin was only able to suppress p-rS6 (Figure 2E). Rapamycin consistently increased phosphorylation of Akt in MM1S and OPM2 cells, whereas INK128 inhibited activation of Akt in these cell lines (Figure 2E). As in the knockdown experiments, only INK128 was able to decrease the levels of pErk—rapamycin did not affect the activity of Erk (Figure 2E).
These results are in agreement with previous studies showing that rapamycin is not able to decrease the phosphorylation of 4EBP1 at Thr37/46.30
Antimyeloma action of TORC1/2 inhibition in cell lines and freshly isolated plasma cells from patients
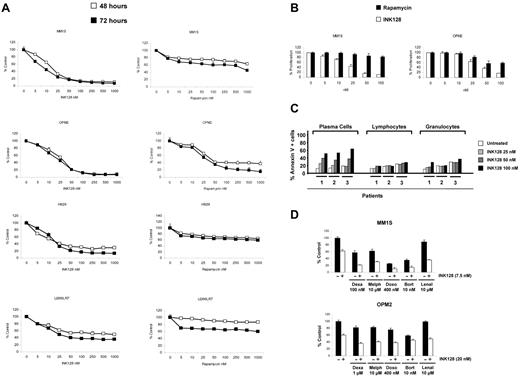
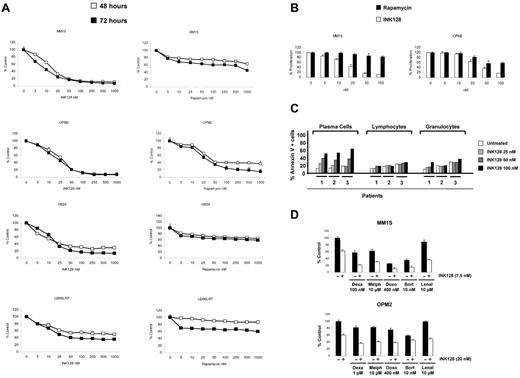
To test the antiproliferative/cytotoxic effect of mTOR1/2 inhibition in MM cells, 7 MM cell lines were treated with increasing doses of INK128 and rapamycin (5-1000nM) for 24, 48, and 72 hours, and viability was analyzed with MTT assays. As shown in Figure 3A and supplemental Figure 2, all cell lines were sensitive to the mTOR1/2 inhibition, with half-maximal inhibitory concentration values at 48 hours ranging from 7.5-25nM. Interestingly, cell lines that expressed Rictor but low levels of Raptor were sensitive to INK128 but more resistant to rapamycin (a Raptor inhibitor), whereas cell lines that expressed both Rictor and Raptor were sensitive to both rapamycin and INK128, but more so to INK128. As shown in Figure 3A, U266LR7 cells with low levels of Raptor and Rictor were the most resistant cell line to both INK128 and rapamycin. However, INK128 showed more activity on U266LR7 compared with rapamycin. These results suggest a strong correlation between Rictor and Raptor expression and response/resistance to TORC1 or TORC1/2 inhibitors.
INK128 triggers cytotoxicity in MM cell lines and primary patient cells without affecting normal cells. (A) Cytotoxicity was assessed with the MTT assay. MM cell lines (MM1S, OPM2, H929, and U266LR7) were cultured with rapamycin (5-1000nM) or INK128 (5-1000nM) for 48 and 72 hours. The average proliferation values of control, untreated samples were taken as 100%. Points indicate the means of quadruplicates of an experiment that were repeated at least twice. (B) BrdU uptake assay. MM1S and OPM2 were cultured with rapamycin (5-100nM) or INK128 (5-100nM) for 48 hours. (C) Action of INK128 in cells from patients with MM. Patient cells were plated in 6-well plates and treated with INK128 at 25, 50, and 100nM concentrations. After 24 hours, cells were stained with annexin V–FITC and 4 mAbs (CD38, CD56, CD138, and CD45), which allowed the distinction between MM plasma cells and the remaining normal BM cells, normal lymphocytes, and granulocytes. (D) INK128 (2.5-20nM) was combined with dexamethasone (250-1000nM), melphalan (2.5-7.5nM), doxorubicin (100-400nM), bortezomib (2.5-10nM), or lenalidomide (1-10 μM). After 48 hours, MTT assays were performed on MM1S and OPM2 cells. Only the most representative doses of each combination are included in the figure.
INK128 triggers cytotoxicity in MM cell lines and primary patient cells without affecting normal cells. (A) Cytotoxicity was assessed with the MTT assay. MM cell lines (MM1S, OPM2, H929, and U266LR7) were cultured with rapamycin (5-1000nM) or INK128 (5-1000nM) for 48 and 72 hours. The average proliferation values of control, untreated samples were taken as 100%. Points indicate the means of quadruplicates of an experiment that were repeated at least twice. (B) BrdU uptake assay. MM1S and OPM2 were cultured with rapamycin (5-100nM) or INK128 (5-100nM) for 48 hours. (C) Action of INK128 in cells from patients with MM. Patient cells were plated in 6-well plates and treated with INK128 at 25, 50, and 100nM concentrations. After 24 hours, cells were stained with annexin V–FITC and 4 mAbs (CD38, CD56, CD138, and CD45), which allowed the distinction between MM plasma cells and the remaining normal BM cells, normal lymphocytes, and granulocytes. (D) INK128 (2.5-20nM) was combined with dexamethasone (250-1000nM), melphalan (2.5-7.5nM), doxorubicin (100-400nM), bortezomib (2.5-10nM), or lenalidomide (1-10 μM). After 48 hours, MTT assays were performed on MM1S and OPM2 cells. Only the most representative doses of each combination are included in the figure.
To further confirm the effect of INK128 and rapamycin, we chose MM1S cells, which only express Rictor, and OPM2 cells, which express both Raptor and Rictor, to investigate their effects on proliferation. As shown in Figure 3B, INK128 was much more effective at inhibiting MM1S and OPM2 proliferation, as measured by the BrdU uptake assay, compared with rapamycin.
The effect of TORC1/2 inhibition was further investigated ex vivo in cells isolated from BM samples obtained from patients with MM. BM aspirates containing tumor plasma cells were incubated with different concentrations of INK128 (25-100nM) for 48 hours, and the induction of apoptosis was analyzed by flow cytometry. As shown in Figure 3C (left panel), all patients analyzed were sensitive to INK128, even at the lowest dose (25nM) of the drug. Moreover, an apparent dose-response effect could be observed. One relevant finding was the low toxicity of INK128 on the normal lymphocytes and granulocytes present in the same samples: no cytotoxicity was induced by INK128 at 25nM (Figure 3C right panel and supplemental Figure 3). INK128 had no activity on CD34 stem cells even at high concentrations of 500nM (supplemental Figure 4).
Because treatment of most cancers, including MM, is based on combinations of drugs with different mechanisms of action, we studied the effect of INK128 in combination with conventional agents used in the treatment of MM, such as dexamethasone, melphalan, doxorubicin, bortezomib, and lenalidomide, in MM1S and OPM2 cells (Figure 3D). Analyses of these data using the Chou and Talalay method28 indicated that in MM1S cells, INK128 was synergistic with melphalan, doxorubicin, and particularly with dexamethasone. In OPM2, INK128 was synergistic with melphalan and additive with doxorubicin and dexamethasone. The combination indexes for bortezomib were in the additive range in both cell lines. INK128 also increased the effect of lenalidomide in both cell lines (supplemental Figures 5 and 6 and supplemental Tables 1 and 2).
TORC1/2 inhibition leads to cell-cycle arrest and cell death
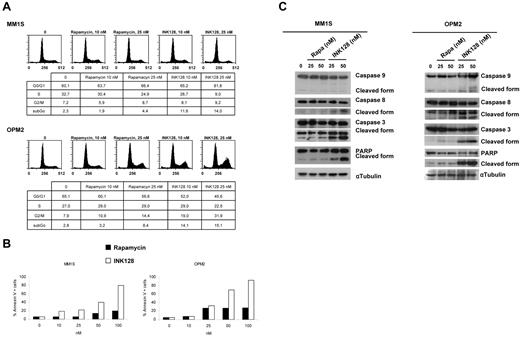
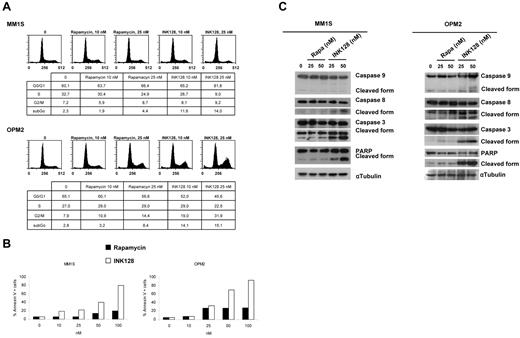
To gain insights into the mechanism of action of mTOR1/2 inhibition on MM cells, we performed biochemical studies of cellular proteins involved in the regulation of the cell cycle, survival, and pathways. We evaluated the effect of INK128 and rapamycin in modulating cell-cycle regulation in MM cells. In MM1S, both drugs induced G1 arrest and reduction of S phase from 32.7% (untreated) to 24.9% and 9.0% with rapamycin and INK128, respectively. In OPM2 cells, rapamycin and INK128 induced G2 arrest and reduction of S phase from 27.0% (untreated) to 29.0% and 22.5%, respectively, Figure 4A. We next investigated the effect on apoptosis. We demonstrated that INK128 induced apoptosis, as evidenced by annexin/propidium iodide staining and flow cytometric analysis. The percentage of apoptotic cells increased from 5.6% (untreated) to 80.0% after 48 hours of treatment with INK128 in MM1S cells. In OPM2 cells, the effect on apoptosis was even higher than in MM1S cells, from 5.2%-93% after 48 hours of treatment with 100nM of INK128 (Figure 4B). Rapamycin did not have significant effect on apoptosis: its effect was primarily cytostatic (Figure 4B). We also observed cleavage of PARP and caspase 3, 8, and 9 in both MM cell lines treated with INK128, but not in those treated with rapamycin, which is consistent with its ability to induce apoptosis (Figure 4C).
INK128 induces cell-cycle arrest and apoptosis in MM cells. (A) MM1S and OPM2 cells were cultured with rapamycin (10 and 25nM) or INK128 (10 and 25nM) for 24 hours, and cell-cycle analysis was performed by propidium iodide staining. (B) MM1S and OPM2 cells were treated with rapamycin (5-100nM) or INK128 (5-100nM) for 48 hours, and apoptosis was determined using annexin/propidium iodide staining and flow cytometric analysis. (C) MM1S and OPM2 cells were cultured with rapamycin (25 and 50nM) or INK128 (25 and 50nM) for 24 hours. Whole-cell lysates were subjected to Western blotting using anti–caspase-9, anti–caspase-8, anti–caspase-3, anti-PARP, and anti–α-tubulin Abs.
INK128 induces cell-cycle arrest and apoptosis in MM cells. (A) MM1S and OPM2 cells were cultured with rapamycin (10 and 25nM) or INK128 (10 and 25nM) for 24 hours, and cell-cycle analysis was performed by propidium iodide staining. (B) MM1S and OPM2 cells were treated with rapamycin (5-100nM) or INK128 (5-100nM) for 48 hours, and apoptosis was determined using annexin/propidium iodide staining and flow cytometric analysis. (C) MM1S and OPM2 cells were cultured with rapamycin (25 and 50nM) or INK128 (25 and 50nM) for 24 hours. Whole-cell lysates were subjected to Western blotting using anti–caspase-9, anti–caspase-8, anti–caspase-3, anti-PARP, and anti–α-tubulin Abs.
Dual inhibition of TORC1/2 abrogates the survival advantage and drug resistance induced by the BM microenvironment
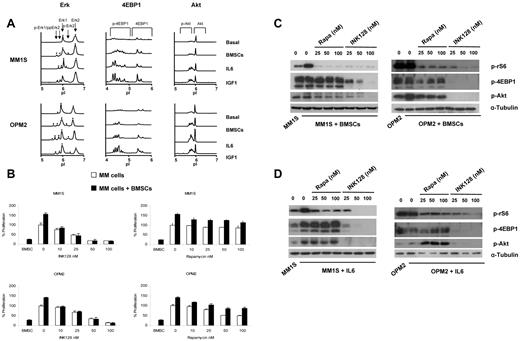
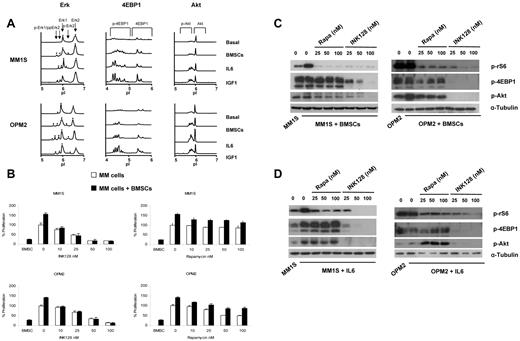
To determine whether the BM microenvironment had any effect on the mTOR pathway and its downstream targets, MM1S and OPM2 cells were incubated with IL-6 (100nM) or IGF-I (100nM) or were cocultured with BMSCs, and protein expression was analyzed by Western blot analysis and the nanofluidic proteomic immunoassay.27
Using the nanofluidic proteomic immunoassay, we showed that BMSCs, IL-6, and IGF1 increased the levels of p-4EBP1 as well as p-Akt and p-Erk (Figure 5A). To determine whether TORC1 or TORC1/2 is a critical regulator of resistance induced by the adhesion of MM cells to the BM microenvironment, MM1S and OPM2 cells were cocultured with BMSCs and treated with increasing concentrations of INK128 or rapamycin for 48 hours. Proliferation was analyzed by BrdU uptake. Despite the proliferative advantage to MM cells conferred by BMSCs, INK128 completely abrogated the effect resulting from adhesion of plasma cells to BMSCs (Figure 5B). In contrast, rapamycin was not able to overcome the BMSC effect (Figure 5B).
Coculture with BMSCs does not protect against INK128-induced MM-cell cytotoxicity. (A) Detection of Erk, 4EBP1, and Akt activation with the nanofluidic proteomic immunoassay in response to coculture with BMSCs, IL-6, or IGF1 in MM1S and OPM2 cells. Peaks on the traces that represent phosphorylated isoforms are indicated with black arrows. All measurements were performed in triplicate. (B) MM1S and OPM2 cells were cultured with rapamycin (10-100nM) or INK128 (10-100nM) for 48 hours in the presence or absence of BMSCs. Cell proliferation was assessed using the BrdU uptake assay. (C) MM1S and OPM2 cells were cultured with rapamycin (25-100nM) or INK128 (25-100nM) for 5 hours in the presence or absence of BMSCs. Whole-cell lysates were subjected to Western blotting using anti–p-rS6, anti–p-4EBP1, anti–p-Akt, and anti–α-tubulin Abs. (D) MM1S and OPM2 cells were cultured with rapamycin (25-100nM) or INK128 (25-100nM) for 5 hours in the presence or absence of IL-6. Whole-cell lysates were subjected to Western blotting using anti–p-rS6, anti–p-4EBP1, anti–p-Akt, and anti–α-tubulin Abs.
Coculture with BMSCs does not protect against INK128-induced MM-cell cytotoxicity. (A) Detection of Erk, 4EBP1, and Akt activation with the nanofluidic proteomic immunoassay in response to coculture with BMSCs, IL-6, or IGF1 in MM1S and OPM2 cells. Peaks on the traces that represent phosphorylated isoforms are indicated with black arrows. All measurements were performed in triplicate. (B) MM1S and OPM2 cells were cultured with rapamycin (10-100nM) or INK128 (10-100nM) for 48 hours in the presence or absence of BMSCs. Cell proliferation was assessed using the BrdU uptake assay. (C) MM1S and OPM2 cells were cultured with rapamycin (25-100nM) or INK128 (25-100nM) for 5 hours in the presence or absence of BMSCs. Whole-cell lysates were subjected to Western blotting using anti–p-rS6, anti–p-4EBP1, anti–p-Akt, and anti–α-tubulin Abs. (D) MM1S and OPM2 cells were cultured with rapamycin (25-100nM) or INK128 (25-100nM) for 5 hours in the presence or absence of IL-6. Whole-cell lysates were subjected to Western blotting using anti–p-rS6, anti–p-4EBP1, anti–p-Akt, and anti–α-tubulin Abs.
We also evaluated the effect of INK128 and rapamycin on mTOR1/2 downstream targets in the presence of BMSCs or IL-6. In contact with BMSCs, both INK128 and rapamycin were able to decrease the levels of p-rS6, but only INK128 was able to decrease p-4EBP1 and p-Akt (Figure 5C). In cells incubated with IL-6 (100nM), only INK128 was able to abrogate the effect on p-rS6, p-4EBP1, and p-Akt (Figure 5D).
In vitro and in vivo adhesion
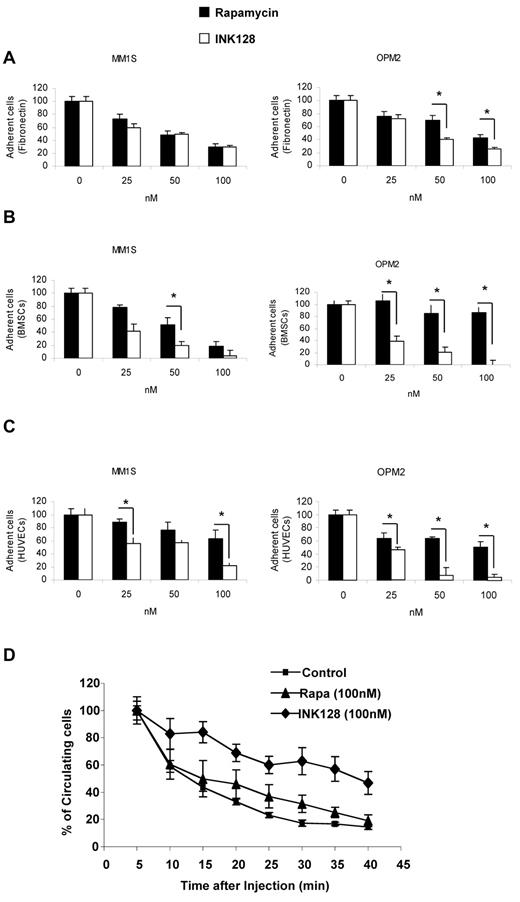
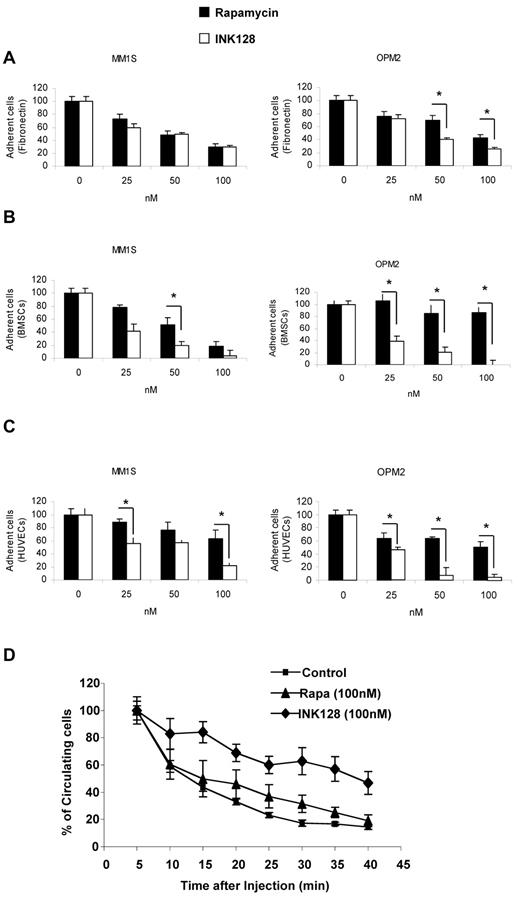
We next tested the effect of INK128 and rapamycin on adhesion of MM1S and OPM2 cell lines to fibronectin, BMSCs, or HUVECs. Cells were treated for 2 hours and no apoptosis was detected at this time point. We found that INK128 had a similar effect to rapamycin in adhesion of MM1S to fibronectin, but in OPM2 cells, the effect of INK128 was higher than rapamycin (P = .01; Figure 6A). Regarding adhesion to BMSCs and HUVECs, the effect of INK128 was higher than rapamycin in MM1S cells and markedly superior in OPM2 cells (P ≤ .01; Figure 6B-C).
Effect of INK128 on adhesion of MM cells. Adhesion assays with MM1S and OPM2 in the presence or absence of rapamycin (25-100nM) or INK128 (25-100nM). (A) Adhesion of MM cells to primary fibronectin. (B) Adhesion of MM cells to primary BMSCs. (C) Adhesion of MM cells to HUVECs. All data represent means ± SD of triplicate experiments. (D) Effect of rapamycin and INK128 on OPM2 cell homing after tail-vein injection in mice detected by circulating OPM2 cells using in vivo flow cytometry. All data represent means ± SD of triplicate experiments.
Effect of INK128 on adhesion of MM cells. Adhesion assays with MM1S and OPM2 in the presence or absence of rapamycin (25-100nM) or INK128 (25-100nM). (A) Adhesion of MM cells to primary fibronectin. (B) Adhesion of MM cells to primary BMSCs. (C) Adhesion of MM cells to HUVECs. All data represent means ± SD of triplicate experiments. (D) Effect of rapamycin and INK128 on OPM2 cell homing after tail-vein injection in mice detected by circulating OPM2 cells using in vivo flow cytometry. All data represent means ± SD of triplicate experiments.
To further investigate the role of TORC1/2 on adhesion in vivo, we tested the effect of the rapamycin and INK128 inhibitors on the number of circulating OPM2 cells after tail-vein injection by the use of in vivo flow cytometry. As shown in Figure 6D, almost all control and rapamycin-treated cells exited from the circulation 30 minutes after the injection, indicating homing to the BM, as was also shown in our prior studies.25,26 However, pretreatment of the cells with INK128 prolonged the time of circulation of OPM2 cells, and approximately 60% of the cells were still circulating 35 minutes after injection (Figure 6D).
In vivo antimyeloma efficacy of INK128
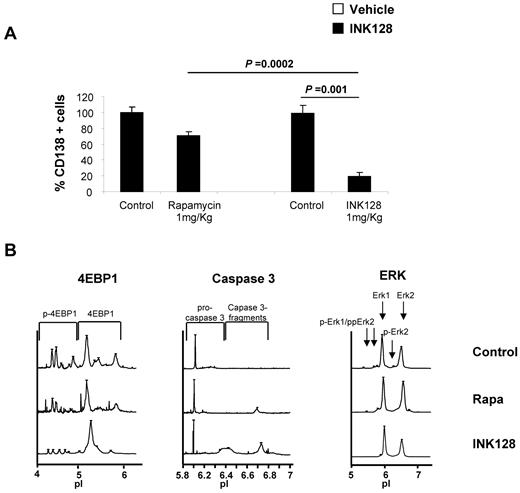
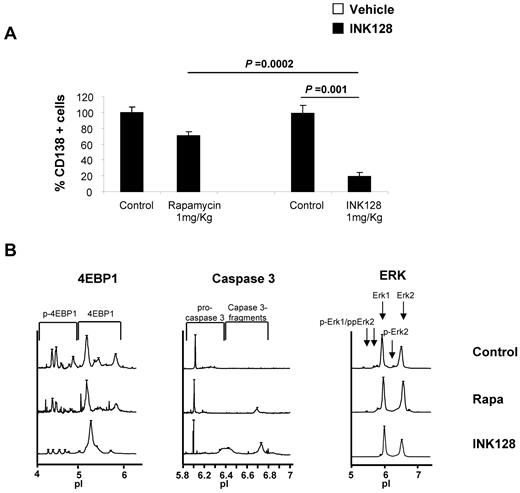
To examine the in vivo pharmacodynamic activity of INK128 or rapamycin on mTOR activity in MM cell lines, 9 mice were treated with vehicle (n = 3), rapamycin 1 mg/kg/d IP (n = 3), and INK128 1 mg/kg by oral gavage (n = 3). Treatment was continued for 1 week to determine the early activity of these agents on mTOR pathway proteins in MM cells. BM cells and mononuclear cells were collected. Human CD138+ cells were detected by flow cytometry from the femurs of all mice treated. As shown in Figure 7A, rapamycin-treated mice showed a minimal decrease in the number of OPM2 cells obtained from the BM, whereas INK128-treated mice showed a significant decrease in the number of cells (P = .0002 and P = .001 for rapamycin vs INK128 and control vs INK128, respectively). Furthermore, cells were collected and CD138+ cells were isolated to perform nanofluidic proteomic immunoassay studies. INK128 reduced the levels of p-Akt and p-4EBP1 and induced caspase-3 cleavage (Figure 7B). Consistent with the “in vitro” results in MM1S and OPM2 cell lines, rapamycin induced an increase in p-Akt and was not be able to reduce the p-4EBP1 levels, whereas INK128 showed significant in vivo inhibition of both Akt and p4-EBP1, as well as caspase 3 activation in the cells tested. Erk was used as a control and showed no change in rapamycin- and INK128-treated mice, indicating that there was no activation of Erk on inhibition of the TORC1/2 pathways.
In vivo antimyeloma effect of INK128. Vehicle, rapamycin, or INK128 was administered by oral gavage at 1 mg/kg/d for 10 days. (A) Detection of CD138+ cells in the BM of mice assessed by flow cytometry. (B) Nanofluidic proteomic immunoassay analyses for Akt, p-4EBP1, and caspase-3 were performed in CD138+ cells isolated from femurs of 9 mice.
In vivo antimyeloma effect of INK128. Vehicle, rapamycin, or INK128 was administered by oral gavage at 1 mg/kg/d for 10 days. (A) Detection of CD138+ cells in the BM of mice assessed by flow cytometry. (B) Nanofluidic proteomic immunoassay analyses for Akt, p-4EBP1, and caspase-3 were performed in CD138+ cells isolated from femurs of 9 mice.
Discussion
In the present study, we examined the activity of the TORC1/2 complexes in different MM cell lines and patient samples and showed that the pathway and its regulators are activated in most cell lines, either because of c-maf translocation and activation of the pathway through Deptor (as in MM1S) or because of PTEN mutation and activation of Akt (as in OPM1 and OPM2). These data reveal the complex levels of activation of the PI3K/Akt/mTOR pathway; specifically, they demonstrate that Deptor activation and c-maf translocation cannot be the only factors that predict response to TORC1/2 inhibitors. Indeed, the level of Rictor and Raptor can be used to predict response to TORC1 and TOCR1/2 inhibitors. TORC1 (Raptor) controls cell growth in part by phosphorylating S6 kinase 1 (S6K1) and the eIF-4E–binding protein 1 (4E-BP1), whereas TORC2 (Rictor) modulates cell survival in response to growth factors by phosphorylating its downstream effectors Akt/PKB.
Prior studies have shown that the activation of Akt that results from TORC1 inhibitors such as rapamycin may contribute to the limited success of this class of agents as cancer therapies in MM. Our loss-of-function data demonstrate that dual inhibition of function of both Rictor and Raptor is essential for the induction of apoptosis in MM cells that harbor Rictor or Rictor and Raptor. This effect becomes more evident when cells are cocultured with primary BM stromal cells that induce activation of the PI3K/Akt/mTOR pathway. Rictor and Raptor knockdown led to complete abrogation of proliferation of MM cells, even in the presence of growth stimulation by BM stromal cells.
We have demonstrated that INK128, a potent and selective TORC1/2 kinase inhibitor, showed the most potent inhibition of proliferation and cytotoxicity in 8 MM cell lines. INK128 showed significant activity on cells lines that had TORC1 activity or both TORC1 and TORC2 activities. INK128 did not have any cytotoxic effects on normal lymphocytes or granulocytes, indicating that it should have a therapeutic window in clinical trials of patients with MM. INK128 also enhanced the effect of several treatments that are currently used in MM, including dexamethasone, bortezomib, melphalan, doxorubicin, and lenalidomide. It was especially interesting to observe that the lenalidomide effect was highly potentiated with INK128 given the recent reports showing that high levels of Deptor predict response to thalidomide.31 Future studies to examine the mechanisms of synergy between immunomodulatory agents and TORC1/2 inhibitors are warranted.
We also examined the mechanism of activity of this novel agent on MM cells that harbor TORC1 (MM.1S) or TORC1/2 activity (OPM2). INK128 inhibits p-4EBP1 whereas rapamycin failed to inhibit this downstream protein in our cell lines. This effect could potentially be used in clinical trials as pharmacodynamic studies to determine the in vivo activity of this agent. We confirmed that p-4EBP1 is a marker of response in vivo using nanofluid proteomics from human plasma cells obtained from the BM of SCID mice in a xenograft model system, indicating that INK128 can better inhibit p-4EBP1 in vivo compared with rapamycin.
In addition, TORC1 and TORC2 have been implicated in the regulation of cell migration and adhesion.32-35 These processes are critical for cell dissemination and invasion in MM. We therefore examined the role of INK128 in inhibiting the adhesion of MM cells to primary stromal cells, endothelial cells, or fibronectin, representing the extracellular matrix. We showed that knockdown of Rictor and Raptor significantly inhibited the adhesion of MM cells to BM stromal cells. INK128 significantly inhibited the adhesion of MM cells to the extracellular matrix and to BM microenvironmental cells, whereas rapamycin did not have the same activity, indicating that both TORC1 and TORC2 are critical for regulating cell dissemination in MM. We further examined the in vivo activity of this agent and showed that it inhibited in vivo homing of MM cells compared with control or rapamycin.
There have been many attempts to target the PI3K/Akt/mTOR pathway in MM. Prior studies using TORC1 inhibitors have shown minimal activity in MM. Similarly, Akt inhibitors have shown limited activity in patients, partly because of the lack of specificity of these agents. In the present study, we describe a new molecule that showed significant activity in vitro and in vivo in MM cells by inhibiting the TORC1/2 complexes. Other inhibitors that target PI3K and mTOR may have similar activity, but may be more toxic on normal hematopoietic cells.30 This study therefore forms the basis for the study of INK128 in clinical trials on patients with MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by the National Institutes of Health (R01CA125690-01) and by the Multiple Myeloma Research Foundation.
National Institutes of Health
Authorship
Contribution: P.M. designed and performed research, analyzed data, and wrote the paper; B.M., A.K.A., F.A., A.S., and A.M.R. performed the research and analyzed data; Y.L. designed the INK128 molecule; P.R., M.B.M., and C.R. revised the manuscript; S.J.R., C.P.L., Y.Z., Y.L., H.N., and P.Q. analyzed the data; and I.M.G. designed the research and wrote the paper.
Conflict-of-interest disclosure: Y.L., P.R., M.B.M., and C.R. are employees of Intellikine Inc. I.M.G. is on the advisory board of Millennium, Celgene, Novartis, Genzyme, and Onyx. The remaining authors declare no competing financial interests.
Correspondence: Irene M. Ghobrial, MD, Medical Oncology, Dana-Farber Cancer Institute, 44 Binney St, Mayer 1B, Boston, MA 02115; e-mail: irene_ghobrial@dfci.harvard.edu.