Abstract
In vitro RBC production from stem cells could represent an alternative to classic transfusion products. Until now the clinical feasibility of this concept has not been demonstrated. We addressed the question of the capacity of cultured RBCs (cRBCs) to survive in humans. By using a culture protocol permitting erythroid differentiation from peripheral CD34+ HSC, we generated a homogeneous population of cRBC functional in terms of their deformability, enzyme content, capacity of their hemoglobin to fix/release oxygen, and expression of blood group antigens. We then demonstrated in the nonobese diabetes/severe combined immunodeficiency mouse that cRBC encountered in vivo the conditions necessary for their complete maturation. These data provided the rationale for injecting into one human a homogeneous sample of 1010 cRBCs generated under good manufacturing practice conditions and labeled with 51Cr. The level of these cells in the circulation 26 days after injection was between 41% and 63%, which compares favorably with the reported half-life of 28 ± 2 days for native RBCs. Their survival in vivo testifies globally to their quality and functionality. These data establish the proof of principle for transfusion of in vitro–generated RBCs and path the way toward new developments in transfusion medicine. This study is registered at http://www.clinicaltrials.gov as NCT0929266.
Introduction
The generation of RBCs in vitro with the use of biotechnologies could represent an interesting alternative to classic transfusion products in that it would combine adequate supplies with the specific production of blood products of a particular phenotype and reduce the risks posed by infection and reduce the risks posed by transfusion-transmitted infectious agents.1-3 The chronic difficulty of maintaining an RBC supply is supported by the high annual requirement of RBCs of nearly 90 million units in the world. In this context, new sources of hemoglobin have to be designed. Because of the disappointing results with oxygen-carrier substitutes, the production of bioengineered RBCs is a promising route that has to be tackled in the coming years to fulfill the public health issues.
It is now possible in vitro to obtain complete maturation of the erythroid line to the stage of enucleation, starting from HSCs from peripheral blood, BM,4-6 umbilical cord blood7 fetal liver,8 from embryonic cells,9-11 or adult pluripotent stem cells (induced pluripotent stem cells).12-14 However, until now the clinical feasibility of this concept has not been demonstrated, whatever the origin of the cells and the experimental protocol. Our objective was thus to evaluate the in vitro functionality and the in vivo behavior in animal model as well as in humans of these cultured RBCs (cRBCs) produced by cell engineering. We report here the first injection of cRBCs, into a human, that were generated under good manufacturing practice (GMP) conditions in an autologous situation.
We initially described a methodology for the ex vivo culture of HSCs15 that partially reproduces the conditions of the hematopoietic microenvironment in a serum-free medium in the presence of recombinant growth factors. However, the complexity of a coculture system is a hindrance to the scaling-up of this process for industrial development.16 Interestingly, replacement of the microenvironment by either FCS17 or human serum in the presence18 or absence of VEGF and IGF-II19 permits the maturation of erythroid precursors into enucleated cells. Overall these data support the hypothesis of enucleation signals mediated by soluble factors probably secreted by the microenvironment. Such observations are of a nature to simplify the manufacture of RBC for transfusion purposes, provided it is possible to demonstrate the functionality of these cells, a crucial point that had not yet been investigated.
First, we addressed the following questions: (1) what is the stage of maturity of the enucleated cells generated under these conditions; (2) are they functional; (3) can they be stored in vitro until use; and (4) what is their fate after injection in vivo in the animal model of the nonobese diabetes/severe combined immunodeficiency (NOD/SCID) mouse? The collection of these data provided the rationale for in vivo injection of homogeneous sample of cRBCs labeled with 51Cr into humans. Hence, we can report for the first time the persistence of cRBCs for several weeks in vivo in humans. These results establish the feasibility of the concept of the transfusion of cRBCs.20
Methods
Cell culture
CD34+ cells were isolated by supermagnetic microbead selection by the use of Mini-MACS columns (Miltenyi Biotec; 94% ± 3% purity). The cells were cultured in erythroid differentiation medium (EDM) on the basis of IMDM supplemented with stabilized glutamin (Biochrom), 330 μg/mL holo-human transferrin (Scipac), 10 μg/mL recombinant human insulin (Incelligent SG; CellGen), 2 IU/mL heparin Choay, and 5% solvent/detergent virus-inactivated plasma (Etablissement Français du Sang).
The expansion procedure comprised 3 steps. In the first step (day 0 to day 7), 104/mL CD34+ cells were cultured in EDM in the presence of 10−6 M hydrocortisone (Upjohn),100 ng/mL SCF (PeproTech), 5 ng/mL IL-3 (PeproTech), and 3 IU/mL Epo (Eprex, kindly provided by Janssen-Cilag). On day 4, 1 volume of cell culture was diluted in 4 volumes of fresh medium containing SCF, IL-3, Epo, and hydrocortisone. In the second step (day 7 to day 11), the cells were resuspended at 105/mL in EDM supplemented with SCF and Epo. In the third step (day 11 to day 18), the cells were cultured in EDM supplemented with Epo alone. Cell counts were adjusted to 7.5 × 105 to 1 × 106 and 5-10 × 106 cells/mL on days 11 and 15, respectively. Beyond day 18, the culture medium containing Epo was renewed twice a week. The cultures were maintained at 37°C in 5% CO2 in air, and results are presented in terms of the actual rate of expansion after plating.
Cells were stained with May-Grünwald-Giemsa and new methylene blue (NMB) reagents (Sigma-Aldrich) for morphologic analyses. For NMB staining, 2-3 × 105 cells were washed in PBS (pH 7.4) and incubated with 2 μL of NMB in a glass tube for 10 minutes. The cells were then spun onto a glass slide by cytocentrifugation and examined under a microscope. Cells with at least 2 granules were scored as reticulocytes.
Standard hematologic parameters (mean cell volume [MCV, fL], mean corpuscular hemoglobin concentration [MCHC, %], and mean cell hemoglobin [MCH, pg/cell]) were evaluated with the use of an XE2100 automate (Sysmex; Roche Diagnostics) in the day 18 enucleated population purified by passage through a deleukocyting filter (Leucolab LCG2; Macopharma).
Flow cytometry
Cells were labeled with unconjugated or FITC- or PE-conjugated antibodies. Anti-CD235 (glycophorin A)–PE, anti–CD45-FITC, anti–CD71-PE or -FITC, anti–CD36-FITC, and anti–CD34-PE antibodies (Beckman Coulter) were used for phenotyping. A primary human anti-RhD antibody and a secondary PE-conjugated goat anti–human antibody (Beckman Coulter) were used for RhD determination. Analyses were performed on a FACSCalibur flow cytometer (BD Biosciences) with Cell Quest software.
Vital nucleic acid dye.
We detected nucleic acid by staining cells with LDS-751 (Laser Dye Styryl; Invitrogen).7 Then, 2 × 106 cells were incubated for 20 minutes at 4°C with 0.04 μg of LDS. The supernatant was removed by centrifugation, and the cells were resuspended in PBS for flow cytometric analysis (FL3 channel).
Reticulocyte count by flow cytometry.
Reticulocyte count by flow cytometry was performed with 3 × 105 cells washed in PBS (pH 7.4) and incubated with 300 μL of Retic-count solution (Retic-count/Thiazole-Orange; BD Biosciences) for 30 minutes at room temperature. A negative control was carried out by incubating the cells with PBS alone.
Enzyme activities
Digitonin (0.2%) was added to the erythrocytes obtained after leukocyte depletion, and Hb was quantified by spectrophotometry with the use of Drabkin reagent. Glucose-6-phosphate dehydrogenase and pyruvate kinase activities were determined by measurement of the rate of increase in NADPH absorbance at 340 nm with the use of a Synchron CX4 Beckman spectrophotometer and reagents from Randox Laboratories and Roche Diagnostics, respectively. Results were expressed in units per gram of Hb.
Deformability measurements
The deformability of purified cRBC populations was measured by a laser diffraction technique (LORCA [laser-assisted optical rotational cell analyzer], R&R Mechanotrics).
The measuring principle of LORCA has been described in depth elsewhere.21 In brief, a highly diluted suspension of cells is sheared in a Couette system with a gap of 0.3 mm between 2 cylinders, one of which is able to rotate to induce shear stresses. A laser beam is passed through the suspension, and the diffraction pattern is measured at 37°C. At low shear stress, the cells are circular disks, whereas at high shear stress, the cells become elliptical. The cell deformability is expressed in terms of the elongation index (EI), which depends on the ellipticity of the deforming cells. EI is defined as (L − W)(L + W), where L and W are the major and minor axes of the ellipse, respectively. EI is recorded continuously at various shear stresses in the range 0.3-30 Pa.
Aliquots containing 12.5 μL of pelleted RBC pellets were diluted in 5 mL of polyvinylpyrrolidone solution (molecular weight 360 000). The osmolality and viscosity of the solution were 300 mOsm/kg and 31 mPa-s, respectively. The EI values at 30 Pa (referred to as EImax) and 3 Pa were selected as representative values of the deformability for easy comparison between samples at various shear stresses. Peripheral blood controls were collected from healthy donors (n = 10). Native reticulocytes were isolated from cord blood units (n = 3) by an immunomagnetic method (Miltenyi Biotec). In brief, the cells were incubated with anti-CD71 microbeads (Miltenyi Biotec), and the labeled cells were enriched on Mini-MACS columns. The reticulocyte content of the CD71-purified population was controlled by NMB staining (> 90% of cells with intense staining). To evaluate the impact of the immunomagnetic separation on cell deformability, purified day 18 cRBCs were selected by the immunomagnetic method and their deformability was compared with that of nonselected cRBC (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Hb analyses
Hb fractions were separated and quantified by ion-exchange HPLC as extensively previously reported.15 Analyses were performed on washed cell pellets with use of the Bio-Rad Variant II dual program (Bio-Rad Laboratories) according to the manufacturer's instructions.
Oxygen binding curves
Equilibrium oxygen binding curves at 37°C were determined in a tonometer linked to a 1-cm path length cuvette. Spectral measurements were performed with a spectrophotometer (Cary 50; Variant Inc), and the temperature was controlled with a Peltier module. Analyses were performed in 50mM bis-Tris buffer (pH 7.2) containing 140mM NaCl and 2mM glucose. After thorough deoxygenation under nitrogen, the red cell suspensions were equilibrated at different partial pressures of oxygen by injection of known volumes of pure oxygen into the tonometer through a rubber cap with a Hamilton syringe. The fractional saturation was estimated by simulation of the absorption spectra in the visible and Soret regions as a linear combination of the fully deoxygenated and oxygenated spectra of the RBC suspension by the use of a least-squares fitting routine of the software Scientist (Micromath Scientific Software).
Blood group antigen expression
The expression of 43 RBC antigens belonging to 28 blood group systems was studied serologically by agglutination (reference method of the CNRGS, Center National de Référence pour les Groupes Sanguins, Paris, France) by the use of specific antibodies. The expression of these antigens at the surface of cRBC was compared with that at the surface of native RBC from the same donor. The labeling intensity obtained was assessed visually for each antigen and quantified in increasing order from 1 to 4+.
Studies in the NOD/SCID mouse model
All procedures for animals are conformed to the French Ministry of Agriculture regulations and approved by the ethic committee for animals (CCP Ile de France V). NOD/SCID/-LtSz-scid/scid (NOD/SCID) mice were raised under sterile conditions. Mice 6-8 weeks of age were initially conditioned by sublethal irradiation with 2.5 Grays from a 137Cs source (2.115 Gy/min) and intraperitoneal injection of 5 × 109 human RBCs per mouse. After 24 hours, the mice were injected intraperitoneally with day 18 cRBCs (5 × 109 cells per mouse) previously washed, filtered through a deleukocyting filter, and labeled with CFSE (Invitrogen).22 The enucleated human cells were followed for up to 5 days after injection as CFSE+ cells by flow cytometry in 5-μL samples of heparinized blood drawn by retro-orbital vein puncture. The cells from peripheral blood were washed and colabeled with LDS-751 or anti-CD71 or anti-RhD antibodies. On day 3, CFSE+ cells were sorted and examined by confocal laser scanning microscopy.
Determination of the lifespan of cRBCs in humans
The clinical protocol was approved by the required French regulatory administrations: the Afssaps (Agence française de sécurité sanitaire des produits de santé, ref TC245) and the ethical committee of Paris (CCP Ile de France V, reference 08742). The donor of HSC gave his informed consent. This study is registered at http://www.clinicaltrials.gov as NCT00929266.
To remain in an autologous situation in this phase 1 study, the cRBCs were generated from CD34+ cells isolated by cytapheresis from a healthy donor of HSCs.23 CD34+ cells were isolated under GMP conditions with Clinimacs via the use of a 150 tubing set (Miltenyi Biotec). Then, 106 CD34+ cells were expanded to 1 × 1010 enucleated cells by the use of 13 L of medium. The cells were cultured in a closed system in 300 mL of LifeCell flasks (Baxter) until day 7 and then in Cellstacks (Macopharma) until day 18. The erythroid culture media were identical to those described previously. After filtration through a deleukocyting filter, cRBCs were stored overnight at 4°C before 51Cr labeling.
The use of chromium as a gold standard labeling technique24 is justified by (1) the nature of the studied population, which is pure in RBCs, allowing specific labeling; (2) homogeneous labeling as a function of the age of the cells; and (3) an absence of reuptake by living cells of chrome that is released by lysed cells. Aliquots containing 1010 cRBCs were labeled in vitro with a solution of 51Cr sodium chromate (activity 37 MBq/mL at calibration; GE Healthcare Medical Diagnostics). The cells were washed to eliminate free 51Cr and then resuspended in 0.9% NaCl solution before autologous injection intravenously into the same donor of HSC. After injection of the labeled cells, 5 blood samples were taken (at 1 hour, 3 hours, 5 days, 14 days, and 26 days), and each was divided into 3 aliquots. The triplicates of the 5 samples were analyzed simultaneously in a radioactive counter (Wizard 1480; Perkin Elmer) to avoid corrections related to the physical decay of the isotope. The radioactivity of blood samples was not measured beyond day 26 because of the decay of 51Cr (physical half-life of 51Cr: 27.7 days) and the sensitivity limits of the counter. The loss of labeled cRBCs, estimated at 23% at the 3rd hour, was extrapolated from the expected specific activity on the basis of the amount of transfused cells and the donor blood volume (assumed as normal). It should be noted that the measurement uncertainties of the loss of labeled cRBC were related to (1) the multiple ex vivo manipulations, (2) the small amount of cells processed, and (3) the cell loss for injection. Therefore, results are expressed as the mean percentage survival at the different time points, with respect to the survival at 1 and 3 hours (referred to as day 0).
Results
We have developed a cell-culture protocol permitting erythroid differentiation in the absence of a microenvironment. The sequential addition of SCF, IL3, and Epo ensures the cell proliferation and erythroid differentiation of CD34+ HSCs obtained by leukapheresis (LK) after mobilization with G-CSF, with the informed consent of the donor.
In vitro generation of functional RBCs from peripheral CD34+ cells
Cell proliferation and differentiation.
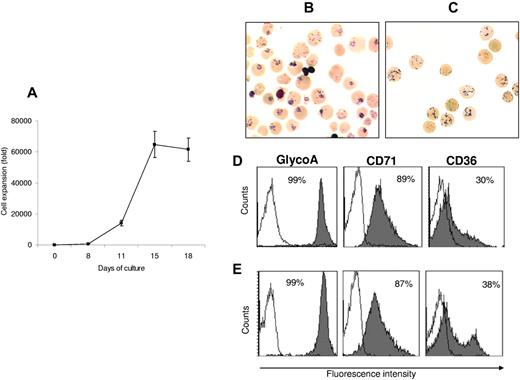
Nine assays from 9 donors were processed to set up the RBC production protocol that has finally been applied for the human assay. By day 18, a plateau was reached with a mean cell amplification of 61 500 ± 7600-fold for CD34+ cells (n = 9; Figure 1A). The percentage of morphologically enucleated cells was 81% ± 2%. At this stage, the cells displayed reticulocyte characteristics as assessed by NMB staining (Figure 1B-C) and flow cytometry with thiazole orange staining (87% ± 4.2%, n = 9, Retic-count; BD).
Expansion and differentiation of cRBCs. (A) Amplification of human CD34+ cells obtained by G-CSF–mobilized LK. Results are expressed as the mean ± SD (n = 9). (B) NMB staining from cRBC and (C) from native reticulocytes (original magnification ×50). (D) Flow cytometric analysis of one representative experiment from 9 independent experiences: expression of glycophorin A-PE, CD71-PE, and CD36-FITC from cRBC and (E) from native reticulocytes. Solid histograms represent relevant mAbs and open ones negative controls with irrelevant mAbs.
Expansion and differentiation of cRBCs. (A) Amplification of human CD34+ cells obtained by G-CSF–mobilized LK. Results are expressed as the mean ± SD (n = 9). (B) NMB staining from cRBC and (C) from native reticulocytes (original magnification ×50). (D) Flow cytometric analysis of one representative experiment from 9 independent experiences: expression of glycophorin A-PE, CD71-PE, and CD36-FITC from cRBC and (E) from native reticulocytes. Solid histograms represent relevant mAbs and open ones negative controls with irrelevant mAbs.
MCV, MCHC, and MCH of the generated cells were 138 ± 4.2 fL, 25 ± 0.5% and 35 ± 1.6 pg, respectively (n = 9). cRBCs had a slightly increased MCV compared with native Ret, related to induced stress erythropoiesis.1 Immunophenotypic characterization of the enucleated cells confirmed their reticulocyte profile because they expressed glycophorin A at 99.7% ± 0.1% (n = 9), the transferrin receptor (CD71) at 88% ± 3% (n = 9), and the thrombospondin receptor (CD36) at 16% ± 3.2% (n = 9; Figure 1D-E).
Prolonging the culture beyond day 18 caused a decrease in cell production of the order of 10% by day 21 and 40% by days 24-28, which affected both nucleated and enucleated cells (supplemental Figure 2A). At the same time, 78% ± 2% and 62% ± 1% of the cells expressed the transferrin receptor, by days 24 and 28, respectively. One representative experiment is shown in supplemental Figure 2B. Therefore, day 18 cells with reticulocyte characteristics were retained for subsequent studies.
Enzyme content.
The cRBCs had a glucose-6-phosphate dehydrogenase content of 51 ± 6 units and a pyruvate kinase level of 86 ± 6 units per gram of Hb, in keeping with the nature of a reticulocyte population (n = 6).25
Deformability.
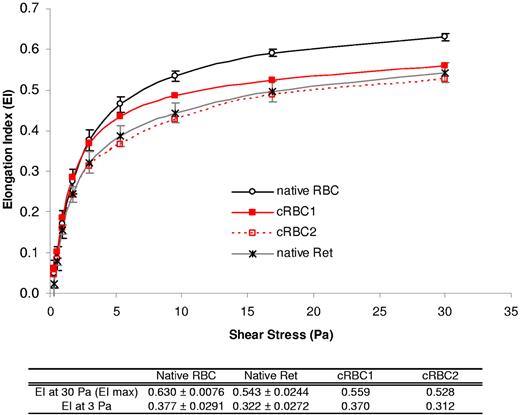
The deformability profiles of cRBC suspensions are shown in Figure 2 and were compared with those of native mature RBC and native reticulocytes. The deformability was decreased by 11%-17% for cRBCs and by 9%-17% for native reticulocytes compared with native RBCs. Thus, the cRBC curves were similar to those obtained with native reticulocytes.
Deformability of cRBCs studied by LORCA. Deformability of cRBCs was studied by LORCA. The deformability profile of day 18 cRBCs (n = 2, red curves) was compared with those of (1) mature peripheral RBCs from healthy donors (native RBC) as the control (n = 10, black circles) and (2) native reticulocytes (n = 3, gray crosses). The EI at 3 and 30 Pa is given in the table for each group and results are expressed as the mean ± SEM.
Deformability of cRBCs studied by LORCA. Deformability of cRBCs was studied by LORCA. The deformability profile of day 18 cRBCs (n = 2, red curves) was compared with those of (1) mature peripheral RBCs from healthy donors (native RBC) as the control (n = 10, black circles) and (2) native reticulocytes (n = 3, gray crosses). The EI at 3 and 30 Pa is given in the table for each group and results are expressed as the mean ± SEM.
Hb content.
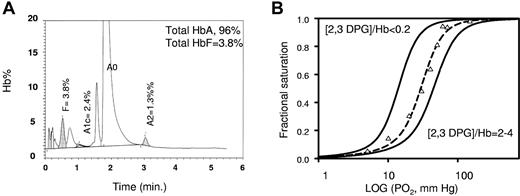
The cRBCs contained 88% ± 2.7% and 10.6% ± 2.8% (n = 9) adult hemoglobin A (HbA) and fetal Hb (HbF), respectively (Figure 3A). HbF increase is a common feature of stress erythropoiesis as observed in cultures in which a high level of proliferation is induced by growth factors. It is notably known that SCF is a major stimulator of HbF.26 Such level is responsible for any functional alteration (eg, deformability, O2 release) as observed in numerous experiments with cord blood derived RBC (data not shown).
Functionality of cRBC hemoglobin. (A) Hemoglobin status of cRBCs determined by HPLC (Bio-Rad Variant II). The percentage of hemoglobin in the elution peak is indicated for the Hb0, HbF, HbA1c, and HbA2 fractions. One representative graph from 9 independent experiments is shown. (B) Tonometric oxygen binding curves at 37°C for a cRBC (triangles) and a control RBC suspension (dotted line) at different DPG/Hb4 ratios in 10mM HEPES buffer (pH 7.4) containing 150mM NaCl. The RBC isotherms were simulated from the average MWC parameters for 10 different blood samples (black line).
Functionality of cRBC hemoglobin. (A) Hemoglobin status of cRBCs determined by HPLC (Bio-Rad Variant II). The percentage of hemoglobin in the elution peak is indicated for the Hb0, HbF, HbA1c, and HbA2 fractions. One representative graph from 9 independent experiments is shown. (B) Tonometric oxygen binding curves at 37°C for a cRBC (triangles) and a control RBC suspension (dotted line) at different DPG/Hb4 ratios in 10mM HEPES buffer (pH 7.4) containing 150mM NaCl. The RBC isotherms were simulated from the average MWC parameters for 10 different blood samples (black line).
Functionality of hemoglobin.
The functionality of hemoglobin from cRBCs was studied by the use of tonometry (Figure 3B). Oxygen equilibrium curves showed that a suspension of cRBC binds O2 reversibly in the same manner as a suspension of native RBC. The oxygen affinity (P50) was 29 ± 2 mmHg for the reticulocytes compared with 27 ± 2 mmHg for native RBCs,27,28 whereas the Hill coefficient (n50) was equal to 2.4 ± 0.1 for both samples. In Figure 3B we also present the O2 binding isotherms at different intracellular 2,3 diphosphoglycerate (2,3 DPG)/Hb ratios. These curves arise from the simulation of O2 binding isotherms for a red cell suspension either depleted of 2,3 DPG or conversely incubated with glucose and other glycolysis metabolites to increase the level of 2,3 DPG.28 As expected for a normal glycolysis rate in our cultured reticulocytes, the O2 binding curve obtained from a cRBC suspension corresponds well to that for almost stoichiometric concentrations of 2,3 DPG and Hb.
In vivo maturation of cRBC in the NOD/SCID mouse model.
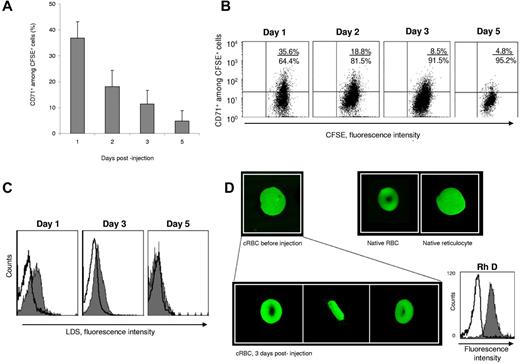
Before injection into the NOD/SCID mice, 95% and 83% of the cRBCs were CD71+ and LDS+, respectively. To follow the in vivo evolution of these cells, we injected CFSE-labeled cRBC intraperitoneally into 4 NOD/SCID mice. Retro-orbital blood samples were taken daily. CFSE+ cells were detected for 5 days with a peak between the 2nd and 3rd days representing 1.5%-20% of the total peripheral blood cells of the animal. The kinetics of CD71+ cells among CFSE+ cells showed a regular decrease of the antigen: each day, the expression of CD71 diminished by 50%. On the 5th day after injection, almost 95% of the CFSE+ cells were CD71− (Figure 4A-B). Extrapolation calculations established that the CD71− fraction increased between the starting injection time and the 3rd day, thus traducing the maturation of the injected cells (supplemental Tables 1-2). At the same time, we observed the conversion of CFSE+/LDS+ cells into CFSE+/LDS− cells, reflecting the absence of nucleic acids in the samples (Figure 4C). On the 3rd day after injection, we sorted the human cells by using the criterion of CFSE positivity, and confocal images were compared with those of the reticulocytes injected. All the sorted cells had the form of a biconcave disk (Figure 4D). This transformation of the reticulocytes into discocytes by the 3rd day after injection, together with the decrease in the size of the cells, provides evidence for the level of differentiation achieved in vivo.
In vivo maturation of cRBCs in the NOD/SCID mouse model. (A) Kinetics of CD71+ cells among CFSE+ cells in mouse blood after injection of purified CFSE+ cRBCs. Results are expressed as the mean ± SEM (n = 4). (B) CD71 expression on CFSE+ cells in 1 representative experiment. Quadrant statistics are given in each dot plot. The percentage of CFSE+ cells on days 1, 2, 3, and 5 was 2.65%, 19.2%, 20.5%, and 1.2%, respectively. (C) Kinetics of LDS expression on CFSE+ cells in 1 experiment. (D) Confocal microscopy images of CFSE+ cells before (top left) and after injection into mice (bottom) compared with native RBCs (top middle) and native reticulocytes (top right). On the bottom are front, profile, and side views of cells recovered 3 days after their injection. The cell diameter before and after 3 days injection was 11 μm and 7.5 μm, respectively. Magnification ×500. On day 3, sorted CFSE+ cells were colabeled with an anti-RhD antibody (solid histogram) or its isotype control (open histogram).
In vivo maturation of cRBCs in the NOD/SCID mouse model. (A) Kinetics of CD71+ cells among CFSE+ cells in mouse blood after injection of purified CFSE+ cRBCs. Results are expressed as the mean ± SEM (n = 4). (B) CD71 expression on CFSE+ cells in 1 representative experiment. Quadrant statistics are given in each dot plot. The percentage of CFSE+ cells on days 1, 2, 3, and 5 was 2.65%, 19.2%, 20.5%, and 1.2%, respectively. (C) Kinetics of LDS expression on CFSE+ cells in 1 experiment. (D) Confocal microscopy images of CFSE+ cells before (top left) and after injection into mice (bottom) compared with native RBCs (top middle) and native reticulocytes (top right). On the bottom are front, profile, and side views of cells recovered 3 days after their injection. The cell diameter before and after 3 days injection was 11 μm and 7.5 μm, respectively. Magnification ×500. On day 3, sorted CFSE+ cells were colabeled with an anti-RhD antibody (solid histogram) or its isotype control (open histogram).
Blood group antigen expression
A total of 43 RBC antigens, included in 28 blood group systems, 1 blood group collection, and the 901 blood group series, were investigated in cRBCs and native RBCs from the same donor (Table 1). There were no notable differences in antigen expression between these 2 populations, except for lower antigen H intensity. However, this did not prevent significant ABO antigen expression, as confirmed on cRBCs from a B-positive donor (data not shown). As expected, the Lewis antigens were absent from the surface of cRBC.
Determination of the lifespan of cRBC in humans
A total of 106 CD34+ of initial LK cells were expanded to 3.7 × 1010 total cells with a 68% enucleation rate. Cells were purified with a deleukocyting filter. The characteristics of the cRBCs submitted to chromium labeling and subsequent infusion into the human recipient were as follows: MCV, MCHC, and MCH were 130 fL, 26%, and 34 pg, respectively. They expressed CD235 at 100%, CD71 at 87%, and CD36 at 28%. Cell morphology established on 10 000 cells showed 84% reticulocytes (NMB staining), 0.08% orthochromatic cells, and no leukocytes. The hemoglobin content was 75% HbA and 25% HbF.
The survival of cRBCs labeled with 51Cr was determined between 1 hour and 26 days. The results were expressed as the percentage survival during the period of analysis. By using the reported mean rates of elution of chromium ranging from 0.6 to 2.2%/d,29 we found that the fraction of cells surviving on the 26th day ranged from 41% to 63% (Table 2).
Long-term storage of cRBCs
Purified cRBCs were stored at 4°C for up to 4 weeks in a Sag-M preservative-based solution (saline adenine glucose mannitol). cRBCs were conserved, as were native reticulocytes (n = 6, Figure 5A). The Hb content of cRBC was maintained throughout storage (Figure 5B). After 4 weeks of storage, the status of the cells was unchanged as was confirmed by their CD71 expression and reticulocyte content (Figure 5C). The cell deformability of cells stored for 4 weeks was close to that of fresh cRBCs (Figure 5D). Finally, cRBCs stored for 15 days behaved in a manner comparable with fresh cells in vivo in the NOD/SCID mouse, notably in terms of the disappearance of membrane CD71 and the acquisition of a biconcave form (Figure 5E).
Long-term storage of cRBCs. Purified cRBCs and native reticulocytes were stored at 4°C for up to 4 weeks in a Sag-Mannitol–based preservative solution. (A) Comparative kinetics of cell recovery during long-term storage of cRBCs and native reticulocytes (native Ret). Mean ± SEM of 9 and 6 experiments, respectively. (B) Evolution of the hemoglobin content during storage of cRBCs. Mean ± SEM and the number of experiments is indicated on each bar. (C-D) CD71 expression, reticulocyte content (C) and deformability (D) were evaluated before (fresh cRBCs) and after 4 weeks of storage (stored cRBC). Mean of 2 experiments. (E) Comparative kinetics of CD71+ cells among CFSE+ cells in mouse blood after injection of fresh (n = 4 mice) or stored (n = 2 mice) purified cRBC. Data for fresh cRBC are from Figure 4A, and the results are expressed as the mean ± SEM. Confocal microscopy image shows CFSE-labeled stored cells 3 days after injection into mice.
Long-term storage of cRBCs. Purified cRBCs and native reticulocytes were stored at 4°C for up to 4 weeks in a Sag-Mannitol–based preservative solution. (A) Comparative kinetics of cell recovery during long-term storage of cRBCs and native reticulocytes (native Ret). Mean ± SEM of 9 and 6 experiments, respectively. (B) Evolution of the hemoglobin content during storage of cRBCs. Mean ± SEM and the number of experiments is indicated on each bar. (C-D) CD71 expression, reticulocyte content (C) and deformability (D) were evaluated before (fresh cRBCs) and after 4 weeks of storage (stored cRBC). Mean of 2 experiments. (E) Comparative kinetics of CD71+ cells among CFSE+ cells in mouse blood after injection of fresh (n = 4 mice) or stored (n = 2 mice) purified cRBC. Data for fresh cRBC are from Figure 4A, and the results are expressed as the mean ± SEM. Confocal microscopy image shows CFSE-labeled stored cells 3 days after injection into mice.
Discussion
We report that cRBCs generated in vitro from HSCs under GMP conditions encounter in vivo the conditions required for their maturation and that they persist in the circulation for several weeks in humans. We report for the first time that cRBCs with reticulocyte features are functional with regard to the following major points: (1) their capacity to correctly bind oxygen in a reversible manner, to ensure their transport function; (2) normal contents of glucose-6-phosphate dehydrogenase and pyruvate kinase, to reduce glutathion and maintain a level of ATP sufficient to prevent the accumulation of 2,3 DPG, which lowers the affinity for hemoglobin; and (3) their deformability, which conditions the survival of the cells in vivo on account of their repeated passages through microvessels.30 As expected, the deformability is slightly diminished compared with that of adult RBCs but similar to that of native reticulocytes from a healthy donor. These results are in accordance with the data of Chasis et al,31 who demonstrated that the deformability of native immature reticulocytes is decreased to approximately 10% of the normal. Our data further show that the rheologic properties are conserved, which is a prerequisite indispensable for the maintenance of the cells in vivo.
By using the NOD/SCID mouse model, we confirm that the passage in vivo of these reticulocytes induces their complete maturation to adult RBCs, as shown by the disappearance of membrane CD71 and nucleic material and the acquisition of biconcavity. Indeed, it is now established that the maturation of reticulocytes takes place in vivo in 48-72 hours and involves a succession of events: loss of organelles,32,33 rheologic modifications,31 changes in membrane permeability34,35 and cell mobility,30 a decrease in surface area,36,37 changes in membrane protein organization,38 and elimination of numerous membrane components through exosomes (such as the transferrin receptor and α4β4 integrins).39,40 The RBC then adopts its typical form of a biconcave disk indispensable for efficient gaseous exchange.
All the results obtained in vitro and in vivo constituted the preclinical rationale for the injection into humans of cRBCs generated under GMP conditions and without reconstitution of the microenvironment. Our objective was to study the behavior in vivo of a homogeneous sample of cultured reticulocytes labeled with 51Cr. Sodium chromate binds preferentially to the β chains of Hb without destroying the red cells. There nevertheless exists, quite apart from any cell death, an elution of chromium, which must be taken into account to interpret the curve describing the decrease in circulating radioactivity. Although the commonly adopted coefficient of spontaneous elution (k) is 1.2%/d,41,42 it varies in the literature from 0.6% to 2.2%/d from one person to another.29 The literature also shows that k increases with the age of the cells43 and their content of fetal Hb.44
Because of these uncertainties, we can assume that (1) cRBCs survive in great numbers during the 5 first days after injection into humans (94%-100% survival) and (2) their level in the circulation at 26 days is between 41% (if k = 0.6%/d) and 63% (if k = 2.2%/d; Table 2). Starting from peripheral blood, we confirmed in vitro these rates of random loss of 51Cr (from 1.25% to 2.4%/d; supplemental Table 3). Their in vivo behavior therefore compared favorably with the half-life of 28 ± 2 days commonly reported for normal native RBCs. From a purely theoretical point of view, one would expect to observe a greater lifespan value of a homogeneous red cell population, of which most are at the stage of reticulocytes, compared with a mixture of red cells of all ages. However, the pattern of cultured red cell survival we observed in this experiment is very similar to a conventional one. Beyond the factors contributing to measurement uncertainties of the loss of labeled cRBCs already mentioned previously, we have few if any information about the impact on 51Cr binding in very young red cells containing a high proportion of HbF, and its rate of elution in vivo from RBC in these conditions. If because of the quantities injected our study we were not able to evaluate the transfusion efficacy of cRBCs, it nevertheless remains that their survival in vivo testifies globally to their quality and functionality.
In the field of transfusion, the storage of labile blood products is a major preoccupation that reflects the necessity of a rigorous management of the stocks of blood cell units. The future products of cell engineering will have to meet these same requirements. In this context, we show that cRBCs stored for several weeks at 4°C conserve their deformability and their capacity to undergo terminal maturation in vivo in the NOD/SCID mouse.
One of the objectives is the generation of a maximum number of units of packed red cells from one apheresis. If the aim is to generate RBCs from HSC for transfusion purposes, we immediately face the problem of the quantity of cells to be produced: 1 unit of conventional packed RBCs contains some 2000 billion cells. The challenge is therefore to exploit to the maximum the proliferation/differentiation capacity of HSC to reach terminal maturation as far as possible. Although peripheral HSC can be of interest for the production of autologous RBCs, in our experience the best source for RBC production is clearly cord blood–derived HSCs. These cells in fact generate 5- to 10-fold more RBCs than HSCs derived from peripheral blood and have an increased proliferation capacity, whereas the enucleation capacity is similar. Under our experimental conditions, combining a first phase of 50-fold amplification of CD34+ HSC with a second phase of erythroid differentiation would enable us to generate 4-30 million RBCs from 1 CD34+ (data not shown).
Concerning blood group antigens, our work shows that cell culture does not induce any modification of their expression, except for the decreased intensity of H antigen, which is, however, not considered to cause any transfusion consequence. As expected, the Lewis antigens were absent from the surface of cRBC because they are not produced by the erythroblast itself but are secondarily adsorbed on the surface of RBCs from glycolipids transported in plasma. As blood groups were tested here for the first time on a very wide panel of antigens, this study offers important perspectives for the constitution of banks of progenitors, with blood phenotypes specifically chosen to match most alloimmunized patients, as well as those with a rare blood type.3,45 In demonstrating the survival in humans of RBCs generated in vitro from autologous HSCs, the present work establishes the proof of principle of cRBC transfusion.
This clinical in vivo study is, in our opinion, of major interest despite it being focused on a single person. This observation is a kind of go/no go step. Indeed, if the results had been disappointing, this concept of cultured RBCs for transfusion would have been questionable. The results validate the approach and more experiments would not modify the message. Indeed, our present aim was not to evaluate the variability that might be associated with such procedures because the challenge is now the scaling-up of this approach whose clinical applications would be considerable.45-47
A similar approach should be made with the use of cord blood cells as an easily available source of HSCs or more likely the use of induced pluripotent stem cells as an unlimited source of stem cells.45,48 Even if this approach does not claim to replace blood transfusion in its entirety, it is clear that it could at least provide a solution for patients who are carriers of rare blood groups or are polyimmunized, which represent no less than 1%-3% of the transfused population.3 The future of this concept is, however, not conceivable unless the cost of production can be rendered comparable with that of conventional blood units. The ultimate and decisive challenge is therefore to design a cost-effective automated industrial cell culture system capable of maintaining a self-renewing progenitor population, which provides an environment for efficient erythroid differentiation and allows sorting/purification and packaging of the end-product RBCs in a manner directly suitable for transfusion. Such a system does not exist. Implementing an industrial production process will without a doubtlessly require a huge technical and financial investment. Several industrial consortia are presently working on this throughout the world, and crucial answers to the scaling-up feasibility are expected within the next few years.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Unité de recherche clinique de l'Est Parisien, Dr Laura Wakselman (URCEST) Hôpital St Antoine; Dr Beny Charbit, Investigation Center Paris EST of Pitié-Salpêtrière University Hospital; and Dr Anne Léon (Etablissement Français du Sang [EFS]) and Dr Marie-Thérèse Rubio (Hôpital St Antoine) for their monitoring of the recipient. They are indebted to Mickael Lecuyer (EFS) for expert technical assistance, to Dr Guy Benoit (Hôpital Trousseau) for GMP preparation of cell culture medium, and Dr Marion Lambert (U561, Hôpital St Vincent de Paul) for cell sorting.
This work was supported by the Etablissement Français du Sang, the association Laurette Fugain, the association Combattre La Leucémie, the Association pour la Recherche en Transfusion, and the fondation Jérôme Lejeune. It was supported by a grant in aid from the Assistance Publique-Hôpitaux de Paris and Inserm at the Clinical Investigation Centre Paris EST of Pitié-Salpêtrière University Hospital.
Authorship
Contribution: M.-C.G. was the project leader, contributed to all aspects of the work, and wrote the manuscript; H.R. was the project leader for GMP production and clinical protocol and wrote the manuscript; A.D. was the project leader for chromium labeling procedure; L.K. analyzed oxygen binding properties for red cell suspensions; I.S. performed the deformabilty analysis; P.-Y.L. performed blood group antigen determination/expression; S.F. monitored NOD/SCID mice; G.T. performed confocal analysis; T.P. performed blood group antigen determination/expression; T.M. provided expert technical assistance; S.J., N.H., and L.H. provided expert technical assistance; C.M. provided quality control; N.M. provided analyses of hemoglobin (HPLC) and enzyme content; H.L. provided quality control; J.-Y.D. designed and supervised the chromium labeling procedure; and L.D. was head of the laboratory, designed and supervised the overall research, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Luc Douay, UMR_S938 CDR Saint-Antoine, 27 rue Chaligny, F-75012 Paris, France; e-mail: luc.douay@sat.aphp.fr.
References
Author notes
M.-C.G. and H.R. contributed equally to this work.