To the editor:
The initiator of coagulation, tissue factor (TF), resides on the cell surface in an inactive (cryptic) state that binds factor VIIa with reduced affinity and cannot activate factor X, or in an active (decrypted), conformation.1,2 Protein disulfide isomerase-dependent regulation of the TF allosteric Cys186-Cys209 disulfide has been proposed as a key event in TF decryption, but this remains controversial.3-7 Although elimination of the TF disulfide by Cys209-to-Ala conversion reduces affinity for VIIa and abolishes procoagulant activity, Kothari et al have questioned the validity of the disulfide switching model of TF decryption.8 They showed that mutation of either Cys-residue to Ser led to 10-fold lower cellular TF expression compared with wild-type TF, but coagulant activity normalized per TF molecule at supraphysiologic VIIa concentrations was indistinguishable between mutants and wild-type TF.
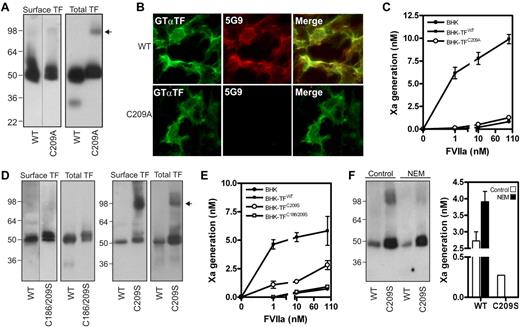
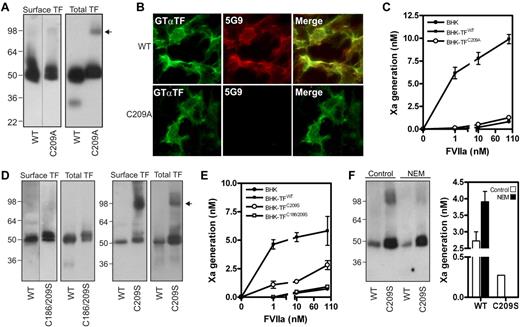
We reasoned that excessive membrane surface in cells expressing lower levels of TF mutants led to an overestimation of procoagulant activity. To exclude this variable, we created BHK cells expressing similar amounts of surface wild-type TF (TFWT) or TF containing single or double Cys-to-Ala mutations. Analysis of 40 clones per mutant showed that Cys186 mutation led to intracellular retention, as described before,1 while a pair of TFWT and TFCys209Ala clonal cell lines with similar surface expression was identified. TFCys209Ala expression was approximately 60% of TFWT (Figure 1A), supported by surface-immunostaining with an antibody with similar reactivity for TFWT and TFCys209Ala (Figure 1B). In contrast, mAb-5G9, which has reduced affinity for disulfide-mutated TF, only recognized surface TFWT. Consistent with previous results in HUVEC,1 BHK cells expressing TFWT efficiently activated X, whereas TFCys209Ala-expressing BHK cells did not differ from untransfected BHK cells, even at supraphysiologic VIIa concentrations (Figure 1C).
Absence of the TF allosteric disulfide results in abolished coagulant function. (A) BHK cells were stably transfected to express TFWT or TFCys209Ala. TF cell surface exposure was determined by labeling with 1mM NHS-biotin in HBS, and subsequently precipitation of biotinylated TF. To obtain total TF protein, immunoprecipitation with the TF mAb 9C3 was performed with n-octyl β-D-glucopyranoside (OG) cell lysates. Western Blotting was performed using Goat anti-TF antibody. The arrow indicates TF dimers. (B) Reactivity of a polyclonal goat anti-TF and 5G9 (both 10 μg/mL) with BHK cell surface levels of TFWT or TFCys209Ala. (C) TF procoagulant activity on nontransfected BHK cells, BHK-TFWT and BHK-TFCys209Ala was measured kinetically after addition of the indicated concentrations of VIIa and 100nM FX. (D) BHK cells expressing TFWT, TFCys209Ser and TFCys186/209Ser. Cell surface expression was determined after NHS-biotin labeling and total TF expression was determined in total lysate. Note the absence of a dimer fraction in case of the TFCys186/209Ser mutant. The arrow indicates TF dimers. (E) TF procoagulant activity on nontransfected BHK cells, BHK-TFWT, BHK-TFCys209Ser and BHK-TFCys186/209Ser was determined as described before. (F) BHK-TFWT and BHK-TFCys209Ser were incubated with 10mM N-ethylmaleimide for 1 hour. Presence of dimer factions was determined on Western blot and procoagulant activity was performed as described before, using 1nM VIIa.
Absence of the TF allosteric disulfide results in abolished coagulant function. (A) BHK cells were stably transfected to express TFWT or TFCys209Ala. TF cell surface exposure was determined by labeling with 1mM NHS-biotin in HBS, and subsequently precipitation of biotinylated TF. To obtain total TF protein, immunoprecipitation with the TF mAb 9C3 was performed with n-octyl β-D-glucopyranoside (OG) cell lysates. Western Blotting was performed using Goat anti-TF antibody. The arrow indicates TF dimers. (B) Reactivity of a polyclonal goat anti-TF and 5G9 (both 10 μg/mL) with BHK cell surface levels of TFWT or TFCys209Ala. (C) TF procoagulant activity on nontransfected BHK cells, BHK-TFWT and BHK-TFCys209Ala was measured kinetically after addition of the indicated concentrations of VIIa and 100nM FX. (D) BHK cells expressing TFWT, TFCys209Ser and TFCys186/209Ser. Cell surface expression was determined after NHS-biotin labeling and total TF expression was determined in total lysate. Note the absence of a dimer fraction in case of the TFCys186/209Ser mutant. The arrow indicates TF dimers. (E) TF procoagulant activity on nontransfected BHK cells, BHK-TFWT, BHK-TFCys209Ser and BHK-TFCys186/209Ser was determined as described before. (F) BHK-TFWT and BHK-TFCys209Ser were incubated with 10mM N-ethylmaleimide for 1 hour. Presence of dimer factions was determined on Western blot and procoagulant activity was performed as described before, using 1nM VIIa.
We next addressed the possibility that the differences observed in Kothari's8 and our study were because of the specific replacements for these Cys-residues and generated BHK cells stably expressing TF containing Cys-to-Ser conversions. Surface biotinylation identified TFCys209Ser and TFCys186/209Ser clones with expression equal to the TFWT clone. Interestingly, TFCys209Ser showed substantial surface-exposed dimers compared with TFCys209Ala, while TFCys186/209Ser did not form dimers, as expected from a TF mutant lacking unpaired cysteines (Figure 1D). While procoagulant activity of the TFCys186/209Ser clone did not differ from untransfected BHK cells, TFCys209Ser showed residual activity (Figure 1E). To prevent dimerization, TFCys209Ser-expressing BHK cells were incubated with N-ethyl-maleimide. Blocking free thiols impaired dimerization and also completely abolished TFCys209Ser activity, while TFWT activity was up-regulated (Figure 1F), consistent with previously reported effects of NEM on flippase-induced PS exposure.9
These data with controlled surface expression of TF support previous conclusions that disulfide-mutated TF is impaired in adopting a procoagulant conformation. Of note, Kothari et al observed dimers when expressing TF mutated at both Cys-residues.8 Because our data demonstrate that dimerization can increase procoagulant activity of single-free-thiol mutants, their assay conditions may have favored TF dimerization by mechanisms unrelated to the mutated Cys-residues. In conjunction with increased membrane availability, residual dimer activity may have led to overestimation of TF-specific procoagulant activity. Thus, we consider general dismissal of the disulfide switching hypothesis as proposed by Kothari et al, unjustified.
Authorship
Acknowledgments: H.H.V. is supported by The Netherlands Organization for Scientific Research (nr 17.106.329)
Contribution: L.G.v.d.H. and B.K. performed the experiments; and L.G.v.d.H., P.H.R., W.R. and H.H.V. analyzed the data and wrote the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Henri H. Versteeg, Ph.D., Einthoven Laboratory for Experimental Vascular Medicine, Leiden University Medical Center, Albinusdreef 2, Leiden, 2333 ZA, The Netherlands; e-mail: h.h.versteeg@lumc.nl.