Abstract
High levels of von Willebrand factor (VWF) are associated with an increased risk for cardiovascular disease (CVD). Although VWF levels are strongly heritable and genetic susceptibility is an important risk factor for CVD, information on the contribution of common VWF gene variants to VWF levels and CVD risk is limited. In a case-control study of 421 young patients with a first event of acute coronary heart disease (CHD) or ischemic stroke (IS), and 409 healthy control participants (men aged ≤ 45 years, women aged ≤ 55 years), 27 haplotype-tagging single-nucleotide polymorphisms (ht-SNPs), covering the total common VWF gene variation, were selected and genotyped. The associations between these SNPs, VWF antigen (VWF:Ag) levels, VWF collagen-binding (VWF:CB) activity, and CVD risk was investigated. Two new associations were identified. For ht-SNP rs4764478 (intron 45), the increase in VWF:Ag levels and VWF:CB activity per minor allele was 0.082 (± 0.026) IU/mL (P = .001) and 0.096 (± 0.030) IU/mL (P = .002), respectively. ht-SNP rs216293 (intron 17) was associated with CVD risk (odds ratio, 1.44; 95% confidence interval [CI], 1.12-1.86 per minor allele). We confirmed the association between rs1063857 and CVD risk. Our data show that common variants in the VWF gene are associated with VWF levels and with the risk for CVD.
Introduction
Von Willebrand factor (VWF) plays a key role in the formation of arterial thrombus because it facilitates platelet adhesion and aggregation at sites of vascular injury.1 For this reason, an association between VWF plasma levels and cardiovascular disease (CVD) is anticipated and has been investigated extensively. Many studies have shown that high VWF levels are positively associated with the risk for coronary heart disease (CHD) and ischemic stroke (IS).2-4
Plasma levels of VWF are not only influenced by nongenetic factors such as hormones and inflammation but are also strongly heritable, with estimates of heritability ranging from 53%-75%.5,6 As genetic susceptibility is an important risk factor for CVD,7,8 several candidate studies have addressed whether single nucleotide polymorphisms (SNPs) in the VWF gene determine VWF levels and subsequently contribute to the genetic susceptibility for CVD.
Because the VWF promoter region is involved in the regulation of gene transcription and is therefore expected to be a determinant of VWF plasma levels, most studies have focused on SNPs in the promoter region of the VWF gene. In a population of healthy individuals, homozygotes for the minor allele of the VWF promoter variant rs7966230 had significantly higher VWF antigen (VWF:Ag) levels than homozygotes for the common allele.9 In a case-control study, nested within a large, prospective, population-based cohort (Rotterdam study), the minor allele of this promoter variant was also associated with the risk for CHD, specifically in the subgroup of individuals with advanced atherosclerosis.10 However, because several other studies could not demonstrate an association with VWF levels or CVD,5,11 it remains unclear whether SNPs in the VWF promoter region contribute significantly to VWF:Ag levels and CVD risk.
SNPs in coding regions of the VWF gene are also of interest, as they may be related to protein changes that influence VWF structure and function. Several SNPs, located in exonic regions known to be involved in the regulation of VWF multimer size (exon 18 and exon 28), have been identified.12,13 Although VWF multimeric composition is a strong determinant of its functional activity,14 the limited studies available on coding regions of the VWF gene mainly focused on the contribution of common variants to VWF:Ag levels and not on their contribution to VWF activity or CVD.12,13
We investigated the association between common variation spanning the total VWF gene (including 12kb of the 5′and 3′ flanking regions), VWF:Ag levels, VWF collagen-binding activity (VWF:CB), and the risk for CVD. A total of 27 haplotype-tagging SNPs (ht-SNPs) representing common variation in the VWF gene were selected and were subsequently genotyped in young patients with a first event of acute CHD or IS and in healthy control participants.
Methods
Study population
In a single-center case-control study (Arterial Thrombosis at a young age: the role of TAFI and other Coagulation factors [ATTAC] study), we have included consecutive patients with arterial thrombotic disease at a young age and healthy control participants.15 Male patients aged 18 to 45 years and female patients aged 18 to 55 years, who experienced a first acute ischemic complication in either the heart or the brain, were eligible for inclusion. The patients had coronary heart disease (CHD), including acute myocardial infarction (AMI) or unstable angina pectoris, or ischemic stroke (IS) including patients with transient ischemic attacks. Patients were included 1 to 3 months after the event to avoid the effects of an acute phase response. Control participants were friends, neighbors, or partners of the patients fulfilling the same age criteria but did not have a history of CVD and were not related to the patients. For this study, we included only patients with European ancestry. The study design has been described previously in more detail.15
The study protocol is in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of Erasmus MC. Written informed consent was obtained from each participant.
Definitions
AMI was defined as typical chest pain, with elevated cardiac markers (creatine kinase-MB, troponin T) and/or characteristic electrocardiographic findings. Unstable angina pectoris was defined as typical chest pain while at rest, which is confirmed by characteristic electrocardiographic findings and normal levels of cardiac markers. IS was defined as a suddenly occurring cerebral deficit, which cannot be explained otherwise than as local cerebral ischemia, and that lasted for longer than 24 hours after onset. Transient ischemic stroke was defined similarly, but symptoms had to be temporary and last less than 24 hours after onset. Brain imaging by computed tomography or magnetic resonance imaging was required to confirm the initial diagnosis.
Clinical data were collected by means of a standard medical questionnaire, and a physical examination was performed by a research physician. European ancestry was self-reporting. To meet the definition of European ancestry, grandparents of the included individuals had to be born in northern or western Europe. Smoking was defined as previous or current smoking. Hyperlipidemia was defined as either a plasma total cholesterol level of > 5.0mM or the use of lipid-lowering treatment on the day of the ischemic event. Patients with a medical history of diabetes, or patients using oral antidiabetic medication or insulin on the day of the ischemic event, were considered to have diabetes. Hypertension was defined as a systolic blood pressure of > 140 mm Hg, a diastolic blood pressure of > 90 mm Hg, or the use of antihypertensive medication on the day of inclusion. Body mass index was calculated for each participant by dividing weight (in kilograms) by the square of height (in meters). A positive family history was noted if the patient had a first-degree relative with a positive history of CVD before the age of 60 years.
Blood sampling procedure and plasma measurements
Under standardized conditions, blood was collected into citrate (0.105M) with use of the Vacutainer system. Citrated blood was centrifuged at 2000g for 10 minutes at 4°C. Plasma was additionally centrifuged at 20 000g for 10 minutes at 4°C and stored at −80°C until analysis. For DNA isolation, blood was collected in tubes containing EDTA. Genomic DNA was extracted and stored at 4°C for genetic analysis.
Von Willebrand factor antigen (VWF:Ag) levels were determined with an in-house enzyme-linked immunosorbent assay, with use of polyclonal rabbit anti–human VWF antibodies (DakoCytomation) for capturing and detecting. The intra-assay coefficient of variation was 5.7%, and the interassay coefficient of variation was 7.8%.
Von Willebrand factor collagen-binding (VWF:CB) activity was measured by an in-house enzyme-linked immunosorbent assay using bovine Achilles tendon collagen type 1 for capture (Sigma-Aldrich) and polyclonal rabbit anti–human VWF antibodies (DakoCytomation) for detection. The intra-assay coefficient of variation was 5.8%, and the interassay coefficient of variation was 9.1%.
Both assays used commercial reference plasma (normal reference plasma, Precision BioLogic, Kordia), which were standardized against the World Health Organization standard by the manufacturer.
Selection of SNPs in the VWF gene
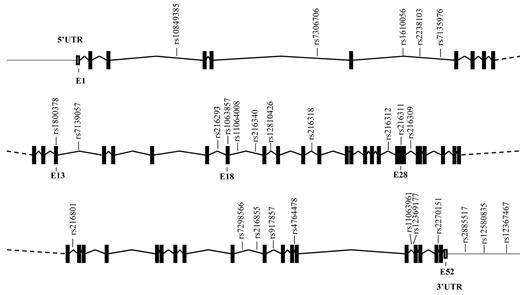
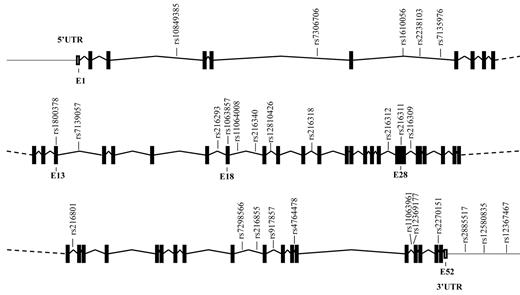
The VWF gene, spanning 175.8 kbp, is located on the short arm of chromosome 12 (p13.3) and contains 52 exons. Thus far, more than 950 SNPs in this gene have been annotated in the National Center for Biotechnology Information's SNP database (dbSNP, build 130). In our study, we considered only SNPs that were present in populations with northern and western European ancestry, with a minor allele frequency of at least 5%. From these SNPs, we selected haplotype tagging SNPs (ht-SNPs) in the VWF gene, including 12kb of the 5′ and 3′ flanking regions. The selection of ht-SNPs was performed on the basis of the linkage disequilibrium (LD) map of the VWF locus provided by the International HapMap Project (phase II, April 2007; www.HapMap.org). For the VWF gene, blocks of haplotypes with a frequency > 5% were defined, and their ht-SNPs were selected as implemented in the Haploview software (version 4.1; www.broadinstitute.org/mpg/haploview).16 This resulted in the selection of 27 ht-SNPs described in Table 1 and presented in Figure 1. The ht-SNPs cover all of the common variation in the VWF gene, which is 70%-95% of the total variation, depending on the LD in the haplotype blocks.
Schematic representation of the VWF gene and the location of the ht-SNPs. Boxes are exons, and lines connecting boxes are introns. Filled or darkened boxes are coding sequence, whereas empty, unfilled boxes are untranslated region (UTR). E indicates exon.
Schematic representation of the VWF gene and the location of the ht-SNPs. Boxes are exons, and lines connecting boxes are introns. Filled or darkened boxes are coding sequence, whereas empty, unfilled boxes are untranslated region (UTR). E indicates exon.
Genotyping
A total of 26 SNPs were genotyped with use of predesigned TaqMan Genotyping Assays (Applied Biosystems). One SNP was genotyped with a custom-designed TaqMan Genotyping Assay.
The nucleotide sequences of the primers and probes used for each assay are available on request. The context sequences for the SNPs are presented in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Endpoint fluorescence was measured on the ABI 7900HT instrument (Applied Biosystems) and was clustered according to genotype with use of SDS 2.2.2 software (Applied Biosystems). For 25 SNPs, genotyping was successful for > 95% of the 830 participants. SNPs rs7135976 and rs11064008 had call rates below 95% and were therefore excluded from the analyses.
A random selection of 10% of the samples was reanalyzed for every genotyping assay. This finding showed that the assays had a reproducibility of more than 99%. To ensure DNA quality, only participants who were successfully genotyped for more than 90% of the 25 SNPs (n = 753) were included in the analysis. For the 25 SNPs, patients and control participants were equally successfully genotyped; therefore, missing genotype data were not patient related.
Statistical analysis
Data on population demographics are presented as means and SDs for continuous variables and as counts and percentages for categoric variables. To test whether the genotype distributions deviated from that expected for a population in Hardy-Weinberg equilibrium, we used a χ2 test with 1 degree of freedom. In the original study population, individuals without European ancestry were also included. The allele frequencies in the different ethnic populations were compared with use of the Fisher exact test. The allele frequencies of SNPs and the LD patterns between SNPs were significantly different between participants with and those without European ancestry. Therefore, for this study, the analyses were conducted in individuals with European ancestry only.
Because of nonnormality, VWF:Ag levels and VWF:CB activity were rank normal transformed, as implemented in GenABEL.17 The association between genotypes and VWF:Ag levels and VWF:CB activity were analyzed with use of linear regression models (under the assumption of an additive genetic model). The covariates included in the multivariable model were age, sex, case status, blood group, hormone use, and time of blood sampling after the event. The β represents the change per minor allele in SDs of the transformed outcomes. Because of this transformation, the change in VWF:Ag levels and VWF:CB activity in international units per milliliter is an estimation of the real change.
The associations between genotypes for each SNP and the risk for CVD were analyzed with use of logistic regression models (under the assumption of an additive genetic effect), and 2 models were fitted. The first multivariable model included genotype, and the covariates were age and sex. The second multivariable model additionally included the covariates smoking, hypertension, diabetes, hyperlipidemia, and blood group. Missing values in covariates were imputed at the series mean. Logistic regression analysis was additionally conducted for subgroups of CHD and patients with IS.
We implemented multiple testing corrections by calculating the false discovery rate (q-value) for each SNP. The q-value of a test measures the proportion of false positives incurred (the false-positive rate) when the particular test is significant.18
We inferred haplotypes using the R package haplo.stats (Mayo Foundation for Medical Education and Research, Version 1.4.4, http://mayoresearch.mayo.edu/mayo/research/schaid_lab/software.cfm).19,20 The association between the haplotypes and the risk for arterial thrombosis was determined by weighted logistic regression analysis. This haplotype analysis calculates posterior probabilities for each possible haplotype for an individual and assigns an appropriate weight to account for this uncertainty in subsequent risk estimation.20 The associations between the haplotypes and VWF:Ag levels and VWF:CB activity were similarly determined by linear regression analysis accounting for posterior uncertainty. The models used in the single SNP analyses were also used in the haplotype analysis. The most common haplotype was taken as the reference. Haplotype analysis was conducted in 2 ways. The first analysis was based on the haplotype block pattern in the VWF gene provided by the International HapMap Project (phase 2, April 2007; www.HapMap.org). In the second analysis, haplotypes in the VWF gene were analyzed with a sliding-window approach.
We conducted the analyses using R, SPSS Version 15 (SPSS Inc), Haploview, and the haplo.stats package. In statistical analyses, a P value of < .05 (2-sided) was considered significant.
Results
Characteristics of the study participants
A total of 421 patients and 409 control participants with European ancestry were included in the study. The characteristics of the study participants are presented in Table 2. Of the patient group, 271 participants had CHD, and 150 participants had IS. Patients were somewhat older (43.2 ± 6.7 years) than control participants (39.6 ± 7.6 years, P = .001). A total of 59% of the included individuals in the patient group and 65% of the included individuals in the control group were female (P = .09). The distribution of blood group was similar in patients and control participants (P = .78). As expected, the prevalence of classical CVD risk factors was significantly higher in patients than in control participants. In addition, at the time of inclusion, most patients used medications including antiplatelet drugs, antihypertensive drugs, and statins. The VWF:Ag levels (mean ± SD) were significantly higher in patients than in control participants (1.26 ± 0.53 IU/mL vs 1.09 ± 0.37 IU/mL, P < .001). In addition, VWF:CB activity was significantly higher in patients than in control participants (1.38 ± 0.54 IU/mL vs 1.25 ± 0.42 IU/mL, P < .001). There was no difference in VWF:CB/VWF:Ag ratio between patients and control participants (1.15 ± 0.31 vs 1.17 ± 0.25, P = .40).
VWF genotypes
The genotype distributions of 24 of the 25 VWF SNPs were in Hardy-Weinberg equilibrium. In the control group, rs216311 was not in Hardy-Weinberg equilibrium (P < .001) and was therefore excluded from the analyses. The allele frequencies for participants with European ancestry were similar to those reported by dbSNP.
Common genetic variation, VWF:Ag levels, and VWF:CB activity
A total of 4 SNPs were associated with VWF:Ag levels and 2 with VWF:CB activity after adjustment for age, sex, case-status, blood group, hormone use, and time of sampling (Table 3). The SNP rs7306706 was nominally associated with a decrease in VWF:Ag level (−0.043 ± 0.021 IU/mL per minor allele, P = .04). The SNP rs1063857 was marginally associated with an increase in VWF:Ag levels (0.039 ± 0.022 IU/mL per minor allele, P = .08). Rs216318 was nominally associated with a decrease in VWF:Ag levels (−0.068 ± 0.036 IU/mL, P = .05) and with a decrease in VWF:CB activity (−0.093 ± 0.042 IU/mL, P = .03). There was no association between rs216318 and the VWFCB/VWF:Ag ratio (P = .83). Finally, rs4764478 was nominally associated with an increase in VWF:Ag levels (0.082 ± 0.026 IU/mL, P = .001) and an increase in VWF:CB activity (0.096 ± 0.030 IU/mL, P = .002) but was not associated with the VWFCB/VWF:Ag ratio (P = .88). After adjustment for multiple testing, only SNP rs4764478 remained associated with a significant increase in VWF:Ag levels (q = 0.03) and with a significant increase in VWF:CB activity (q = 0.03). For the respective common homozygotes, heterozygotes, and rare homozygotes for SNP rs4764478, the mean VWF:Ag levels were 1.12 IU/mL, 1.20 IU/mL, and 1.28 IU/mL, and the mean VWF:CB activity was 1.26 IU/mL, 1.34 IU/mL, and 1.38 IU/mL.
Common genetic variation and the risk for CVD
After adjustment for the full model, 2 SNPs were nominally associated with an increased risk for CVD (Table 4). For rs216293, the estimated increase in risk (OR, 95% CI) per minor allele was 1.44 (95% CI, 1.12-1.86). For SNP rs1063857, the estimated increase in risk per minor allele was 1.32 (95% CI, 1.02-1.70). After adjustment for multiple testing, only rs216293 remained associated with a higher risk for CVD (q = 0.03). It is interesting to note that the associations between the 2 SNPs and the risk for CVD were predominantly seen in the subgroup of patients with IS. In this subgroup, the estimated increase in risk per minor allele was 1.50 (95% CI, 1.17-1.8) for SNP rs216293 and 1.35 (95% CI, 1.03-1.68) for SNP rs1063857. In the subgroup of patients with CHD, the estimated increase in risk per minor allele was 1.26 (95% CI, 0.94-1.58) for SNP rs216293 and 1.15 (95% CI, 0.82-1.48) for SNP rs1063857.
VWF haplotypes
Haplotype analysis confirmed the single SNP analyses; in 3-SNP sliding windows, haplotypes containing the associated SNPs were also related to VWF:Ag levels and to CVD risk. Neither type of haplotype analysis, however, provided additional information on the contribution of common variation in the VWF gene to VWF:Ag levels, VWF:CB activity, and the risk for CVD (data not shown).
Discussion
We genotyped common variants in the total VWF gene in a unique case-control study of young patients with a first AMI or IS. Our data show that the minor allele of SNP rs4764478 is associated with a significant increase in VWF levels and that the minor allele of SNP rs216293 is associated with a significantly increased risk for CVD. In addition, we confirmed the association between rs1063857 and the risk for CVD. The associations with CVD risk were predominantly seen in the subgroup of patients with IS.
This candidate gene study identified an association between ht-SNP rs4764478 and VWF:Ag levels (q = 0.04). After adjustment for multiple testing, rs4764478, which is located in intron 45, was significantly associated with VWF:CB activity as well (q = 0.03). The VWF:CB activity assay measures the binding of plasma VWF to collagen, which is particularly dependent on the quantity of high molecular weight multimers.14 If the multimer composition is changed (eg, because of a reduction of HMW multimers, as is seen in patients with von Willebrand disease type 2A), a lower VWF:CB activity to VWF:Ag ratio is found. For SNP rs4764478, the increase in VWF:CB activity per minor allele was similar to the increase in VWF:Ag levels. Therefore, our study suggests that ht-rs4764478 itself, or SNPs tagged by rs4764478, is not specifically involved in the regulation of VWF multimer size. SNP rs4764478 was not associated with the risk for CVD. However, in consideration of the minor allele frequency of this SNP of 0.25 and an 8% increase of VWF, a study with larger statistical power is needed to significantly detect this relationship.
Ht-SNP rs216293, which is located in intron 17, was associated with an increased risk for CVD, independent of other classic CV risk factors. Yet, rs216293 was not located in intronic regions and did not tag any known nonsynonymous SNP in the VWF gene, and the associations with VWF:Ag levels and the risk for CVD are probably not the result of a change in amino acid composition. However, previous studies on proteins involved in hemostasis such as fibrinogen show that other structural alterations exist (eg, alternations because of alternative splicing).21 Such a structural alteration may also be the underlying mechanism for the association between rs4764478 and VWF:Ag levels and rs216293 and CVD risk. Functional studies are needed to elucidate the mechanism underlying these 2 new identified associations.
The synonymous ht-SNP rs1063857, which is located in exon 18, was associated with an increased risk for CVD. Previously, it was shown that SNP rs1063856, which is also located in exon 18, and the only known SNP in this region resulting in a change in amino acid composition, was associated with the risk for CHD in a young Caucasian population of patients with diabetes mellitus type 1.13 This SNP is in perfect LD with SNP rs1063857 (D' = 1.0; R2 = 1.0) and is therefore likely to be the functional variant responsible for the association between SNP rs1063857 and the risk for CVD in our study population. It is noteworthy that, in the candidate gene study of patients with diabetes mellitus type 1, SNP rs1063856 was also associated with VWF:Ag levels. The Cohort for Heart and Aging Research in Genome Epidemiology (CHARGE) Consortium recently published a meta-analysis of 5 studies in which the association between variation in the total human genome and VWF:Ag levels was investigated. This study confirmed the association between rs1063856, rs1063857, and VWF:Ag level.22 In our study, rs1063857 was borderline significantly associated with VWF:Ag levels, possibly because of the limited power of the study. Another explanation may be that at the time of inclusion, all patients with CVD used statins that are known to decrease VWF levels, probably by influencing VWF secretion.23,24 Therefore, use of statins may have attenuated the association between genetic variation and VWF:Ag levels. Thus far, SNP rs1063857 was associated with an increase in VWF:Ag levels and with the risk for disease in 2 independent studies, which suggests that VWF may be a causal factor in the development of CVD.
It is also interesting to note that although the subgroup of patients with IS was smaller (n = 150) than the subgroup of patients with CHD (n = 371), the associations between SNP rs1063857, SNP rs216293, and CVD appeared to be driven largely by the subgroup of patients with IS. Common genetic variants in other important proteins involved in arterial thrombus formation, such as the GpIbα platelet receptor, show different effect estimates for the risk for CHD and IS as well.25 This finding indicates that genetic variants may have a different contribution to the development of CHD than to the development of IS.
Common variants in the VWF promoter region are tagged by the SNPs in introns 3 and 5. The previously identified association between promoter variants, VWF:Ag levels, and the risk for CVD among other investigated in the Rotterdam study,9,10 could not be replicated in our study. Differences in study design and heterogeneity of the study population may have contributed to these contradictory findings.
A limitation of the case-control design of our study was that only survivors of CVD were included. This may have led to an underestimation of the effect of genetic variation on the risk for CVD. Another limitation of the study was that we investigated variation in the VWF gene only in a population of individuals with European ancestry. Risk factors for CVD, allele frequencies, and LD patterns were dissimilar between different ethnic populations; therefore, the results of this study could not be generalized to other ethnic populations.
Several studies have shown that the genetic contribution to VWF levels and to the pathogenesis of CVD is higher in younger than in older patients.5,26 The strength of our study was that we investigated variation in the VWF gene in a young population with early-onset CVD that was well documented. In contrast to previous studies that focused on individual SNPs and VWF:Ag levels, we investigated ht-SNPs covering the entire VWF gene and studied associations with VWF:Ag levels, VWF:CB activity, and CVD risk. The results of such studies of genetic associations may initiate translational studies and may contribute to the understanding of VWF function and its role in the development of CVD.
In conclusion, our study showed that variants in the VWF gene are associated with VWF:Ag levels and with the risk for CVD. The association with the risk for CVD was largely driven by the subgroup of patients with IS. SNP rs1063857, known to be associated with VWF:Ag levels, was also associated with CVD risk, which suggests a causal role of VWF in the development of CVD. However, polymorphisms that contributed to the risk for CVD were not always associated with VWF:Ag levels or VWF:CB activity. This finding suggests that other mechanisms than increased VWF levels might also mediate the association between VWF gene variation and the risk for CVD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank E. L. de Bruijne, T. N. Bongers, and J. E. van Loon for their contribution to data collection. We thank M. Dieterich, K. van Gaalen, P. van Randwijk, and N. Farhou for their excellent laboratory assistance.
This work was supported by the Erasmus MC (MRACE grant 2006, FWGL) and the Netherlands Heart Foundation (grant no. 2007B159, FWGL).
Authorship
Contribution: F.W.G.L., D.W.J.D., and J.W.D. designed the research; M.C.v.S. performed the research and contributed to data collection; A.I, M.C.v.S., and C.M.v.D. contributed to the statistical analysis; M.C.v.S., M.P.M. de M., F.W.G.L., and A.I. interpreted the data; and M.C.v.S., M.P.M. de M., and A.I. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Frank W. G. Leebeek, Department of Hematology (Room L-435), Erasmus University Medical Centre, PO Box 2040, Rotterdam, The Netherlands; e-mail: f.leebeek@erasmusmc.nl.