Kindlin-3 is a key lymphocyte function–associated antigen-1 (LFA-1) coactivator deleted in leukocyte adhesion deficiency-III (LAD-III). In the present study, we investigated the involvement of this adaptor in lymphocyte motility and TCR-triggered arrest on ICAM-1 or on dendritic cells (DCs). Kindlin-3–null primary T cells from a LAD-III patient migrated normally on the major lymph node chemokine CCL21 and engaged in normal TCR signaling. However, TCR activation of Kindlin-3–null T lymphocytes failed to trigger the robust LFA-1–mediated T-cell spreading on ICAM-1 and ICAM-1–expressing DCs that is observed in normal lymphocytes. Kindlin-3 was also essential for cytoskeletal anchorage of the LFA-1 heterodimer and for microclustering of LFA-1 within ventral focal dots of TCR-stimulated lymphocytes spread on ICAM-1. Surprisingly, LFA-1 on Kindlin-3–null lymphocytes migrating over CCL21 acquired normal expression of an epitope associated with the conformational activation of the key headpiece domain, β I. This activated LFA-1 was highly responsive to TCR-triggered ICAM-1–driven stop signals in normal T cells locomoting on CCL21, but not in their Kindlin-3–null T-cell counterparts. We suggest that Kindlin-3 selectively contributes to a final TCR-triggered outside-in stabilization of bonds generated between chemokine-primed LFA-1 molecules and cell-surface ICAM-1.
Introduction
The lymphocyte function–associated antigen-1 (LFA-1) integrin is the best-studied adhesion molecule involved in the arrest of lymphocytes on their various antigen-presenting partners.1 T cells entering the T zone of lymph nodes must scan a large area for rare antigen-presenting dendritic cells (DCs)2,3 before they encounter sufficient antigen-triggered TCR signals to stop and spread on DCs.2 Recent in vivo imaging of antigen-triggered T-cell arrest on DCs suggests a key role for the LFA-1 ligand ICAM-1 in long-lasting adhesion and spreading of T cells on antigen-presenting DCs.4 TCR-stimulated LFA-1 is also responsible for directing T-cell orientation during cytolytic responses.5 LFA-1 is maintained in a range of nonadhesive conformational states in motile lymphocytes before their encounter with cognate antigens.6 The molecular basis for the ability of TCR signals to switch LFA-1 from a nonadhesive to a highly adhesive state is still unclear.7,8
Previous data implicated the key integrin adaptor Talin1 in TCR-triggered LFA-1 activation.9 Kindlin-3 is an additional integrin coactivator that, together with talin, supports a variety of inside-out and outside-in activation processes underlying the adhesive functions of integrins in platelets, neutrophils, and lymphocytes.10,–12 In T lymphocytes, Kindlin-3 was recently found to be essential for rapid LFA-1 and VLA-4 by chemokine signals in the context of shear forces.13 Nevertheless, LFA-1 on resting T cells from a leukocyte adhesion deficiency-III (LAD-III) patient lacking Kindlin-3 developed considerable shear-resistant adhesion in response to brief TCR stimulation.13 These findings suggested that Kindlin-3 may not be required for a subset of TCR-stimulated LFA-1 activation processes, and therefore also for T-cell arrest on antigen-presenting cells.
T-cell motility on chemokines is a key step in the ability of T cells to scan antigen-presenting cells for TCR-specific stop signals.14 To explore the involvement of Kindlin-3 in this motility and in the generation of high LFA-1 adhesiveness by TCR signals, we analyzed primary peripheral blood (PB) T cells from a LAD-III patient lacking Kindlin-3 expression13 and compared them with control primary lymphocytes. Although Kindlin-3–deficient LAD-III T cells retained intact TCR-signaling responses and displayed normal locomotion on the major T-cell–attracting chemokine CCL21, LAD-III cells failed to arrest on ICAM-1–bearing surfaces in response to various TCR-activation signals. Interestingly, when Kindlin-3–null T cells were spread on immobilized CCL21 or on a substrate coated with anti-CD3 mAb and ICAM-1, LFA-1 on these cells readily expressed the 327C epitope, and this was associated with opening of the β I domain and LFA-1 headpiece activation.15,16 Nevertheless, this headpiece-primed LFA-1 failed to concentrate in focal dots during the spreading of Kindlin-3–null T cells on ICAM-1 and could not engage in LFA-1–mediated arrest in response to TCR stop signals delivered to motile Kindlin-3–null T cells. These results suggest that, rather than acting as a general regulator of LFA-1 headpiece activation, Kindlin-3 contributes to a critical step of LFA-1/ICAM-1 bond stabilization, driving motile lymphocytes to firmly arrest on ICAM-1–expressing DCs in response to TCR-stimulatory signals.
Methods
Reagents and antibodies, human lymphocyte isolation and DC preparation, flow cytometry, motility assays, immunostaining procedures, scanning and transmission electron microscopy, and statistical analysis are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All experiments using human blood samples were approved by the Weizmann Institute of Science review board, and informed consent was provided according to the Declaration of Helsinki.
ICAM-1 bead-binding assay
To examine the encounter rate and duration between T lymphocytes and ICAM-1–coated beads, magnetic protein-A beads (2.8-μ diameter Dynabeads, Dynal; Invitrogen) were coated with recombinant ICAM-1/Fc and blocked with 100 μg/mL of human IgG. CAM site densities were assessed using 125I-labeled anti–ICAM-1 (HA58), as described previously.17 T cells were either left untreated or pretreated with OKT3 mAb (10 μg/mL) and immediately injected together with the beads into microslides (IBIDI) coated with either human serum albumin (2 μg/mL) or CCL21 (2 μg/mL). T cell–bead encounters were recorded for 15 minutes at 6 frames/min using a 40×/0.95 numerical aperture differential interference contrast (DIC) objective. In some experiments, XVA143 (1μM) was included to block the β2 integrin of LFA-1 from reaching its fully activated conformation during the entire assay period. Encounters were defined as any T cell–bead contact lasting > 2 seconds, and a productive contact was defined as any T cell–bead encounter lasting at least 60 seconds. Over 95% of the TCR-triggered productive contacts remained stable for > 5 minutes.
TCR stimulation of LFA-1–dependent lymphocyte spreading on ICAM-1
Control or Kindlin-3–null PB T cells were either left untreated or incubated with OKT3 (10 μg/mL), and immediately perfused into a flow chamber mounted on a polystyrene or glass slide coated with human ICAM-1–Fc overlaid on immobilized protein A (20 μg/mL) and subsequently blocked with human IgG. In other assays, protein A plates were coated with either ICAM-1–Fc or IgG control (1.5 μg/mL), followed by rabbit anti–mouse antibody (10 μg/mL) on which OKT3 (0.2 μg/mL) was captured. T cells were settled on the substrate and their spreading was monitored by videomicroscopy for 10 minutes. To monitor T-cell spreading on DCs associated with CCL21, cultured DCs were seeded on immobilized CCL21 for 45-60 minutes. T cells were perfused over the DC/CCL21 substrate, and both their motility toward and spreading over individual DCs were tracked for 15 minutes by DIC videomicroscopy. Lymphocyte morphology (circumference, area, and darkening) was determined using NIS-Elements D Version 3.0 software (Nikon). T cells that underwent both darkening for at least 3 minutes accompanied by an at least 40% increase in original cell circumference and a 2-fold increase in area were considered spread.
Analysis of integrin resistance to detachment developed during short static contacts with surface-bound mAbs
Purified mAbs were coated on polystyrene plates as described previously.18 The polystyrene plates were each assembled on the lower wall of the flow chamber (260-μm gap).18 Cells were washed with cation-free H/H medium, resuspended in binding medium (H/H medium supplemented with 1mM CaCl2 and 1mM MgCl2), perfused into the flow chamber, and allowed to settle onto the substrate for 1 minute. Flow was then initiated and increased stepwise every 5 seconds through a programmed set of flow rates. At the end of each 5-second interval, the number of cells that remained bound was expressed relative to the number of cells originally settled on the substrate. All cellular interactions with the adhesive substrates were analyzed by MatLab-based computerized tracking of individual cell motion within at least 2 fields of view (each one 0.17 mm2 in area).
Fluorescence image acquisition and analysis
Fluorescence microscopy was carried out with the DeltaVision system (Applied Precision) using an oil 60×/1.4 PlanApo DIC objective. All images of fixed cells were acquired as serial Z-stacks (0.2 μm apart) and subjected to digital deconvolution and 3D reconstruction using the SoftWoRx software Version 3.7.0 (Applied Precision). Microclusters were quantitatively analyzed using 2D polygon modeling of the SoftWoRx software (Applied Precision). A 327C cluster was defined according to size (> 0.4 μm) and fluorescence intensity (at least 4-fold > the cell mean fluorescence intensity).
Results
Lymphocyte Kindlin-3 is not required for lymphocyte motility on lymph node chemokines
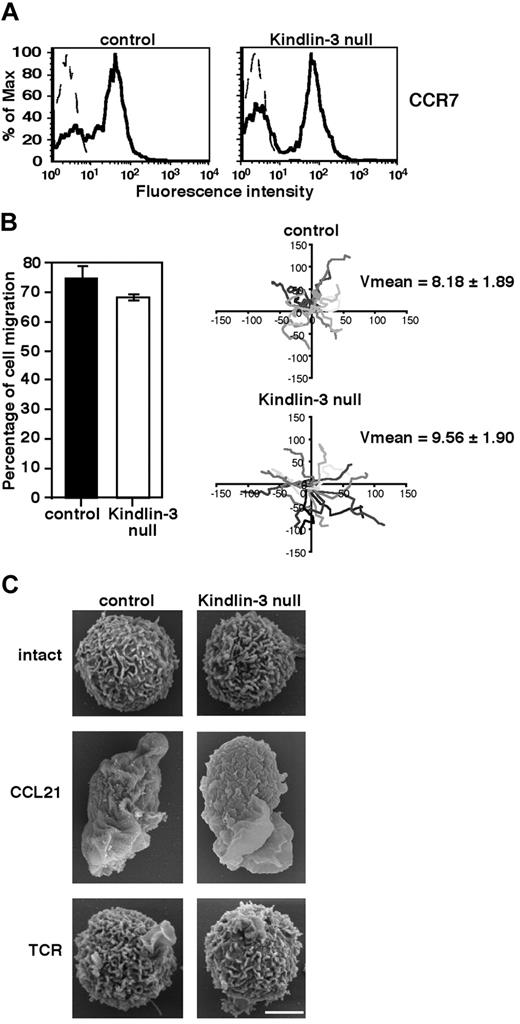
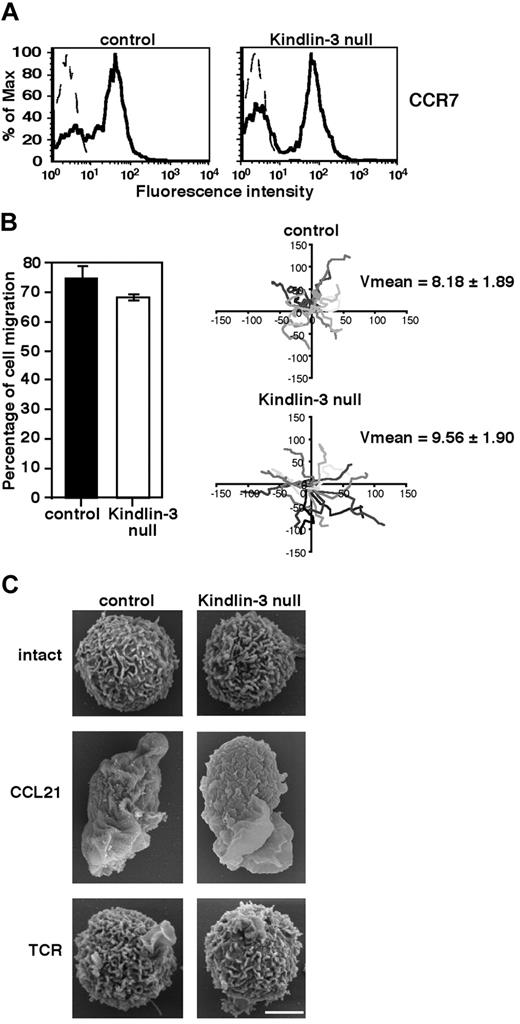
Absence of Kindlin-3 in human EBV–transformed cells derived from LAD-III patients12 and knock-down of Kindlin-3 by siRNA in K562 erythroleukemia10 were recently shown to impair cell motility or spreading on surface-bound fibronectin or ICAM-1. Because primary T cells readily migrate on surface-bound chemokines both in vitro19 and in vivo,14 we first investigated whether the loss of Kindlin-3 in LAD-III patient–derived primary T cells impairs their motility on the major CCR7-specific lymph node chemokine CCL21. Freshly isolated PB CD3+ T cells derived from a LAD-III patient (herein referred to as LAD-III PB T cells), which lack Kindlin-3 expression,13 expressed normal levels of CCR7 (Figure 1A). The fraction of LAD-III PB T cells capable of randomly migrating on a high density of uniformly immobilized CCL21 was practically indistinguishable from that of healthy PB T cells (Figure 1B). Furthermore, the mean migration velocity of Kindlin-3–deficient LAD-III T cells was similar to that of normal T cells (Figure 1B right). The ability of soluble CCL21 to trigger collapse of all lymphocyte microvilli, a key step in chemokine-mediated actin-remodeling events that is essential for leukocyte spreading,20,21 was also largely intact in LAD-III T cells (Figure 1C). Nevertheless, soluble CCL21 failed to trigger expression of the 327C epitope associated with β I domain opening and headpiece activation of LFA-1 in LAD-III cells (supplemental Figure 1), which is in agreement with previous data in LAD-III effector cells stimulated by soluble CXCL12.13 Therefore, Kindlin-3 is not required for the ability of primary T lymphocytes to respond to CCL21 signals, collapse their microvilli, spread, and rapidly migrate over the surface-bound chemokine, but is critical for headpiece activation of LFA-1 by soluble chemokines.
Kindlin-3 is not required for chemokine-mediated T lymphocyte motility or microvillar collapse. (A) CCR7 expression on control and LAD-III Kindlin-3–null PB T cells determined by FACS analysis. (B) Left: The fraction of control or Kindlin-3–null PB T cells persistently locomoting over immobilized CCL21 (2 μg/mL). Results shown are the means of 4 fields of view. Right: Tracks of individual lymphocytes depicted in different shades of gray during 10-minute tracking periods. Average velocities (mean ± SD) in each experimental group are indicated (n = 15). (C) CCL21, but not TCR, signals induce similar microvillar collapse in both control and Kindlin-3–null T lymphocytes. Scanning electron microscopic images of control and Kindlin-3–null T lymphocytes untreated or stimulated for 2 minutes with the TCR-ligating mAb OKT3 (10 μg/mL) or with soluble CCL21 (10nM). Scale bar represents 3 μm. Micrographs are representative of > 55 cells analyzed in each experimental group.
Kindlin-3 is not required for chemokine-mediated T lymphocyte motility or microvillar collapse. (A) CCR7 expression on control and LAD-III Kindlin-3–null PB T cells determined by FACS analysis. (B) Left: The fraction of control or Kindlin-3–null PB T cells persistently locomoting over immobilized CCL21 (2 μg/mL). Results shown are the means of 4 fields of view. Right: Tracks of individual lymphocytes depicted in different shades of gray during 10-minute tracking periods. Average velocities (mean ± SD) in each experimental group are indicated (n = 15). (C) CCL21, but not TCR, signals induce similar microvillar collapse in both control and Kindlin-3–null T lymphocytes. Scanning electron microscopic images of control and Kindlin-3–null T lymphocytes untreated or stimulated for 2 minutes with the TCR-ligating mAb OKT3 (10 μg/mL) or with soluble CCL21 (10nM). Scale bar represents 3 μm. Micrographs are representative of > 55 cells analyzed in each experimental group.
Kindlin-3 is not required for TCR-mediated signaling or integrin-independent spreading
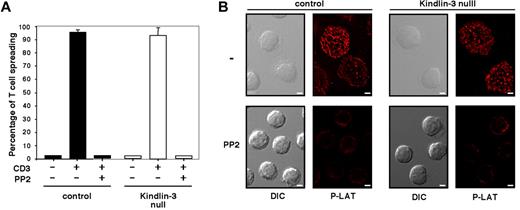
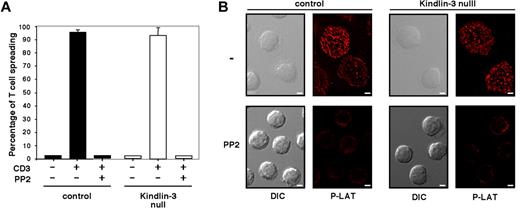
We recently showed that primary human lymphocytes readily spread on the TCR-activating mAb OKT3 through a process driven by actin polymerization,22 which is consistent with similar findings reported for lymphocyte lines and T blasts.23 Normal and LAD-III T cells express comparable levels of CD3 (supplemental Figure 2). When each of these cell populations was applied to a substrate coated with OKT3, nearly all settled lymphocytes rapidly spread over the TCR-stimulatory mAb in both normal and LAD-III groups (Figure 2A). In contrast to a recent study,24 no significant defects in this integrin-independent, TCR-dependent spreading process were observed on substrates coated with either high- or low-density OKT3 (Figure 2A and data not shown). Lymphocyte spreading on the immobilized anti-CD3 mAb was eliminated by lymphocyte pretreatment with the Src family kinase (SFK) inhibitor PP2 (Figure 2A-B). Furthermore, the SFK-dependent spreading of both normal and LAD-III T cells on immobilized OKT3 was associated with robust tyrosine phosphorylation of the proximal TCR adaptor LAT in both normal and LAD-III T cells (Figure 2B top row). Therefore, SFK-dependent TCR signaling to LAT and consequent lymphocyte spreading on surface-bound anti-CD3 mAb were fully conserved in LAD-III T cells, suggesting that Kindlin-3 is not required for TCR signaling to the actin cytoskeleton. These results demonstrate that integrin-independent T-cell spreading triggered by either CCR7 or TCR signals does not require Kindlin-3.
Kindlin-3 is not required for SFK-dependent TCR-mediated signaling and spreading. (A) Control (black bars) or Kindlin-3–null (white bars) PB T cells were pretreated with carrier (DMSO 0.1%) or PP2 (10μM for 30 minutes at 37°C) and allowed to spread on immobilized anti-CD3 mAb (OKT3, 20 μg/mL). Results are the mean ± range of 2 fields. The experiment shown is representative of 2. (B) PB T cells spread on anti-CD3 mAb as in panel A were fixed and stained with anti–phospho LAT antibodies. DIC and fluorescence images of cells are shown in a representative field. Scale bar represents 3 μm.
Kindlin-3 is not required for SFK-dependent TCR-mediated signaling and spreading. (A) Control (black bars) or Kindlin-3–null (white bars) PB T cells were pretreated with carrier (DMSO 0.1%) or PP2 (10μM for 30 minutes at 37°C) and allowed to spread on immobilized anti-CD3 mAb (OKT3, 20 μg/mL). Results are the mean ± range of 2 fields. The experiment shown is representative of 2. (B) PB T cells spread on anti-CD3 mAb as in panel A were fixed and stained with anti–phospho LAT antibodies. DIC and fluorescence images of cells are shown in a representative field. Scale bar represents 3 μm.
TCR signals fail to stabilize conformationally activated LFA-1 within ICAM-1–dependent focal dots in Kindlin-3–null T cells
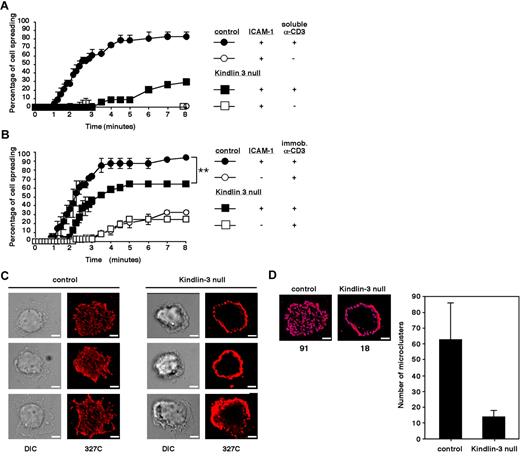
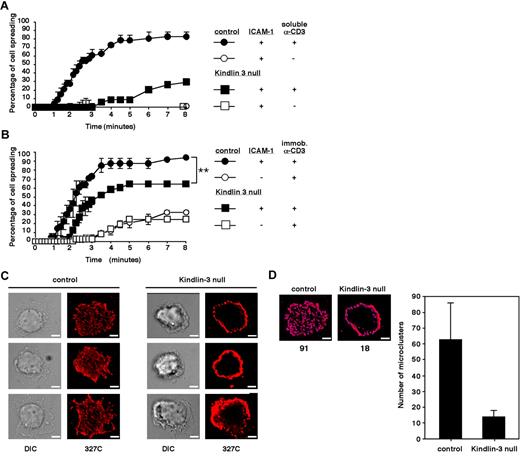
We recently showed that LFA-1 expressed on LAD-III T cells settled briefly on ICAM-1 can generate shear-resistant adhesion when triggered by OKT3.13 In the present study, we explored the involvement of Kindlin-3 in a more physiologic setting of TCR-stimulated spreading of T cells on ICAM-1 under shear-free conditions, mimicking the extravascular environment in which T cells encounter TCR-stimulatory signals and arrest on ICAM-1–expressing antigen-presenting cells. Adhesion and spreading of LAD-III T cells and of normal T cells were compared on a substrate coated with high-density ICAM-1 before or after brief stimulation with saturating levels of soluble OKT3. Despite their ability to generate shear-resistant TCR-stimulated LFA-1 adhesions,13 Kindlin-3–deficient LAD-III T cells exhibited dramatically reduced LFA-1 spreading compared with their normal counterparts, suggesting a severe defect in LFA-1 activation by TCR signals (Figure 3A). Interestingly, saturating levels of the CD3-ligating mAb failed to drive microvillar collapse, as opposed to CCL21 (Figure 1C), suggesting a critical role for LFA-1–driven actin remodeling in this spreading. Therefore, although integrin-independent T-cell spreading on a TCR-ligating mAb is Kindlin-3 independent, optimal TCR stimulation of LFA-1/ICAM-1 adhesions, which are critical for T-cell spreading on ICAM-1, appears to be Kindlin-3 dependent.
Kindlin-3–null cells display defective TCR-triggered spreading on ICAM-1 and fail to generate scattered focal dots on immobile ICAM-1 enriched with headpiece-activated LFA-1. (A) Time course of normal and Kindlin-3–null PB T lymphocyte spreading on ICAM-1 (600 sites/μm2) triggered by TCR ligation with soluble OKT3. (B) Time course of normal and Kindlin-3–null T cells spreading on ICAM-1–IgG (600 sites/μm2) or IgG, each co-immobilized with rabbit anti–mouse antibody. Where indicated, OKT3 was immobilized on the rabbit antibody. Values in panels A and B are each the mean ± range of 2 fields of view. **P < .005 (C) Control and Kindlin-3–null T cells spread on the immobilized OKT3/ICAM-1 surface shown in panel B were fixed and stained with Alexa Fluor 568–labeled 327C mAb specific for the active (open) I domain of the LFA-1 β2 subunit. Scale bar represents 3 μm. Experiments in panels A-C are each representative of 2. (D) Shown is a representative control and Kindlin-3–null cell (taken from panel B, top row) quantitatively analyzed for microclustering by 2D polygon modeling. The number of individual clusters in the indicated cells is shown below the images in the left panels, and the mean number of clusters per cell is shown in the right panel. Eight cells representative of 100 were analyzed.
Kindlin-3–null cells display defective TCR-triggered spreading on ICAM-1 and fail to generate scattered focal dots on immobile ICAM-1 enriched with headpiece-activated LFA-1. (A) Time course of normal and Kindlin-3–null PB T lymphocyte spreading on ICAM-1 (600 sites/μm2) triggered by TCR ligation with soluble OKT3. (B) Time course of normal and Kindlin-3–null T cells spreading on ICAM-1–IgG (600 sites/μm2) or IgG, each co-immobilized with rabbit anti–mouse antibody. Where indicated, OKT3 was immobilized on the rabbit antibody. Values in panels A and B are each the mean ± range of 2 fields of view. **P < .005 (C) Control and Kindlin-3–null T cells spread on the immobilized OKT3/ICAM-1 surface shown in panel B were fixed and stained with Alexa Fluor 568–labeled 327C mAb specific for the active (open) I domain of the LFA-1 β2 subunit. Scale bar represents 3 μm. Experiments in panels A-C are each representative of 2. (D) Shown is a representative control and Kindlin-3–null cell (taken from panel B, top row) quantitatively analyzed for microclustering by 2D polygon modeling. The number of individual clusters in the indicated cells is shown below the images in the left panels, and the mean number of clusters per cell is shown in the right panel. Eight cells representative of 100 were analyzed.
Normal T cells adhered on a substrate coated with a low-density of CD3-ligating mAb undergo much faster spreading if they encounter co-immobilized ICAM-122 (Figure 3B). We next investigated whether LAD-III/Kindlin-3–null T cells adhered to low-density CD3-ligating mAb (Figure 2) can also spread more rapidly when encountering ICAM-1. Interestingly, despite their poor spreading on ICAM-1 (Figure 3A) in response to signals from soluble CD3-ligating mAb, Kindlin-3–null T cells, which adhered to low-density CD3-ligating mAb, exhibited increased spreading in response to ICAM-1, although to a lesser degree and with slower kinetics than normal T cells (Figure 3B). Furthermore, within the fraction of LAD-III/Kindlin-3–null T cells that successfully spread on the OKT3/ICAM-1–bearing substrate, a large number of LFA-1 molecules expressed the 327C epitope associated with the activated open β I domain (Figure 3C). Because this conformational state is stabilized in an LFA-1 subset of T cells spread on ICAM-1 but not in T cells spread on VCAM-1 in response to TCR-stimulating signals,22 Kindlin-3 appears to be dispensable for the ability of the LFA-1 β I domain to acquire an open conformation (ie, to be recognized by the 327C mAb) when encountering ICAM-1. Nevertheless, all of the LFA-1 molecules carrying this 327C β I domain activation epitope remained in a thick peripheral ring on Kindlin-3–null T cells (Figure 3C right panels). In contrast, characteristic focal dots enriched with 327C-bearing LFA-1 were readily generated within the ventral surface of normal T cells spread on identical ICAM-1 and co-immobilized CD3-ligating mAb (Figure 3C left panels). Therefore, although high levels of LFA-1 bearing the 327C epitope could be detected on Kindlin-3–null lymphocytes during TCR-triggered spreading on ICAM-1, the ability of these conformationally switched LFA-1 molecules to microcluster in evenly scattered focal dots and support rapid spreading on ICAM-1 (Figure 3B) was impaired in Kindlin-3–null T cells. Because these LFA-1/ICAM-1–dependent focal sites are the quantal adhesive units of rapid T-cell spreading on ICAM-1,22 Kindlin-3 appears to be critical for the early organization of active ICAM-1–occupied LFA-1 within these focal assemblies, rather than for conformational LFA-1 headpiece activation by TCR and ICAM-1 signals.
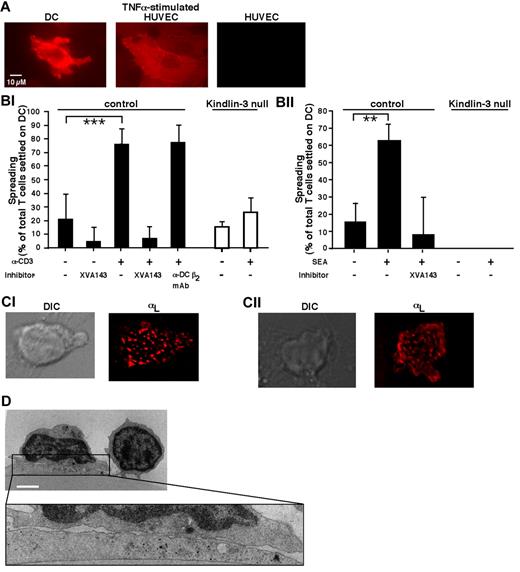
The evenly scattered focal dots of microclustered LFA-1 characteristic of T cells spread on ICAM-1 were also observed under normal T cells spread on ICAM-1–expressing DCs in response to either OKT3-mediated ligation or an encounter with DC-presented superantigen (Figure 4A-C). This multifocal pattern of T cell–DC synapses remained stable for up to 60 minutes of spreading (not shown) and was reflected in multiple closely apposed contacts between the spread T cells and their DC targets (Figure 4D), as was reported previously for murine T cell–DC conjugates.25,26 However, despite their ability to form rings of activated LFA-1 and to spread on anti-CD3 mAb in the presence of co-immobilized ICAM-1 (Figure 3B-C), Kindlin-3–deficient T cells totally failed to spread on DCs in response to either soluble CD3-ligating mAb or DC-presented superantigen (Figure 4Bi-ii). Therefore, the indispensable function of Kindlin-3 in conferring T cells with the ability to organize their activated LFA-1 within focal dots on isolated ICAM-1 (Figure 3C) appeared to also be essential for the ability of T cells to organize their LFA-1 in scattered focal dots and to spread on DC ICAM-1 after TCR stimulation.
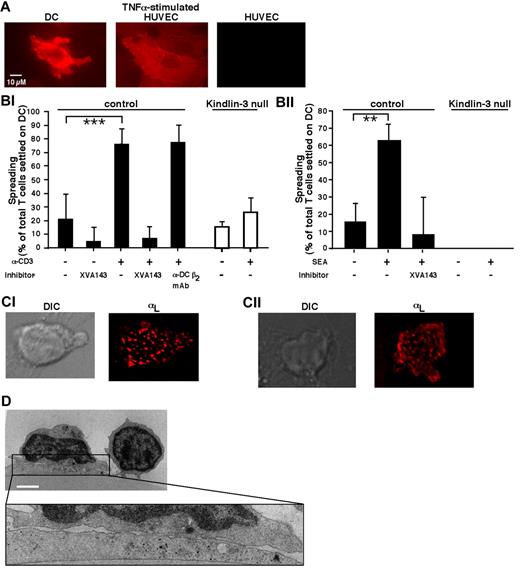
TCR-triggered Kindlin-3–null T cells fail to spread on DCs. (A) ICAM-1 expression on human DCs spread for 1 hour on immobilized CCL21 before being tested as a substrate for PB T cells. DCs were fixed and stained with PE-conjugated α-ICAM-1 mAb. Staining of resting and TNFα-stimulated HUVECs (200 U/mL for 24 hours) is shown for comparison. Scale bar represents 10 μm. (Bi) PB T cells were stimulated with OKT3 (10 μg/mL) or left in medium (–) and settled alone or in the presence of the indicated inhibitors on DCs prespread on immobilized CCL21 as in panel A. For each experimental group, the fractions of settled lymphocytes that spread on the DCs were quantified by live videomicroscopy, as described in “Methods.” Values are the means ± SD of 9 fields of view in 3 independent experiments. ***P < 10−6. (Bii) CD4+ PB T cells were settled for 10 minutes on DCs that had been left untreated or preloaded for 1 hour with staphylococcal endotoxin A (1 μg/mL). All DCs were prespread on immobilized CCL21 as in panel A. The fractions of lymphocytes spread on DCs were quantified as in panel Bi. Values are the means ± SD of 4 fields of view in 1 experiment representative of 3. **P < .005. (Ci) Normal PB T cells were incubated with OKT3 (10 μg/mL), labeled with a trace of Alexa Fluor 568–labeled TS2.4 (anti–αL, 1 μg/mL), and allowed to spread for 5 minutes on DCs before fixation. Shown are a DIC image (left) and a fluorescence image (right) of LFA-1 in a representative T cell. Scale bar represents 3 μm. (Cii) Fluorescence staining of LFA-1 visualized by Alexa Fluor 568–labeled TS2.4 anti–LFA-1 in a normal CD4+ T cell spread for 10 minutes on an staphylococcal endotoxin A–loaded DC prespread on CCL21 and fixed. Scale bar represents 3 μm. (D) Transmission electron microscopic image of a representative OKT3-stimulated normal T cell spread on a DC prespread on immobilized CCL21 as in panel Bi. Because no Kindlin-3–null T cells could be recovered from the DC after fixation, similar fluorescence staining and electron microscopy could not be carried out for these T cells. Scale bar represents 3 μm.
TCR-triggered Kindlin-3–null T cells fail to spread on DCs. (A) ICAM-1 expression on human DCs spread for 1 hour on immobilized CCL21 before being tested as a substrate for PB T cells. DCs were fixed and stained with PE-conjugated α-ICAM-1 mAb. Staining of resting and TNFα-stimulated HUVECs (200 U/mL for 24 hours) is shown for comparison. Scale bar represents 10 μm. (Bi) PB T cells were stimulated with OKT3 (10 μg/mL) or left in medium (–) and settled alone or in the presence of the indicated inhibitors on DCs prespread on immobilized CCL21 as in panel A. For each experimental group, the fractions of settled lymphocytes that spread on the DCs were quantified by live videomicroscopy, as described in “Methods.” Values are the means ± SD of 9 fields of view in 3 independent experiments. ***P < 10−6. (Bii) CD4+ PB T cells were settled for 10 minutes on DCs that had been left untreated or preloaded for 1 hour with staphylococcal endotoxin A (1 μg/mL). All DCs were prespread on immobilized CCL21 as in panel A. The fractions of lymphocytes spread on DCs were quantified as in panel Bi. Values are the means ± SD of 4 fields of view in 1 experiment representative of 3. **P < .005. (Ci) Normal PB T cells were incubated with OKT3 (10 μg/mL), labeled with a trace of Alexa Fluor 568–labeled TS2.4 (anti–αL, 1 μg/mL), and allowed to spread for 5 minutes on DCs before fixation. Shown are a DIC image (left) and a fluorescence image (right) of LFA-1 in a representative T cell. Scale bar represents 3 μm. (Cii) Fluorescence staining of LFA-1 visualized by Alexa Fluor 568–labeled TS2.4 anti–LFA-1 in a normal CD4+ T cell spread for 10 minutes on an staphylococcal endotoxin A–loaded DC prespread on CCL21 and fixed. Scale bar represents 3 μm. (D) Transmission electron microscopic image of a representative OKT3-stimulated normal T cell spread on a DC prespread on immobilized CCL21 as in panel Bi. Because no Kindlin-3–null T cells could be recovered from the DC after fixation, similar fluorescence staining and electron microscopy could not be carried out for these T cells. Scale bar represents 3 μm.
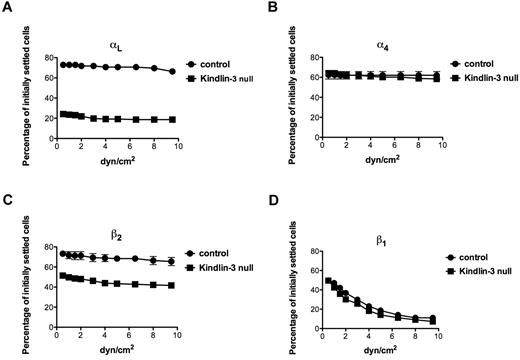
Kindlin-3 is required for increased resistance of ligated αL and β2 subunits to detachment forces
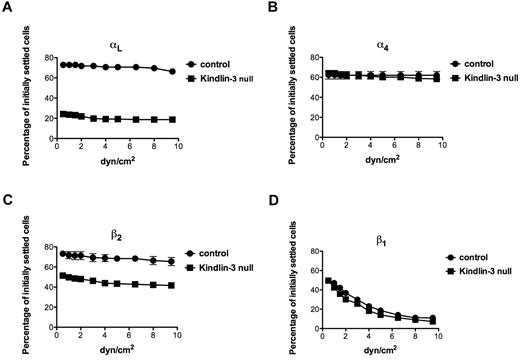
We next considered that the inability of LFA-1 to microcluster in focal dots in Kindlin-3–null T cells spread on anti-CD3 mAb and ICAM-1, despite its normal acquisition of activated headpiece conformation, resides in either a specialized (TCR-dependent) or a more general (TCR-independent) defect in the cytoskeletal anchorage of ligated LFA-1. In a previous study, we used an assay based on shear force application to assess the degrees of selectin and integrin anchorage to the cortical cytoskeleton by determining the relative resistance of intact and tail-mutated selectins and integrin subunits to detachment forces in lymphocytes occupied by surface-bound mAbs specific to the ectodomains of these subunits.27,28 For example, an α4 cytoplasmic tail mutant with abrogated paxillin binding and cytoskeletal anchoring was normally occupied by a mAb to the extracellular α4 domain, but failed to generate shear-resistant adhesions when occupied by a surface-bound form of this mAb.28 We therefore settled equal numbers of either normal or LAD-III/Kindlin-3–null T cells on substrates coated with identical site densities of the αL I domain–specific mAb TS1.22 or the α4-binding mAb HP1.2. Strikingly, the ability of the αL subunit on Kindlin-3–null T cells to resist detachment from the surface-bound anti-αL mAb was diminished compared with the αL subunit of normal T cells (Figure 5A). Nevertheless, the ability of the α4 subunit or the β1 subunit to resist detachment from an α4-specific or a β1-specific surface-bound mAb, respectively, remained unchanged in Kindlin-3–null T cells. In contrast, paxillin suppression in primary T cells reduces the basal resistance of the α4 subunit to detachment from its cognate mAb.29 Consistent with a specialized role of Kindlin-3 in the resistance of mAb occupied αL to detachment by external forces, the resistance of the β2 subunit to forces was also significantly reduced in Kindlin-3–null T cells compared with control lymphocytes (Figure 5C). Therefore, whereas Kindlin-3 constitutively contributed to optimal resistance of both the β2 and the αL subunits of LFA-1 to detachment forces in resting T cells, it did not seem to increase the mechanical resistance of either the β1 subunit or its associated α4 subunit. The TCR-activation signals that triggered robust LFA-1 adhesion to ICAM-1 (Figure 3A) did not augment the resistance of either mAb-occupied LFA-1 or mAb occupied VLA-4 to detachment forces (not shown). Therefore, TCR signals activate LFA-1 adhesiveness by facilitating its headpiece activation by ICAM-1, rather than by strengthening the cytoskeletal associations of the LFA-1 heterodimer before its occupancy with ICAM-1. These results suggest that Kindlin-3 plays an additional, TCR-independent role in the cytoskeletal anchorage of LFA-1.
Ligation of LFA-1 by immobile mAbs generates stronger shear-resistant adhesions in normal than in Kindlin-3–deficient T cells. Spontaneous adhesion strengthening developed by PB T cells settled for 1 minute on surface-coated TS1.22 (anti-αL; A), HP1.2 (anti-α4; B), TS1.18 (anti-β2; C), or CD29 (anti-β1; D) antibodies. Cells were subjected to detachment by incremented shear forces, and the percentage of initially settled T cells that resisted detachment from the substrate at the indicated shear forces was determined in 2 fields of view. The values represent the means ± range. Each experiment shown is representative of 3.
Ligation of LFA-1 by immobile mAbs generates stronger shear-resistant adhesions in normal than in Kindlin-3–deficient T cells. Spontaneous adhesion strengthening developed by PB T cells settled for 1 minute on surface-coated TS1.22 (anti-αL; A), HP1.2 (anti-α4; B), TS1.18 (anti-β2; C), or CD29 (anti-β1; D) antibodies. Cells were subjected to detachment by incremented shear forces, and the percentage of initially settled T cells that resisted detachment from the substrate at the indicated shear forces was determined in 2 fields of view. The values represent the means ± range. Each experiment shown is representative of 3.
Lymphocyte motility on CCL21 enhances LFA-1 responsiveness to TCR stop signals in normal but not in Kindlin-3–deficient T cells
T cells encounter DCs while migrating on CCL21 presented by fibroblastic reticular cells.14 This chemokine was previously shown to keep LFA-1 in a nonadhesive state both in vitro and in vivo.19 We therefore next investigated whether TCR signals encountered by T cells as they locomote on immobilized CCL21 can readily switch the nonadhesive LFA-1 molecules clustered at the leading edge of T cells locomoting over CCL21,19 triggering LFA-1–dependent T-cell arrest on ICAM-1–bearing surfaces. As reported previously,19 LFA-1 failed to arrest T cells locomoting over CCL21 near ICAM-1 beads or DCs expressing ICAM-1 (Figure 6A and 6Bi-ii, bar 1). Although CCL21-polarized T cells could transiently grab ICAM-1 beads, the duration of these contacts were ICAM-1 independent (supplemental Figure 3), supporting the notion that LFA-1 on these chemokine-polarized T cells is functionally nonadhesive. This result is compatible with an earlier finding that LFA-1 remains nonadhesive in the same T cells allowed to locomote on surfaces presenting both CCL21 and ICAM-1 unless shear stress is applied on the locomoting T cells.19 Surprisingly, however, a subset of these LFA-1 cells acquired an activated headpiece conformation carrying the open 327C β I domain epitope and remained in clusters at the leading edge (Figure 6C). This population of LFA-1 molecules appeared highly sensitive to TCR signals, because nearly all T cells migrating on CCL21 firmly bound ICAM-1 beads, but not control nonadhesive beads, shortly after exposure to a TCR-ligating mAb (supplemental Video 1). Furthermore, T-cell motility on CCL21 was found to increase LFA-1 responsiveness to TCR activation by > 200% after normalizing T-cell/ICAM-1 bead-binding events to the number of overall T-cell–bead encounters (Figure 6A bars 2 and 4). Therefore, TCR signals not only overcame the apparent LFA-1 silencing exerted by immobilized CCL21, but in fact triggered LFA-1 adhesiveness much more efficiently in T cells polarized and migrating on CCL21 (Figure 6A). Consistent with these results, T cells locomoting on CCL21 readily arrested after collision with DCs in response to TCR stimulation by either anti-CD3 mAb or DC-presented superantigen (Figure 6Bi-ii).
Motility on CCL21 primes T-cell LFA-1 in both normal and Kindlin-3–deficient T cells, but the primed LFA-1 undergoes TCR-induced activation on ICAM-1 binding only in normal T cells. (A) The fraction of control PB T cells engaged by firm contacts with ICAM-1 (600 sites/μm2)–coated beads during distinct conditions of motility and TCR stimulation. T cells settled on albumin (nonmotile) or allowed to locomote on immobilized CCL21 were exposed to the anti-CD3 mAb (OKT3) or to control mAb. Where indicated, the α/β I allosteric antagonist of LFA-1/ICAM-1 interactions XVA143 (1μM) was included. The number of firm T-cell–bead contacts (> 60 seconds) was divided by the number of total T-cell–bead-encountering events for each experimental condition to yield the fraction of productive contacts. Values are the means ± SD of 4 fields of view. (B) Control (black) compared with Kindlin-3–null (white) PB lymphocytes were allowed to migrate over a CCL21-coated surface toward scattered DCs. (Bi) Each group of T cells was preincubated either in the presence of OKT3 or a control mAb. (Bii) Control (black) or Kindlin-3–null (white) T cells were allowed to migrate on CCL21 toward DCs preloaded with medium or superantigen (staphylococcal enterotoxin A [SEA]). The fractions of T cells that established firm adhesion (> 5 minutes) after colliding into prespread DCs are shown. Values are the means ± SD of 9 fields of view in 3 independent experiments. The frequency of T-cell collision into DCs was comparable in all experimental groups. (C) Control (left) or Kindlin-3–null (right) PB T cells left alone (−, top) or allowed to locomote on immobilized CCL21 (bottom) as in panel A were fixed and stained with either the pan–anti-β2 mAb (TS1.18, left) or the 327C mAb (right) that detects the active open state of the β2 I domain. Merged DIC/fluorescence images of representative T cells are shown. Scale bar represents 3 μm. (D) The fraction of control (black) compared with Kindlin-3–null (white) PB lymphocytes engaged by productive contacts with high-density (4200 sites/μm2) ICAM-1–coated beads under conditions identical to those in panel A. The fraction of T cells suspended in Mg2+/EGTA medium firmly bound to similar ICAM-1 beads is shown for comparison. Values are the means ± SD of 4 fields of view.
Motility on CCL21 primes T-cell LFA-1 in both normal and Kindlin-3–deficient T cells, but the primed LFA-1 undergoes TCR-induced activation on ICAM-1 binding only in normal T cells. (A) The fraction of control PB T cells engaged by firm contacts with ICAM-1 (600 sites/μm2)–coated beads during distinct conditions of motility and TCR stimulation. T cells settled on albumin (nonmotile) or allowed to locomote on immobilized CCL21 were exposed to the anti-CD3 mAb (OKT3) or to control mAb. Where indicated, the α/β I allosteric antagonist of LFA-1/ICAM-1 interactions XVA143 (1μM) was included. The number of firm T-cell–bead contacts (> 60 seconds) was divided by the number of total T-cell–bead-encountering events for each experimental condition to yield the fraction of productive contacts. Values are the means ± SD of 4 fields of view. (B) Control (black) compared with Kindlin-3–null (white) PB lymphocytes were allowed to migrate over a CCL21-coated surface toward scattered DCs. (Bi) Each group of T cells was preincubated either in the presence of OKT3 or a control mAb. (Bii) Control (black) or Kindlin-3–null (white) T cells were allowed to migrate on CCL21 toward DCs preloaded with medium or superantigen (staphylococcal enterotoxin A [SEA]). The fractions of T cells that established firm adhesion (> 5 minutes) after colliding into prespread DCs are shown. Values are the means ± SD of 9 fields of view in 3 independent experiments. The frequency of T-cell collision into DCs was comparable in all experimental groups. (C) Control (left) or Kindlin-3–null (right) PB T cells left alone (−, top) or allowed to locomote on immobilized CCL21 (bottom) as in panel A were fixed and stained with either the pan–anti-β2 mAb (TS1.18, left) or the 327C mAb (right) that detects the active open state of the β2 I domain. Merged DIC/fluorescence images of representative T cells are shown. Scale bar represents 3 μm. (D) The fraction of control (black) compared with Kindlin-3–null (white) PB lymphocytes engaged by productive contacts with high-density (4200 sites/μm2) ICAM-1–coated beads under conditions identical to those in panel A. The fraction of T cells suspended in Mg2+/EGTA medium firmly bound to similar ICAM-1 beads is shown for comparison. Values are the means ± SD of 4 fields of view.
Because Kindlin-3 was found to be dispensable for T-cell motility on CCL21 (Figure 1B) and for upstream TCR signaling (Figure 2), but to be required for TCR triggering of LFA-1 focal contacts (Figure 3A-B), we next investigated whether the loss of this adaptor would perturb both the switching of the LFA-1 headpiece into a primed headpiece conformation on Kindlin-3–null T cells locomoting on CCL21, as well as the responsiveness of LFA-1 to TCR stop signals. LFA-1 clustered normally at the leading edge of Kindlin-3–null T cells migrating on CCL21, as indicated by similar localization of the LBA-1 β2 subunit in both control and Kindlin-3–null PB T cells (Figure 6C columns 1 and 3). Surprisingly, the fraction of clustered β2 found to express the open β I activation epitope 327C was preserved or even elevated in motile Kindlin-3–null PB T cells (Figure 6C). These results suggest that Kindlin-3 is not required for the generation of primed LFA-1 (Figure 7) on T cells migrating over CCL21. Nevertheless, this primed LFA-1 could not respond to TCR stimuli, because neither CD3-ligating mAb nor DC-presented superantigen could arrest motile Kindlin-3–null T cells on ICAM-1–expressing DCs (Figure 6Bi-ii). Furthermore, the primed LFA-1 on Kindlin-3–null T cells migrating on CCL21 also failed to generate firm adhesion to ICAM-1–coated beads in response to the CD3-ligating mAb (Figure 6D white bars 2 and 3 and supplemental Video 2). As expected, LFA-1 on Kindlin-3–null T cells became locked in a fully adhesive state to ICAM-1 in the presence of activating cations (Figure 6D). These findings suggest that signals from surface-bound CCL21 switch the β I headpiece domain of LFA-1 into an open conformation in a Kindlin-3–independent manner. However, once it encounters TCR signals, the CCL21-primed LFA-1 microclusters in focal dots together with surface-bound ICAM-1 and does so in a Kindlin-3–dependent manner (Figure 7). These focal assemblies are therefore critical both for the arrest of motile lymphocytes and for their subsequent spreading on DC ICAM-1.
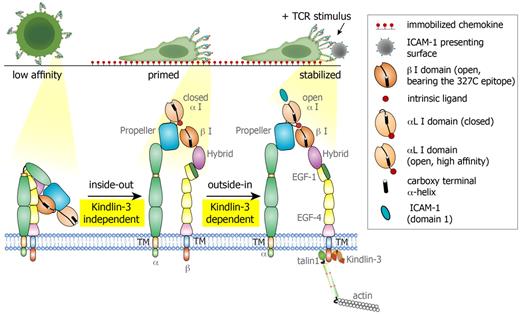
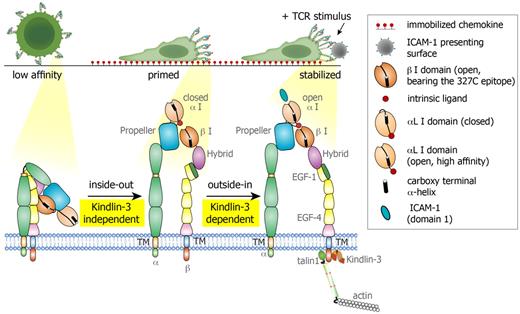
Proposed model for LFA-1 conformational switches triggered during lymphocyte motility on CCL21 and by TCR signals. Resting PB T cells express globally inactive LFA-1 with low affinity to ICAM-1. During motility on the immobilized chemokine, LFA-1 molecules cluster at the leading edge of the polarized T cell, and a subset of these LFA-1 molecules undergo priming and opening of their β2 I domain, exposing the 327C epitope. This priming step is Kindlin-3 independent. The αL I domain on both normal and Kindlin-3–deficient T cells remains in a closed conformation with low affinity to ICAM-1 and is therefore nonadhesive. When a concomitant TCR signal is encountered by a motile lymphocyte, the primed LFA-1 becomes readily responsive to an outside-in, ICAM-1–driven rearrangement event, which stabilizes the αL I domain in an open, high-affinity conformation, resulting in a firm LFA-1/ICAM-1 contact (to a bead or a DC, data not shown). This critical TCR-stimulated LFA-1/ICAM-1 bond stabilization does not take place in Kindlin-3–deficient T cells due to the inability of the LFA-1 heterodimer to properly anchor to the cytoskeleton and undergo final outside-in unclasping.
Proposed model for LFA-1 conformational switches triggered during lymphocyte motility on CCL21 and by TCR signals. Resting PB T cells express globally inactive LFA-1 with low affinity to ICAM-1. During motility on the immobilized chemokine, LFA-1 molecules cluster at the leading edge of the polarized T cell, and a subset of these LFA-1 molecules undergo priming and opening of their β2 I domain, exposing the 327C epitope. This priming step is Kindlin-3 independent. The αL I domain on both normal and Kindlin-3–deficient T cells remains in a closed conformation with low affinity to ICAM-1 and is therefore nonadhesive. When a concomitant TCR signal is encountered by a motile lymphocyte, the primed LFA-1 becomes readily responsive to an outside-in, ICAM-1–driven rearrangement event, which stabilizes the αL I domain in an open, high-affinity conformation, resulting in a firm LFA-1/ICAM-1 contact (to a bead or a DC, data not shown). This critical TCR-stimulated LFA-1/ICAM-1 bond stabilization does not take place in Kindlin-3–deficient T cells due to the inability of the LFA-1 heterodimer to properly anchor to the cytoskeleton and undergo final outside-in unclasping.
Discussion
Integrin activation is tightly regulated by multiple conformational switches that involve unclasping of the integrin heterodimer coupled to allosteric changes in the integrin headpiece, which can take place both before and after ligand binding.30,31 Talins and Kindlins are 2 essential coactivators of these conformational changes and are therefore the most critical regulatory adaptors of integrin-mediated adhesions in both nonhematopoietic and hematopoietic cells.32,–34 In the current study, we investigated the involvement of Kindlin-3 in 2 of the most important adhesive processes that determine the formation of immune synapses: TCR-stimulated LFA-1–mediated arrest and spreading of T cells on DC ICAM-1.7,35 Although the role of Talin1 in these processes is well established,9 the contribution of Kindlin-3 to LFA-1–mediated synapses formed by primary lymphocytes and DCs has not been explored.13 Our present study reveals a critical role for this focal adhesion adaptor in LFA-1 adhesiveness in 2 biologic contexts regulated by TCR activation: (1) LFA-1 adhesiveness to ICAM-1 at the leading edge of primary human lymphocytes migrating over a surface-presented chemokine and triggered by an in situ TCR-activating signal, and (2) LFA-1 adhesiveness to ICAM-1 at the ventral side of these lymphocytes as they spread in response to chemokine-independent TCR signals.
T cells migrate considerable distances on CCL21-presenting stromal cells in search of antigen-presenting DCs.14 We provide the first evidence that T-cell motility over the lymph node chemokine CCL21 primes LFA-1 for subsequent TCR-mediated activation in normal T cells. Kindlin-3 is critical for the coupling of the LFA-1–priming chemokine signals and the TCR arrest signal, which drives an otherwise nonadhesive LFA-1 molecule that fails to assemble in focal contacts before encountering TCR stimuli19 into ICAM-1–induced conformationally activated LFA-1 microclustered within focal dots. CCL21 signals both promote lymphocyte motility and stabilize the LFA-1 headpiece β I domain on motile T cells in an open conformation (as manifested by the appearance of the 327C epitope on the LFA-1 headpiece), and they do so in a Kindlin-3–independent manner. Three major GTPases, RhoA, Rac1, and Rap-1, are involved in the induction of the 327C epitope on lymphocyte LFA-1 in response to chemokine signals.16,36 Each of these GTPases is also readily triggered by chemokine signals.16,36 Kindlin-3 is apparently not required for the activation of these GTPases, because its loss does not interfere with chemokine-stimulated Rap-1 activation10 and does not perturb microvillar collapse, spreading, or contractility (this study), processes tightly dependent on chemokine-stimulated Rac1 and RhoA activation.21,37,38 These chemokine-activated GTPases were reported to recruit Talin1 or activate its headpiece at the leading edge of motile T cells.16,19,32 Therefore, the amount of activated Talin1 proximal to LFA-1 at the leading edge of both normal and Kindlin-3–null lymphocytes may be sufficiently high to stabilize a fraction of LFA-1 in a primed conformation bearing the open β I epitope 327C, which was previously shown to be tightly regulated by Talin1.16,39 In contrast, rapid induction of this epitope by soluble chemokine signals encountered by T cells is a Kindlin-3–dependent process, suggesting that LFA-1–priming in T cells locomoting on surface-bound chemokine is qualitatively different from LFA-1 priming in suspended T cells globally activated by the same chemokine. Nevertheless, the clustering of primed LFA-1 molecules with an open β I domain is obviously insufficient to confer LFA-1 adhesiveness, as evident from the complete failure of these LFA-1 molecules to bind ICAM-1–coated beads even in normal T cells, despite the low stringency conditions of this cell-bead adhesive assay.22 In the presence of TCR signals, however, this primed LFA-1 expressing the 327C epitope undergoes a critical outside-in activation by ICAM-1, which in contrast to the initial LFA-1 headpiece–priming event, requires Kindlin-3. Therefore, Kindlin-3 is dispensable for T-cell motility on the major lymph node chemokine CCL21 for the enrichment and microclustering of LFA-1 with open β I domain (acquiring the 327C epitope) in T cells polarized on immobilized CCL21 and for early TCR signaling. However, Kindlin-3 is essential for the final outside-in activation of the integrin headpiece by ICAM-1, a critical step in LFA-1/ICAM-1 bond stabilization and lymphocyte arrest on ICAM-1 (Table 1). A previous study showed that the ability of LFA-1 to acquire the 327C epitope and undergo opening of its β I domain in response to chemokine stimuli is directly linked to successful LFA-1 binding of ICAM-1.16 Our results, however, demonstrate the complexity of full LFA-1 activation by inside-out signals by providing a first example of LFA-1 that successfully acquires the 327C epitope in response to chemokine motility signals but still fails to engage ICAM-1 due to the absence of a critical integrin coactivator.
The observation that T-cell motility over lymph node chemokines can prime LFA-1 for a subsequent TCR-mediated activation is novel, because CCR7 ligands were previously reported to exert both negative and positive effects on LFA-1–dependent adhesions triggered by TCR stimuli. Whereas interfering with stable antigen–stimulated effector T-cell adhesion to ICAM-1 in their soluble states,40 CCR7 ligands presented to T cells in juxtaposition to ICAM-1 and immobilized antigen were reported to augment LFA-1 adhesiveness.41 T cells also use CCL21 immobilized on B cells and DCs to form antigen-independent LFA-1–dependent tethers.42 Our results suggest that CCL21, while keeping LFA-1 nonadhesive to ICAM-1 in the absence of any TCR occupancy,19 in fact sensitizes this LFA-1 to a subsequent TCR arrest signal on T cells locomoting over CCL21 and approaching a target DC. T-cell motility on CCL21 can therefore be considered an essential preparatory step that primes LFA-1 for a subsequent arrest signal. Conversely, soluble gradients of CCL21 or CCL19 may provide “go” signals that destabilize TCR-dependent formation of immune synapses in certain microenvironments.40,43 T-cell polarization on surface-bound CCL21, in addition to stabilizing conformationally primed LFA-1 in microclusters at the leading edge, is also likely to increase the sensitivity of individual TCR molecules for antigen signals.44,45 Therefore, fibroblastic reticular cell–presented CCL21 may not only promote the motility and scanning capacity of infiltrating T cells,14 but may also dramatically lower the threshold of the TCR signals necessary for LFA-1–mediated arrest on DC ICAM-1. Because recent 2-photon imaging studies suggested that in several settings T cells fail to stop on antigen-presenting DCs immediately after entry to the T zone,4,46 it is possible that CCR7 activity on these early infiltrating T cells may be initially desensitized by the abundant CCR7 ligands that these cells encounter. At later time points, CCR7 may recycle and regain an optimal activity required for the LFA-1 headpiece–priming event used by TCR signals to arrest motile T cells on DC ICAM-1.
A second process in which the role of Kindlin-3 was investigated in the present study is LFA-1–mediated T-cell spreading on ICAM-1 and on DCs, which is stimulated by different TCR stimuli independently of chemokine signals. Our results provide the first evidence that T-cell spreading on DC ICAM-1 involves LFA-1 microclustering within scattered focal dots rather than macroclustering within a peripheral pSMAC ring.47,48 We have recently shown that human T-cell spreading on isolated ICAM-1 also requires headpiece-activated LFA-1 to microcluster and engage with surface-bound ICAM-1 within scattered focal adhesive contacts (ie, focal dots).22 Our new results suggest that the inability of Kindlin-3–null human T cells to arrange ICAM-1–occupied LFA-1 into these submicron adhesive units is tightly correlated with the loss of antigen-stimulated, LFA-1–dependent T-cell arrest and spreading on DCs. Electron microscopic analysis further supports the multifocal nature of the human T cell–DC synapses studied here, which is in agreement with a previous study on murine T cell–DC synapses.25
Investigation of the molecular basis of this spreading defect showed that in T cells settled on an immobilized form of the TCR-ligating mAb OKT3 co-immobilized with ICAM-1, acquisition by LFA-1 of the 327C open β I domain epitope did not require the presence of Kindlin-3. Nevertheless, in Kindlin-3–deficient T cells, these 327C-bearing LFA-1 molecules could not assemble within the typical focal adhesive units of T cells spreading on ICAM-122 or on DCs (the present study). Therefore, the assembly of microclustered, ICAM-1–occupied LFA-1 in scattered focal dots, where final outside-in activation of the integrin headpiece by ICAM-1 takes place in TCR-stimulated T cells, is Kindlin-3 dependent. Because cytoskeletal anchorage of ligated LFA-1 is also Kindlin-3 dependent, we propose that Kindlin-3 functions as an essential focal adhesion adaptor necessary to anchor and stabilize the otherwise labile LFA-1/ICAM-1 bond.49 Why is such cytoskeletal linkage critical for LFA-1/ICAM-1 bond strengthening? Recent data suggest that application of force on an anchored, ligand-occupied integrin is necessary to achieve maximal separation between the integrin subunit tails.50 Force application on the isolated αI domain is also critical for full activation of this domain.51 These multiple examples of chemo-mechanical activation of LFA-1 by ICAM-1 may therefore all share a tight dependence on proper Kindlin-3–regulated anchorage of the ICAM-1–occupied LFA-1 to the cortical cytoskeleton52,53
As summarized in Table 1, the role of Kindlin-3 in LFA-1–dependent T-cell stop signals appears to be more restricted and specialized than previously proposed for affinity modulation of the platelet integrin GpIIbβ354 and for rapid chemokine–triggered LFA-1 and VLA-4 activation in lymphocytes interacting with endothelial ligands under shear flow.13 Whereas in these 2 processes, Kindlin-3 is critical for rapid headpiece opening,33 in the context of TCR stimulation, the major function of Kindlin-3 is to facilitate the final step of LFA-1/ICAM-1 bond strengthening, possibly by optimizing the association of the LFA-1 tail with talin1 and other actin-binding partners. Kindlin-3–facilitated multimerization of LFA-1/ICAM-1 bonds may be also essential to increasing the stability of focal LFA-1 contacts. Future biochemical and biophysical studies will be required to further address how Kindlin-3 and its partners55 cooperate with Talin1 and with other focal adhesion adaptors (including vinculin, filamin, and α-actinin) in linking LFA-1 priming on motile lymphocytes to mechanochemical changes in LFA-1/ICAM-1 bonds critical for the generation and function of different immune synapses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr S. Schwarzbaum for editorial assistance. R.A. is the incumbent of the Linda Jacobs Chair in Immune and Stem Cell Research. This research was supported by the Germany-Israel Science Foundation and the Israel Science Foundation. This manuscript is dedicated in loving memory to Dr Valentin Grabovsky, who passed away during the last phases of this research.
Authorship
Contribution: S.W.F. designed and performed most parts of the study, organized the data, and assisted in writing the manuscript; V.G. performed experiments and assisted in data analysis; E.M.-M. performed the mAb adhesion assays; R.P. assisted in the DC experiments; Z.S. assisted in immunofluorescence staining; V.S. and E.K. assisted with the electron and scanning microscopy experiments, respectively; A.E. contributed to the discussion; M.A. provided blood samples and contributed to the discussion; and R.A. designed and supervised all aspects of the work and wrote major parts of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronen Alon, Department of Immunology, The Weizmann Institute of Science, Rehovot 76100, Israel; e-mail: ronen.alon@weizmann.ac.il.

![Figure 6. Motility on CCL21 primes T-cell LFA-1 in both normal and Kindlin-3–deficient T cells, but the primed LFA-1 undergoes TCR-induced activation on ICAM-1 binding only in normal T cells. (A) The fraction of control PB T cells engaged by firm contacts with ICAM-1 (600 sites/μm2)–coated beads during distinct conditions of motility and TCR stimulation. T cells settled on albumin (nonmotile) or allowed to locomote on immobilized CCL21 were exposed to the anti-CD3 mAb (OKT3) or to control mAb. Where indicated, the α/β I allosteric antagonist of LFA-1/ICAM-1 interactions XVA143 (1μM) was included. The number of firm T-cell–bead contacts (> 60 seconds) was divided by the number of total T-cell–bead-encountering events for each experimental condition to yield the fraction of productive contacts. Values are the means ± SD of 4 fields of view. (B) Control (black) compared with Kindlin-3–null (white) PB lymphocytes were allowed to migrate over a CCL21-coated surface toward scattered DCs. (Bi) Each group of T cells was preincubated either in the presence of OKT3 or a control mAb. (Bii) Control (black) or Kindlin-3–null (white) T cells were allowed to migrate on CCL21 toward DCs preloaded with medium or superantigen (staphylococcal enterotoxin A [SEA]). The fractions of T cells that established firm adhesion (> 5 minutes) after colliding into prespread DCs are shown. Values are the means ± SD of 9 fields of view in 3 independent experiments. The frequency of T-cell collision into DCs was comparable in all experimental groups. (C) Control (left) or Kindlin-3–null (right) PB T cells left alone (−, top) or allowed to locomote on immobilized CCL21 (bottom) as in panel A were fixed and stained with either the pan–anti-β2 mAb (TS1.18, left) or the 327C mAb (right) that detects the active open state of the β2 I domain. Merged DIC/fluorescence images of representative T cells are shown. Scale bar represents 3 μm. (D) The fraction of control (black) compared with Kindlin-3–null (white) PB lymphocytes engaged by productive contacts with high-density (4200 sites/μm2) ICAM-1–coated beads under conditions identical to those in panel A. The fraction of T cells suspended in Mg2+/EGTA medium firmly bound to similar ICAM-1 beads is shown for comparison. Values are the means ± SD of 4 fields of view.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/26/10.1182_blood-2010-12-322859/5/m_zh89991173390006.jpeg?Expires=1770179124&Signature=E3souLqB0Fe-RLJo18UfxdzeQ-RB1BLcjmPGrZc1RRX8EYj3EyF3w4VKLW8M9FFm7zIxnS3r4AddJlqHHhspzegss5dEK644jXIfxF8OIj0hh9EDIfAk-zkJ2IGSZspIHbvSw2Ddrat9~Jn02v-mvnGa0HVpXscWFANxLOm64s7IwnSOa8c7xK5hCt20sTbagqiCNSHVH~0LHCsDvL-GN3YPmqyifd6gNrLHY~PymG6fcD0-MEqCnACTRn10wfGyZ80-yRXhGyNMu1-tF~RSCDEKskmG1dfQbtlmLInQAkO-x-JP5rGlC3K3BWo4BKy4fUQjAar8tYv~6uyPiKXr5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Motility on CCL21 primes T-cell LFA-1 in both normal and Kindlin-3–deficient T cells, but the primed LFA-1 undergoes TCR-induced activation on ICAM-1 binding only in normal T cells. (A) The fraction of control PB T cells engaged by firm contacts with ICAM-1 (600 sites/μm2)–coated beads during distinct conditions of motility and TCR stimulation. T cells settled on albumin (nonmotile) or allowed to locomote on immobilized CCL21 were exposed to the anti-CD3 mAb (OKT3) or to control mAb. Where indicated, the α/β I allosteric antagonist of LFA-1/ICAM-1 interactions XVA143 (1μM) was included. The number of firm T-cell–bead contacts (> 60 seconds) was divided by the number of total T-cell–bead-encountering events for each experimental condition to yield the fraction of productive contacts. Values are the means ± SD of 4 fields of view. (B) Control (black) compared with Kindlin-3–null (white) PB lymphocytes were allowed to migrate over a CCL21-coated surface toward scattered DCs. (Bi) Each group of T cells was preincubated either in the presence of OKT3 or a control mAb. (Bii) Control (black) or Kindlin-3–null (white) T cells were allowed to migrate on CCL21 toward DCs preloaded with medium or superantigen (staphylococcal enterotoxin A [SEA]). The fractions of T cells that established firm adhesion (> 5 minutes) after colliding into prespread DCs are shown. Values are the means ± SD of 9 fields of view in 3 independent experiments. The frequency of T-cell collision into DCs was comparable in all experimental groups. (C) Control (left) or Kindlin-3–null (right) PB T cells left alone (−, top) or allowed to locomote on immobilized CCL21 (bottom) as in panel A were fixed and stained with either the pan–anti-β2 mAb (TS1.18, left) or the 327C mAb (right) that detects the active open state of the β2 I domain. Merged DIC/fluorescence images of representative T cells are shown. Scale bar represents 3 μm. (D) The fraction of control (black) compared with Kindlin-3–null (white) PB lymphocytes engaged by productive contacts with high-density (4200 sites/μm2) ICAM-1–coated beads under conditions identical to those in panel A. The fraction of T cells suspended in Mg2+/EGTA medium firmly bound to similar ICAM-1 beads is shown for comparison. Values are the means ± SD of 4 fields of view.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/26/10.1182_blood-2010-12-322859/5/m_zh89991173390006.jpeg?Expires=1770185333&Signature=N2~BJfBbFSyH3b3O9KztUJqH0KDIK-Vm6zEceDmwZvHL0lTjFozMFviPWsmZOVBiOvGIkQf8Zvv8ncb9D0bhW8cKv1VUNUXUWWndN6FDkt9Ni8Nu9fQD6ezlOc4NQMO-9rH-NKHiJRto1nhWjvx047UfA0q15Ozeyu3-eypBKbHoGXbzbe1SOaSylMGomxZILFTTdRucmYf~HQCuPXQYDoXg5GOPzYo8dqve8NQQnJXOO9T4YzYf111uXE-KHVH0YRF34Cs1L7dmitKCplWNSV8AE-L08h5npU8K9Z2czmHdsBMvtZUrbVkdyjhENPovbqf2-KH2sLNDp8by0L034w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)