Abstract
Aberrant DNA methylation contributes to the malignant phenotype in virtually all types of cancer, including myeloid leukemia. We hypothesized that CpG island hypermethylation also occurs in juvenile myelomonocytic leukemia (JMML) and investigated whether it is associated with clinical, hematologic, or prognostic features. Based on quantitative measurements of DNA methylation in 127 JMML cases using mass spectrometry (MassARRAY), we identified 4 gene CpG islands with frequent hypermethylation: BMP4 (36% of patients), CALCA (54%), CDKN2B (22%), and RARB (13%). Hypermethylation was significantly associated with poor prognosis: when the methylation data were transformed into prognostic scores using a LASSO Cox regression model, the 5-year overall survival was 0.41 for patients in the top tertile of scores versus 0.72 in the lowest score tertile (P = .002). Among patients given allogeneic hematopoietic stem cell transplantation, the 5-year cumulative incidence of relapse was 0.52 in the highest versus 0.10 in the lowest score tertile (P = .007). In multivariate models, DNA methylation retained prognostic value independently of other clinical risk factors. Longitudinal analyses indicated that some cases acquired a more extensively methylated phenotype at relapse. In conclusion, our data suggest that a high-methylation phenotype characterizes an aggressive biologic variant of JMML and is an important molecular predictor of outcome.
Introduction
Epigenetic changes, defined as mitotically heritable changes in gene expression without DNA sequence alterations, accompany the development and progression of malignant disease. A hallmark of the molecular phenotype of malignant transformation is global DNA hypomethylation coupled with regional DNA hypermethylation. Hypermethylation occurs in a tumor-specific pattern at CpG island sequences representing CG-rich regions frequently located at the 5′ end of coding genes. These changes can affect gene-promoter functionality, resulting in stable gene silencing.1 CpG island hypermethylation is found in virtually all kinds of cancer, including myeloproliferative neoplasms (MPNs), myelodysplastic syndrome (MDS), and myeloid leukemia.2
Juvenile myelomonocytic leukemia (JMML) is an aggressive MPN of early childhood. An important feature of the molecular pathophysiology of JMML is the deregulation of the Ras signal transduction pathway, which is caused by mutations in the PTPN11, NRAS, KRAS, CBL, or NF1 genes.3-5 The clinical risk assessment in JMML involves age at diagnosis, thrombocytopenia, and fetal hemoglobin (HbF) as the main prognostic variables.6 However, the molecular basis of the prognostic diversity in JMML remains little understood, and it is unclear if and how specific Ras pathway lesions determine the clinical course.
The available literature on aberrant CpG island methylation in JMML is limited to a small series of patients and a few genes, including CDKN2A (hypermethylation reported in 0 of 18 cases),7 CDKN2B (3 of 18 cases),7 RASSF1A (1 of 5 cases),8 PTPN6 (0 of 5 cases),8 SOCS1 (0 of 5 cases),8 and PTEN (23 of 30 cases in one series but 0 of 90 in another).9,10 In the present study, we investigated the DNA methylation status of 14 gene CpG islands in a large cohort of JMML patients to determine whether aberrant methylation is associated with clinical, hematologic, or prognostic features of the disease. We report that the presence or absence of hypermethylation characterizes subsets of the disease with distinct clinical behavior, and that CpG island hypermethylation is an important molecular marker of prognosis in JMML.
Methods
Patient samples
Clinical samples from 127 children with JMML were collected in the context of the European Working Group on MDS in Childhood (EWOG-MDS) studies in 1998 and 2006 (registered with the National Institutes of Health; www.clinicaltrials.gov identifiers NCT00047268 and NCT00662090, respectively), after obtaining informed consent from parents or legal guardians in accordance with the Declaration of Helsinki and approval from institutional review committees at each participating center. Diagnostic samples included BM in 70 cases and peripheral blood (PB) in 57 cases. Hematopoietic spleen cells were obtained from organs removed for clinical indication in 2 cases, and these were used for flow cytometric cell-sorting experiments. Each BM/PB/spleen cell sample was separated into mononuclear cells (MNCs) and granulocytes by density gradient centrifugation. Unfractionated PB leukocytes from 15 healthy individuals were used as controls.
Nucleic acid extraction
Genomic DNA was isolated using the Puregene (QIAGEN) or Transfast (Peqlab) kits. Total RNA was isolated from MNCs using TRIzol reagent (Invitrogen).
Bisulfite conversion and bisulfite sequencing
Genomic DNA (500 ng) was bisulfite-modified using the EZ Methylation Gold Kit (Zymo Research). Primers used for subsequent amplification are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). PCR products were either gel-purified using GFX columns (GE Healthcare) before ligation or were directly ligated into the pCR 2.1 vector (Invitrogen). Six to 8 clones per sample were sequenced using BigDye terminator chemistry (Applied Biosystems) and capillary electrophoresis. Complete bisulfite modification was confirmed by sequence analysis.
MassARRAY
Quantitative DNA-methylation analysis at single CpG units was performed using MassARRAY EpiTyper, as described previously.11 Briefly, bisulfite-treated genomic DNA was PCR-amplified, in vitro–transcribed, cleaved by RNase A, and subjected to matrix-assisted laser desorption ionization–time of flight mass spectrometry (Sequenom). Primer sequences for PCR amplicons in relation to CpG island positions are listed in supplemental Table 1. Methylation standards (0%, 20%, 40%, 60%, 80%, and 100% methylated genomic DNA) and correction algorithms based on custom scripts for the R statistical computing environment (http://www.r-project.org) were used for data normalization.
Quantitative real-time RT-PCR
The QuantiTect Reverse Transcription kit (QIAGEN) was used, which included a procedure to remove genomic DNA. Quantitative PCR was performed on a Mastercycler EP Realplex (Eppendorf) using the ABsolute QPCR SYBR Green reaction mix (Thermo Scientific) and the primers listed in supplemental Table 1. GAPDH was used as an internal control. RT-PCR reactions were performed in triplicate, including no-template controls. Relative expression was calculated using the comparative CT method.
Flow cytometric cell sorting
Cryopreserved spleen MNCs were thawed using medium supplemented with 20 U/mL of DNAse I, and then 10 μL of CD3-FITC, CD14-PE, CD19-PE, CD34-PE, or CD235a-FITC antibody (BD Biosciences) was added for every 106 cells. Cells were incubated for 30 minutes, washed twice, and sorted on a MoFlo high-speed cell sorter (Dako). The purity of each fraction was checked and found to be greater than 90%.
Pyrosequencing
Specific long interspersed nuclear element 1 (LINE1) primers (QIAGEN) were used for PCR of bisulfite-converted DNA. Biotin-labeled PCR products were bound to Streptavidin Sepharose HP (Amersham). The Sepharose beads were purified, washed, denatured in 0.2M NaOH, and washed again. Pyrosequencing was performed on a PyroMark Q96 MD (QIAGEN). The target CpGs were evaluated by converting the resulting pyrograms to numeric values for peak heights to calculate the percentage of methylation.
Statistical analysis
The χ2 test was used to examine the statistical significance of a relationship between categorized variables. Nonparametric statistics were used to test continuous variables for differences between 2 subgroups (Wilcoxon Mann-Whitney test). Quantitative DNA methylation data derived from MassARRAY were treated as continuous variables, and missing measurements were imputed into multivariable regression analyses using samples with replacement from the nonmissing values (single imputations). Linear associations between 2 continuous variables were quantified by Pearson correlation coefficient. P values < .05 were considered to be statistically significant. The association between percentage of methylation and overall survival (OS), disease-free survival (DFS), and cumulative incidence of relapse (CIR) was assessed univariately for each CpG unit using a Cox proportional hazards regression model. The prognostic power of methylation at each CpG unit was expressed by the 0.632+ bootstrapped time-integrated Brier score measuring the prediction error of the model.12 To build a prognostic model for OS based on CpG methylation, the 127-case dataset was randomly divided into a training set (80 cases) and a validation set (47 cases), with both sets matched for distribution of patient age and follow-up periods. Prognostic models for DFS and CIR, applicable only to patients having received allogeneic hematopoietic stem cell transplantation (HSCT), were based on 97 cases (61 training and 36 validation cases). Prognostic signatures were developed using an L1 penalized least absolute shrinkage and selection operator (LASSO) Cox regression model with incorporated variable selection, with the possibility of including all interrogated CpG units. Models for CIR were fitted by competing risks regression.13 The significance of the prognostic signatures was then verified in the validation set. The Kaplan-Meier method was used to estimate OS and DFS probabilities. Results were expressed as 5-year probability with 95% confidence interval (95% CI). The 2-sided log-rank test was used to test the equality of survivorship functions in different subgroups. CIR curves correspond to the cause-specific hazards adjusted for the competing risk, transplantation-related mortality.14-16 Gray's test was used to compare cumulative incidence curves. Statistical analysis was performed using the R statistical environment Version 2.11.0 (with R packages rms v3.0-0, pec v1.1.1, peperr v1.1-5 penalized v0.9-31, cmprsk v2.2-1) and SPSS for Windows 17.0.
Results
We prescreened the CpG island methylation status of 14 candidate genes in a cohort of 86 children with JMML and 15 healthy controls, using a liquid chromatography–based method (msDHPLC; supplemental Figure 1). The candidate genes were selected based on hypermethylation previously documented in other types of cancer or leukemia (CALCA, CDKN1C, CDKN2B, DAPK1, MGMT, MLH1, RARB, RASSF1, SOCS1, and TP73), or on involvement in the Ras signal transduction pathway, which has a prominent role in JMML pathogenesis (BMP4, PAWR, RASA1, and RECK).17-19 Among these genes, 4 carried aberrant methylation as determined by msDHPLC: bone morphogenetic protein 4 (BMP4) in 28 of 86 JMML cases, calcitonin A (CALCA) in 27 of 86 cases, cyclin-dependent kinase inhibitor 2B (CDKN2B) in 17 of 86 cases, and retinoic acid receptor β (RARB) in 13 of 86 cases (supplemental Table 2). For the other 10 genes studied, there was no evidence of aberrant CpG island methylation in 86 JMML cases. All 14 gene CpG islands exhibited normal methylation in 15 control samples obtained from healthy individuals.
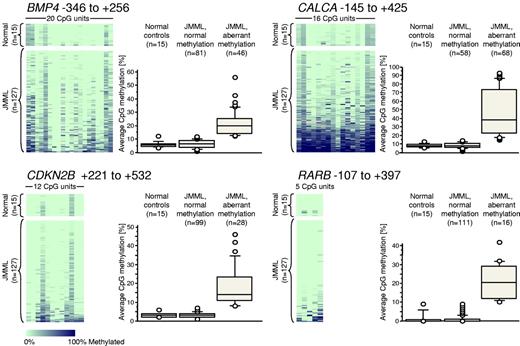
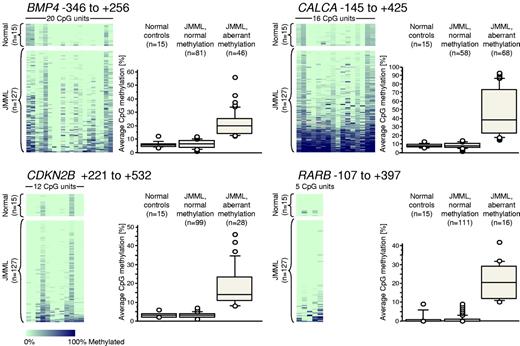
We next used MassARRAY technology to generate quantitative high-resolution CpG methylation patterns of the BMP4, CALCA, CDKN2B, and RARB genes in leukemic cells from an expanded cohort of 127 JMML cases, which included the previous 86 cases (Figure 1). We found that the average levels of CpG methylation at the BMP4 locus ranged from 0.9%-56.1% in JMML but from 3.6%-12.1% in PB leukocytes from 15 healthy individuals (supplemental Table 3). CALCA methylation ranged from 1.3%-92.9% in JMML and from 5.6%-12.9% in the healthy controls (supplemental Table 3). CDKN2B methylation ranged from 0.5%-45.6% in JMML and from 1.5%-6.2% in healthy controls (supplemental Table 3). RARB methylation ranged from 0.0%-42.0% in JMML and from 0.0%-8.8% in healthy controls (supplemental Table 3). Based on the levels of methylation observed in healthy controls, we regarded a CpG island as “hypermethylated” in a JMML sample if its average methylation across all CpGs measured was beyond 3 standard deviations from the mean observed in 15 healthy individuals. Using this definition, 46 of 127 JMML cases (36%) were hypermethylated at BMP4, 68 of 126 JMML cases (54%) were hypermethylated at CALCA, 28 of 127 JMML cases (22%) were hypermethylated at CDKN2B, and 16 of 127 JMML cases (13%) were hypermethylated at RARB (Figure 1). We investigated whether the source material of leukemic cells (BM or PB specimens) had an influence on aberrant methylation, and found no difference when paired BM/PB samples from the same patient were measured (supplemental Figure 2). Similarly, there was no difference in methylation when granulocytes and mononuclear cells derived from the same clinical specimen were compared (supplemental Figure 2).
Quantitative DNA-methylation analysis of BMP4, CALCA, CDKN2B, and RARB CpG islands using MassARRAY in 127 JMML samples and 15 healthy PB samples. Each row represents a sample and each column represents a CpG unit, which is a single CpG site or a combination of CpG sites. The position of the amplified sequence relative to the transcription start site is indicated above each color plot. The data are color-coded according to degree of methylation (light green, 0%; dark blue, 100%; gray, no data). Next to each color plot, the DNA-methylation levels are displayed as box plots in 3 categories: healthy controls, JMML with normal methylation, and JMML with hypermethylation (exceeding the mean level of methylation in healthy controls by more than 3 standard deviations).
Quantitative DNA-methylation analysis of BMP4, CALCA, CDKN2B, and RARB CpG islands using MassARRAY in 127 JMML samples and 15 healthy PB samples. Each row represents a sample and each column represents a CpG unit, which is a single CpG site or a combination of CpG sites. The position of the amplified sequence relative to the transcription start site is indicated above each color plot. The data are color-coded according to degree of methylation (light green, 0%; dark blue, 100%; gray, no data). Next to each color plot, the DNA-methylation levels are displayed as box plots in 3 categories: healthy controls, JMML with normal methylation, and JMML with hypermethylation (exceeding the mean level of methylation in healthy controls by more than 3 standard deviations).
We next investigated whether hypermethylation at the 4 genetic loci was correlated with specific clinical or hematologic features in JMML (Table 1). We found that hypermethylation at each of the 4 genes was strongly associated (P < .001) with older age at diagnosis. Elevated percentage of HbF at diagnosis was associated with methylation at the BMP4 and CALCA genes (P = .008/.004). These observations were intriguing because it is well documented that older age and high HbF percentage at diagnosis are strong predictors of treatment failure in JMML.6,20 The myeloblast count in the BM was associated with CALCA methylation (P = .001). A weaker association was seen between CALCA and CDKN2B hypermethylation and aberrant karyotype (P = .010/.023). There was no correlation between aberrant CpG island methylation at any of the 4 loci with sex, leukocyte count, hemoglobin, monocyte count, blast percentage in PB, spleen size, or mutational JMML subtype (NF1, PTPN11, KRAS/NRAS, or CBL; Table 1).
The association of hypermethylation with known clinical risk factors suggested that the presence of hypermethylation at the time of JMML diagnosis might be predictive of poor outcome. To investigate this possibility using an unsupervised and unbiased approach, we randomly selected a training set of 80 cases from the total 127-patient cohort. This set included 61 children who had received allogeneic HSCT and were thus considered to be uniformly treated. The treatment in children who had not received HSCT was heterogeneous and included supportive care alone, cytoreductive low-dose chemotherapy, high-intensity induction regimens, or more experimental agents. The remaining 47 cases (including 36 HSCT recipients) were used as a validation set. Both sets were matched for distribution of methylation levels, patient age, and follow-up periods (supplemental Table 4 and supplemental Figure 3). Each CpG unit was regarded as an individual disease marker regardless of genetic location, and its predictive power was tested in a univariate Cox model for OS of the 80 training cases. In 61 patients who had received HSCT, the CpG units were also tested for influence on DFS and CIR. The prediction error of the models was controlled using a bootstrapping algorithm, as detailed in “Methods.” We found that 8 of 53 CpG units had strong prognostic value for OS (defined as a prediction error smaller than the Kaplan-Meier prediction error minus twice its standard error), and 18 of 53 CpG units had strong prognostic value for CIR. Ten of 53 CpG units had an intermediate prognostic value for OS (prediction error < Kaplan-Meier prediction error minus one standard error), and 22 of 53 CpG units had intermediate prognostic value for CIR. Among the top 10 prognostic CpG units for OS, 7 were located at the BMP4 gene, 1 at CDKN2B, and 2 at RARB.
Using a LASSO Cox proportional hazards model, prognostic scores for OS were calculated from CpG methylation data using the training set of 80 cases, and for DFS and CIR using 61 post-HSCT cases (supplemental Table 4). The scores were then tested in the independent validation set of 47 patients, of whom 36 had received HSCT. We found that the CpG methylation score was still prognostic in the independent group of JMML patients for both OS (P = .016; likelihood ratio test) and CIR (P = .049). There was no prognostic value for DFS after HSCT (P = .431), probably reflecting the fact that transplantation-related mortality is a confounding variable that is unrelated to gene hypermethylation of leukemic cells at diagnosis.
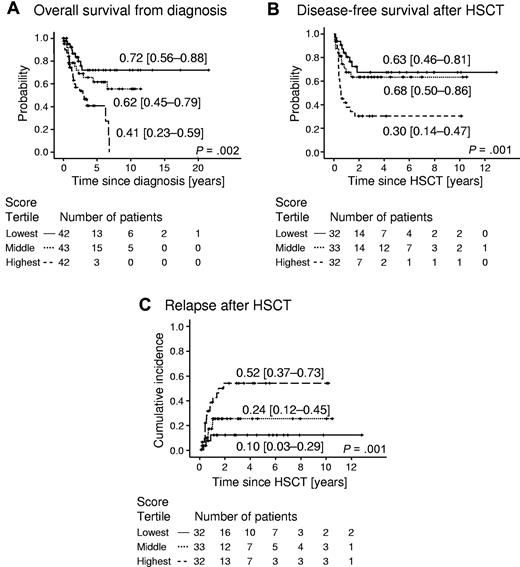
Survival analyses showed that the probability of 5-year OS in 42 of 127 patients representing the lowest tertile of methylation scores was 0.72 (95% CI 0.56-0.88), whereas it was only 0.41 (95% CI 0.45-0.79) in 42 patients with methylation scores in the highest tertile (P = .002, hazard ratio 3.51, 95% CI 1.61-7.65) (Figure 2A). The probability of 5-year DFS among 97 patients who had received HSCT was 0.63 (95% CI 0.46-0.81) in the lowest tertile of scores, but was 0.30 (95% CI 0.14-0.47) in the highest tertile (P = .012, hazard ratio 2.56, 95% CI 1.23-5.32) (Figure 2B). The 5-year CIR among 97 patients who had received HSCT was 0.10 (95% CI 0.03-0.29) for patients in the lowest score tertile, but was 0.52 (95% CI 0.37-0.73) in the top tertile (P = .007, hazard ratio 5.40, 95% CI 1.57-18.56) (Figure 2C). These results indicate that aberrant CpG island methylation in JMML has prognostic implications and predicts the risk of relapse after HSCT. The prognostic power of gene hypermethylation in JMML was reproducible when the nonquantitative msDHPLC methodology was applied (supplemental Figure 4), suggesting that msDHPLC can be useful for methylation assessment in clinical environments in which MassARRAY equipment is not available.
Outcome of 127 JMML patients according to prognostic methylation score divided into tertiles. (A) Probability of survival from diagnosis irrespective of disease status. Patients alive at last follow-up were censored. Death was considered the event. Numbers indicate the probability of 5-year survival and the 95% CI. (B) Probability of DFS in 97 JMML patients who had received HSCT. Numbers indicate the probability of 5-year DFS and the 95% CI. DFS was defined as the probability of being alive and disease-free. Patients alive and disease-free at last follow-up were censored. Death and relapse were considered the events. (C) Cumulative incidence of relapse of 97 patients who had received HSCT. Numbers indicate the 5-year cumulative incidence of relapse and the 95% CI. Relapse incidence was defined as the probability of JMML relapse at a given time. Death without relapse was considered a competing event.
Outcome of 127 JMML patients according to prognostic methylation score divided into tertiles. (A) Probability of survival from diagnosis irrespective of disease status. Patients alive at last follow-up were censored. Death was considered the event. Numbers indicate the probability of 5-year survival and the 95% CI. (B) Probability of DFS in 97 JMML patients who had received HSCT. Numbers indicate the probability of 5-year DFS and the 95% CI. DFS was defined as the probability of being alive and disease-free. Patients alive and disease-free at last follow-up were censored. Death and relapse were considered the events. (C) Cumulative incidence of relapse of 97 patients who had received HSCT. Numbers indicate the 5-year cumulative incidence of relapse and the 95% CI. Relapse incidence was defined as the probability of JMML relapse at a given time. Death without relapse was considered a competing event.
To identify other prognostic variables in our series of JMML patients and to evaluate the relative contribution of CpG methylation to prognosis, the clinical or hematologic parameters listed in Table 1 were tested in univariate analysis for influence on OS in the 80-patient training cohort. Consistent with previous reports on prognostic factors in JMML,6,20,21 age above 2 years was an adverse factor (P = .024, hazard ratio 2.24, 95% CI 1.09-4.59). Hemoglobin concentration at diagnosis (< 10 g/dL) emerged as an additional factor (P = .017, hazard ratio 2.87, 95% CI 1.17-7.05). There was no prognostic value of white blood count, platelet count, blast percentage in BM or PB, monocyte percentage in BM or PB, splenomegaly, cytogenetics, or mutational subtype. To assess whether the prognostic power of the methylation phenotype was independent of age or hemoglobin concentration, these variables were entered into a multivariate Cox regression model for OS that was run on the independent 47-patient validation dataset. Only the presence of aberrant CpG island methylation at diagnosis retained prognostic power in multivariate analysis (Cox regression with backward selection; Pin = 0.05, Pout = 0.10). When the same procedure was carried out with CIR as the outcome end point, the methylation score emerged as the sole independent prognostic factor. The score reached no significant level of prediction when tested for DFS. In summary, CpG island hypermethylation was identified as a robust marker of poor prognosis in JMML.
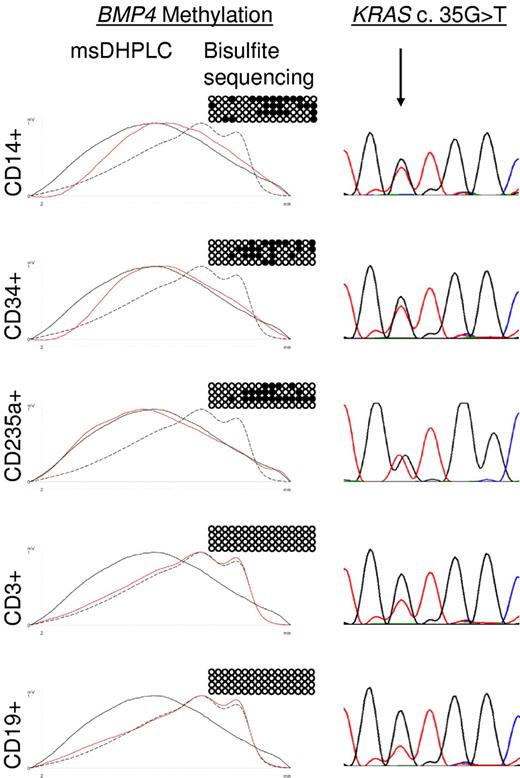
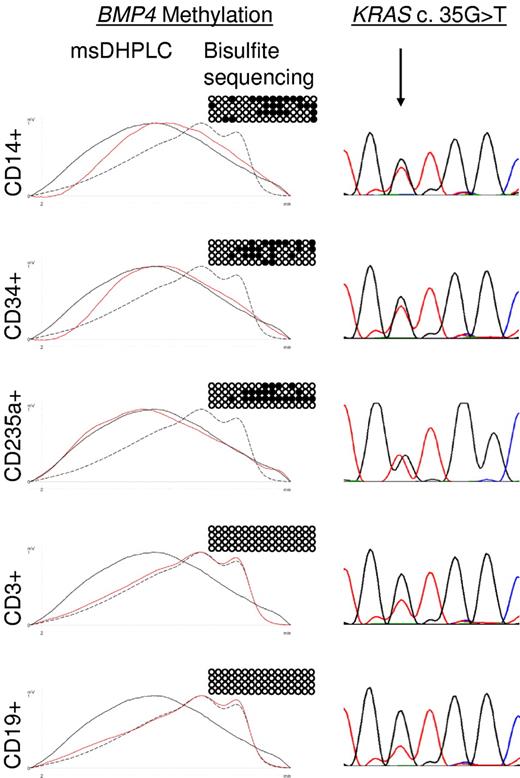
JMML is thought to arise in an early progenitor cell that retains the capability of differentiating into the erythroid, myeloid, and monocytic lineages.22 To determine whether aberrant methylation is specific to leukemic cell progeny in JMML, primary leukemic cells from the spleens of 2 JMML patients with BMP4 hypermethylation (patients D360 and D397 in supplemental Tables 2 and 3) were flow-sorted into monocytes (CD14+), erythroid cells (CD235a+), progenitor cells (CD34+), T lymphocytes (CD3+), or B lymphocytes (CD19+). The leukemic cells of both patients were known to harbor somatically acquired RAS gene mutations (KRAS c.G35T in patient D360 and NRAS c.G38A in patient D397). We found in both cases that CD14+ cells, CD235a+ cells, and CD34+ cells contained the RAS mutation and exhibited increased BMP4 methylation, whereas CD3+ and CD19+ cells carried wild-type RAS and had normal methylation (Figure 3). To quantify the degree of DNA methylation at the BMP4, CALCA, CDKN2B, and RARB CpG islands in normal hematopoietic progenitor cells, we purified CD34+ cells from cord blood of 3 healthy newborns. Bisulfite sequencing revealed normal levels of methylation at each CpG island in all 3 samples (data not shown). These results indicate that aberrant methylation is linked to leukemic hematopoiesis in JMML and can be traced back to the CD34+ progenitor cell compartment. This observation supports the concept that aberrant DNA methylation is clonal and is associated with early pathogenetic events in JMML.
Lineage specificity of aberrant methylation in JMML patient D360. Hypermethylation of the BMP4 CpG island was analyzed by msDHPLC and bisulfite sequencing in flow-sorted CD14+, CD34+, CD235a+, CD3+, and CD19+ spleen cell fractions. On the left are msDHPLC chromatograms in which dotted lines represent healthy control DNA, black lines methylated control DNA, and red lines sample DNA. In the center are the results of bisulfite sequencing in which horizontal lines represent individual alleles (4 per cell sample); ●, methylated cytosines; and ○, unmethylated cytosines. On the right are the results of genomic sequencing; the arrow indicates the position of the heterozygous KRAS c.35 G>T mutation.
Lineage specificity of aberrant methylation in JMML patient D360. Hypermethylation of the BMP4 CpG island was analyzed by msDHPLC and bisulfite sequencing in flow-sorted CD14+, CD34+, CD235a+, CD3+, and CD19+ spleen cell fractions. On the left are msDHPLC chromatograms in which dotted lines represent healthy control DNA, black lines methylated control DNA, and red lines sample DNA. In the center are the results of bisulfite sequencing in which horizontal lines represent individual alleles (4 per cell sample); ●, methylated cytosines; and ○, unmethylated cytosines. On the right are the results of genomic sequencing; the arrow indicates the position of the heterozygous KRAS c.35 G>T mutation.
The common association of older age and increasing methylation with poor prognosis suggested that both features might reflect biologic properties of the underlying disease, and therefore that cases evolving toward refractory disease might be characterized by a higher degree of aberrant methylation. To test this hypothesis, we analyzed longitudinal samples from 3 patients at diagnosis, in remission after HSCT, at relapse, and (if attained) in second remission (Table 2). In patient D003, the RARB CpG island was 2% methylated at diagnosis and progressed to 16% methylation at relapse and CALCA methylation increased from 3% to 61% between diagnosis and relapse. Similarly, in patient D098, the methylation of CALCA had increased from 13% to 80% and that of RARB from 0% to 33% by the time of relapse. In patient D155, the degree of methylation was already substantial at diagnosis and did not progress further (Table 2). In summary, these observations indicate that some JMML cases acquire a higher methylated phenotype at relapse, and support the idea that increasing DNA methylation parallels JMML evolution toward more aggressive disease.
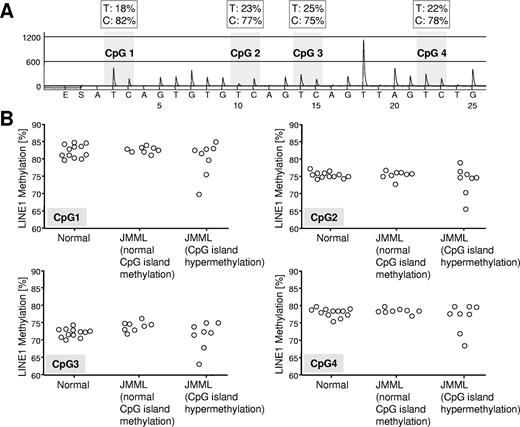
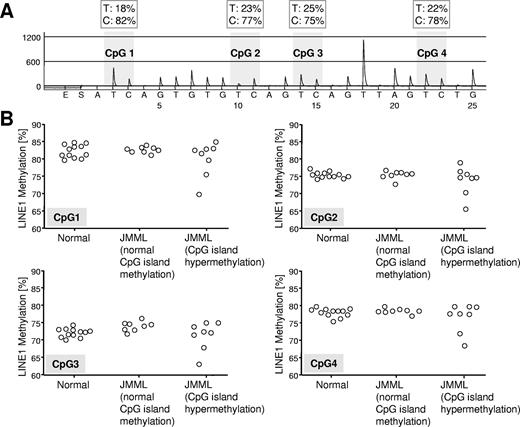
Gene-specific DNA hypermethylation in malignant cells is often paralleled by genome-wide DNA hypomethylation, mainly affecting repetitive elements such as LINE1.23 To determine whether JMML cells were also characterized by global DNA hypomethylation, we used LINE1 bisulfite pyrosequencing24 and assessed the methylation density of genomic LINE1 elements in 8 hypermethylated JMML samples, 8 JMML samples with normal methylation, and 12 healthy controls (Figure 4). Although we found no consistent difference in LINE1 methylation between hypermethylated and normally methylated JMML samples or between JMML cells and normal hematopoietic cells, it was interesting that 2 of 8 hypermethylated JMML cases but 0 of 8 JMML cases with normal methylation exhibited decreased LINE1 methylation (Figure 4).
Quantitative LINE1 bisulfite pyrosequencing. (A) Example of LINE1 pyrogram. The percentage of C and T (corresponding to methylated and unmethylated cytosine, respectively) are shown in boxes above each CpG site (gray shading). Overall LINE1 methylation is estimated from the average proportion of C across 4 CpG sites. (B) LINE1 methylation at 4 CpG sites in hematopoietic cells from 12 healthy controls and 21 JMML cases. The JMML cases are grouped into 8 cases with normal CpG island methylation (patients A054, CH017, D097, D116, D257, D454, D675, and SC102) and 8 cases with hypermethylation in at least 3 of 4 CpG islands (patients D020, D127, D360, D361, D397, D567, D684, and PL019).
Quantitative LINE1 bisulfite pyrosequencing. (A) Example of LINE1 pyrogram. The percentage of C and T (corresponding to methylated and unmethylated cytosine, respectively) are shown in boxes above each CpG site (gray shading). Overall LINE1 methylation is estimated from the average proportion of C across 4 CpG sites. (B) LINE1 methylation at 4 CpG sites in hematopoietic cells from 12 healthy controls and 21 JMML cases. The JMML cases are grouped into 8 cases with normal CpG island methylation (patients A054, CH017, D097, D116, D257, D454, D675, and SC102) and 8 cases with hypermethylation in at least 3 of 4 CpG islands (patients D020, D127, D360, D361, D397, D567, D684, and PL019).
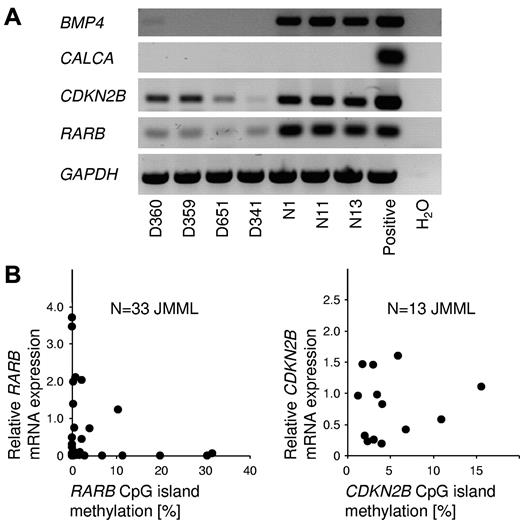
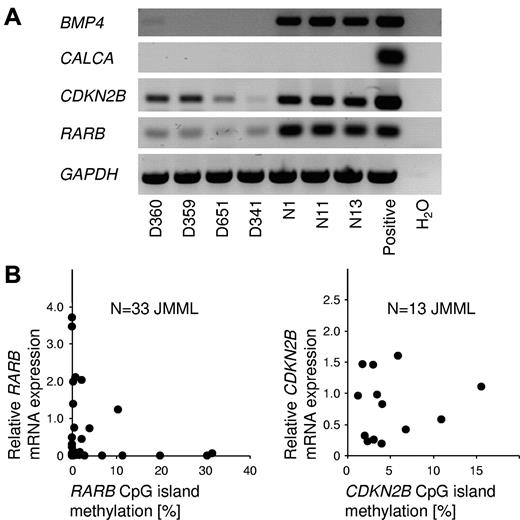
To investigate the relationship between CpG island methylation and gene silencing in JMML cells, the RNA expression of BMP4, CALCA, CDKN2B, and RARB was investigated by RT-PCR in JMML MNCs and healthy blood cells (Figure 5A). BMP4 was expressed at readily detectable levels in normal blood cells but was silent in JMML. CALCA transcripts were not demonstrable in JMML cells or in control cells. The CDKN2B and RARB genes were expressed in JMML and control cells. The latter 2 genes were expressed at considerably lower levels in JMML cells (Figure 5A). We next performed quantitative RT-PCR measurements of CDKN2B and RARB expression in a series of JMML cases, and compared the results with the level of CpG island methylation for each JMML sample. RARB expression was inversely correlated with methylation (Figure 5B), but no such correlation was seen for CDKN2B. It is tempting to speculate that RARB promoter methylation may contribute to the variable clinical response of JMML to isotretinoin.25 We had no opportunity to test this hypothesis further, because none of the patients reported herein had been treated with retinoids. Additional studies will be required to better understand the functional role and therapeutic implications of RARB promoter DNA methylation in JMML.
Relationship between gene silencing and CpG island hypermethylation in JMML. (A) Results of RT-PCR to detect the mRNA expression of BMP4, CALCA, CDKN2B, and RARB in peripheral blood cells from 4 JMML patients (patients D360, D359, D651, and D341) and from 3 healthy controls (patients N1, N11, and N13). (B) mRNA expression levels of the RARB gene (left; N = 33) and the CDKN2B gene (right; N = 13) in JMML PB MNCs in relation to CpG island methylation. Expression levels were measured by quantitative RT-PCR and CpG methylation was measured by MassARRAY. The average RARB mRNA expression in blood cells from 5 healthy controls was used as calibrator and was set to 1.0.
Relationship between gene silencing and CpG island hypermethylation in JMML. (A) Results of RT-PCR to detect the mRNA expression of BMP4, CALCA, CDKN2B, and RARB in peripheral blood cells from 4 JMML patients (patients D360, D359, D651, and D341) and from 3 healthy controls (patients N1, N11, and N13). (B) mRNA expression levels of the RARB gene (left; N = 33) and the CDKN2B gene (right; N = 13) in JMML PB MNCs in relation to CpG island methylation. Expression levels were measured by quantitative RT-PCR and CpG methylation was measured by MassARRAY. The average RARB mRNA expression in blood cells from 5 healthy controls was used as calibrator and was set to 1.0.
Discussion
It is widely recognized that aberrant DNA methylation is a hallmark of malignant disease, including MDS and MPN. However, data on DNA methylation in JMML have been scarce in the literature. In this study, we used MassARRAY to analyze leukemic cells from 127 children with JMML for aberrant DNA methylation at 4 gene loci of interest (BMP4, CALCA, CDKN2B, and RARB), which were identified after prescreening 14 candidate genes. The 4 genes were hypermethylated in 13%-54% of JMML cases. The BMP4 protein, a member of the TGFβ-like family of growth factors,26 participates in the initiation of hematopoietic gene expression and enhances hematopoietic differentiation.27 The present study is the first to implicate hypermethylated BMP4 in a hematologic disorder. The CALCA gene is a well-known target of aberrant methylation in chronic myeloid leukemia progressing to the accelerated phase.28 Those results and ours are consistent with the idea that CALCA methylation marks a more aggressive behavior of MPNs.28 Epigenetic dysregulation of the cell cycle–inhibitor p15/INK4b, which is encoded by the CDKN2B gene, has been implicated in hematologic malignancy in numerous studies.2 Specifically, our findings confirm and extend a previous study reporting CDKN2B hypermethylation in 3 of 18 JMML cases.7 RARB encodes the β polypeptide of the nuclear retinoid acid receptor. A role for epigenetic repression of the retinoid acid signaling pathway in myeloid leukemogenesis was described previously.29 Our data indicate that RARB is frequently underexpressed in JMML cells, and suggest that aberrant RARB methylation may contribute to the silent state. Although based on a limited number of samples, these observations are compatible with the antiproliferative activity of retinoids in some JMML patients.25,30 In the present study, the levels of methylation at the BMP4, CDKN2B, and RARB CpG islands showed some correlation within each case of JMML, whereas the association was less evident for CALCA methylation (supplemental Table 3). The observation of common hypermethylation across different genes may suggest that some JMML cases are marked by a general CpG island methylator phenotype.31 It will be required to complement our findings with genome-wide CpG methylation data to confirm the presence of a CpG island methylator phenotype in JMML.
The most remarkable finding of the present study was that gene hypermethylation at diagnosis was clearly associated with poor OS and a high risk of treatment failure due to relapse after HSCT. These findings establish DNA hypermethylation as a feature of an aggressive JMML variant, which was previously not identifiable through molecular genetic parameters. Whereas the precise target genes of methylation that contribute to the refractory phenotype remain unknown, an overall increase in gene-specific DNA methylation appears to characterize advanced or high-risk disease not only in JMML but also in other malignant disorders of myelopoiesis, such as MDS, acute myeloid leukemia, and chronic myeloid leukemia.32-34
Clinical and hematologic parameters with prognostic significance have been well established in JMML.6,20 Our results suggest that the assessment of DNA methylation at diagnosis may be useful for discriminating low-risk from high-risk JMML cases even within known clinical risk groups, most notably within the unfavorable age group of children above 2 years of age. This was supported by a multivariate Cox model of clinical and hematologic parameters, from which DNA methylation emerged as an independent prognostic determinant. As a note of caution, these conclusions are based on retrospective analyses and prospective data are needed for further validation. A recent study found that patients with JMML could be separated into 2 prognostic groups based on gene-expression profiles.35 Interestingly, the profile of JMML cells in cases with poor prognosis was reminiscent of acute myeloid leukemia.35 If and how this relates to our observation that such cases exhibit a higher methylation phenotype remains to be clarified.
We have recently demonstrated that CBL mutations in JMML are of germline origin in most if not all cases.36 JMML with the CBL mutation appears to have a peculiar clinical phenotype, characterized by a less-aggressive course and a high rate of conversion to stable mixed chimerism after HSCT.36 The mild course is in good agreement with the observations reported here, because CBL-positive cases tended to have normal DNA methylation (data not shown).
Some findings with relevance to clinical practice emerged from the present study. The EWOG-MDS recommends that every JMML patient receive HSCT without unnecessary delay, because a parameter capable of identifying patients who would benefit from non-HSCT strategies has not yet been discovered.37,38 The differential methylation observed in JMML provides no immediate ground to change this policy, because the overall cure rate is far from satisfactory, even in patients with normal DNA methylation. However, our data suggest that high-methylation cases may be good candidates for complementary approaches, for example pre-HSCT window therapies with DNA methyltransferase inhibitors39 or novel strategies exploiting graft-versus-leukemia effects exerted by CD8+ regulatory T cells with specific antileukemia activity without simultaneous capacity of inducing a graft-versus-host reaction.40
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all of the collaborators of the European Working Group of Myelodysplastic Syndromes in Childhood for contributing clinical data and research material.
This work was supported by Deutsche Forschungsgemeinschaft (grant no. KR3473/1-1 to C.F.); by Priority Program SPP1463 “Epigenetic Dysregulation in Myeloid Neoplasia” (to C.P., C.M.N., and C.F.); by Deutsche Krebshilfe (grant no. 108220 to C.M.N. and C.F.); by Deutsche José Carreras Leukämiestiftung (grant no. R08/19 to C.F.); by the Sofia Luce Rebuffat Foundation (to F.L.); by the European Union COST Action BM0801 (EuGESMA); and by the Förderverein für krebskranke Kinder Freiburg.
Authorship
Contribution: C.F. designed research; C.O.-B., A.R.P., P.N., R.C., I.S., T.W., and C.F. performed laboratory research; B.S., H.H., M. Zecca, J.S., E.B., B.D.M., M.T., M.M.v.d.H.-E., D.W., F.L., C.M.N., and C.F. provided study materials and treated patients; all authors collected and assembled experimental or clinical data; C.O.-B., A.R.P., P.N., R.C., M. Zucknick, C.P., C.M.N., and C.F. analyzed and interpreted data; and C.O.-B. and C.F. wrote the manuscript, which was revised and approved by all coauthors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Christian Flotho, Pädiatrische Hämatologie und Onkologie, Zentrum für Kinder- und Jugendmedizin der Universität Freiburg, Mathildenstr 1, 79106 Freiburg, Germany; e-mail: christian.flotho@uniklinik-freiburg.de.