Abstract
Fanconi anemia (FA) is a genetic condition associated with bone marrow (BM) failure, myelodysplasia (MDS), and acute myeloid leukemia (AML). We studied 57 FA patients with hypoplastic or aplastic anemia (n = 20), MDS (n = 18), AML (n = 11), or no BM abnormality (n = 8). BM samples were analyzed by karyotype, high-density DNA arrays with respect to paired fibroblasts, and by selected oncogene sequencing. A specific pattern of chromosomal abnormalities was found in MDS/AML, which included 1q+ (44.8%), 3q+ (41.4%), −7/7q (17.2%), and 11q− (13.8%). Moreover, cryptic RUNX1/AML1 lesions (translocations, deletions, or mutations) were observed for the first time in FA (20.7%). Rare mutations of NRAS, FLT3-ITD, MLL-PTD, ERG amplification, and ZFP36L2-PRDM16 translocation, but no TP53, TET2, CBL, NPM1, and CEBPα mutations were found. Frequent homozygosity regions were related not to somatic copy-neutral loss of heterozygosity but to consanguinity, suggesting that homologous recombination is not a common progression mechanism in FA. Importantly, the RUNX1 and other chromosomal/genomic lesions were found at the MDS/AML stages, except for 1q+, which was found at all stages. These data have implications for staging and therapeutic managing in FA patients, and also to analyze the mechanisms of clonal evolution and oncogenesis in a background of genomic instability and BM failure.
Introduction
Fanconi anemia (FA) is a rare genetic condition characterized by congenital abnormalities, chromosome fragility, progressive bone marrow (BM) failure, and cancer susceptibility.1,2 Thirteen FA genes have been identified to date, the product of which functions in the FA/BRCA DNA-repair pathway.3,4 FA patients often, but not always, present with a combination of various congenital abnormalities, such as short stature, thumb and radius deformities, peculiar facies, cutaneous spots, and organ malformations. Most FA patients develop BM failure throughout the course of the disease, usually during their first and second decades of life and, for the majority of patients, the suspicion of FA is only made after the onset of pancytopenia.1,2 There is a strong predisposition to hematologic and epithelial malignancies, with cumulative probabilities of an FA patient developing myelodysplasia (MDS) or acute myeloid leukemia (AML) being 30% to 40% by the age of 40 years.5-7 In a number of patients, the underlying diagnosis of FA is not known until an MDS/AML occurs. Biologically, MDS in FA often presents as refractory cytopenia with multilineage dysplasia, with or without excess of blasts.8 It is noteworthy that a level of dyserythropoiesis is almost constant in FA, and a mild dyserythropoiesis is not considered as a MDS criterion in this population. Acute leukemia can be diagnosed primarily or after an MDS phase with an increasing fraction of blast cells in the BM. Cytogenetic abnormalities are frequently found, and translocations of chromosome 1q, monosomy 7, and gains of 3q have been reported.5,8-11 Hematopoietic stem cell transplantation is the current treatment in FA to cure aplastic anemia or MDS and to prevent transformation into leukemia.12,13 It has to be carefully considered on clinical and biologic criteria, which include the age, severity of the cytopenia, significant BM dysplasia, excess of blast cells, cytogenetic abnormalities, and immunologic compatibility with the donor.
Here we investigated the pattern of chromosomal and genomic abnormalities in FA and their association to MDS/AML. We used high-density DNA microarray and oncogene sequencing in addition to karyotype and fluorescence in situ hybridization (FISH) to analyze a series of FA patients at the different stages of BM progression. We found, for the first time in FA, recurrent abnormalities of the oncogene RUNX1/AML1 and included them in a highly recurrent pattern of abnormalities in FA. We also found that mutations of other common MDS/AML oncogenes and tumor suppressor genes were rare in FA, as were rarely detected acquired uniparental disomy (UPD) regions. Somatic reversion of the constitutional FANC gene mutations was not found. Importantly, the presence of chromosomal/genomic abnormalities was correlated to the stage of progression to MDS/AML, except for 1q+, which was found at all stages. These data have important implication not only for the cytogenetic staging of the BM cells in FA patients but also as a basis to analyze the stepwise clonal selection and oncogenesis in patients with hypoplastic BM and genomic instability, with potential relevance for non-FA patients.
Methods
Patients
BM cells from 57 FA patients were analyzed in this study. Patients and/or samples were referred between February 2002 and April 2010 to the French Center for Constitutional Bone Marrow Failure of Saint-Louis Hospital, Paris, France. Inclusions in the present study were retrospective, based on the availability of BM frozen cells and on an enrichment of the series in patients more than 18 years old and in patients with morphologic and/or karyotype abnormalities on their BM aspirate follow-up. All patients had a definitive FA diagnosis based on the usual biologic FA criteria, including chromosomal breakage test in blood, which were performed or reviewed at the Fanconi Anemia Laboratory at Saint-Louis Hospital.14,15 FA mutations were searched for in the patient DNAs (in most cases from primary fibroblasts) in collaboration with Oncogenetic Laboratory of Curie Institute, Paris, and mutation data have been sent to the Fanconi Anemia Mutation Database (www.rockefeller.edu/fanconi/mutate). Consanguinity was defined according to the familial history and/or homozygous FANC gene mutation or deletion (when known). Informed consent for sample collection was obtained from the patients and/or their relatives according to the guidelines of the Tumorotheque of Saint-Louis Hospital following the Declaration of Helsinki, and the study was approved by the Institutional Saint-Louis Hospital Review Board.
Clinical characteristics of the 57 FA patients are shown in Table 1. Age was considered at the time of the cytogenetic analysis. BM categories were defined as follows. Aplastic anemia without MDS were diagnosed according to the criteria of the International Study of Aplastic Anemia and Agranulocytosis.16 Two subgroups were further defined: aplastic anemia (AA) when BM cellularity was less than 25% of the normal hematopoietic cellularity, and moderate hypoplastic anemia (MH) when BM cellularity ranged 25% to 50%, based on an evaluation on BM aspiration and smears (BM biopsies were not performed in these FA patients). MDSs were classified according to the World Health Organization classification 2008 (Table 1). Of note, a moderate dyserythropoiesis is usual in FA and is not considered as a criterion for MDS,8 and patients without other morphologic abnormalities were classified as normal, MH, or AA depending of the BM cellularity. Acute leukemia was defined by BM blast cells more than 20%. When several BM samples were obtained from the same patient, the most informative was considered (usually the most recent except technical issues).
Samples
BM samples were obtained from BM aspirate as performed in the systematic follow-up of FA patients, or when a change happened (eg, the onset of a AML). Skin biopsies and primary fibroblast culture were performed at the initial FA diagnosis workup as previously described.14,15 DNAs were extracted using the QIAamp DNA Mini Kit (QIAGEN; www.qiagen.com). The GenomePlex Whole Genome Amplification kit WGA2 was used when necessary according to the manufacturer's instructions (Sigma-Aldrich) before array-comparative genomic hybridization (CGH) processing.
Karyotype and FISH analyses
Cytogenetic analysis was performed using classic procedures. Images were acquired with the Metafer Slide Scanning System and classified on the Ikaros Karyotyping System (MetaSystems). Karyotypes were described according to International System for Human Cytogenetic Nomenclature, 2008. At least 20 metaphases were screened in each patient, except in cases where abnormal mitoses were obtained and in 4 cases with mitosis failure.
Interphase and/or metaphase (when available) FISH were performed from cryopreserved BM pellets, using break-apart probes RUNX1 (Amplitech) and EVI1 (Kreatech). PRDM16 flanking probes were obtained from Amplitech using the BAC RP4–785P20 and RP11–333E3. At least 200 nuclei were scored per sample for each probe using an epifluorescence microscope Axio Imager 2 (Carl Zeiss) and the Isis imaging system (MetaSystems).
SNP array and array-CGH analyses
All the 57 DNA BM samples were analyzed by high-density DNA array technologies: 39 using single nucleotide polymorphism (SNP) arrays and 32 using array-CGH (14 with both technologies), see supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For SNP arrays, the Affymetrix Genome-Wide Human SNP-Array 250K (Nsp and/or Sty, n = 26 patients) or 6.0 (n = 13 patients) were used. Paired fibroblast DNAs were available for 36 patients analyzed in SNP arrays. DNAs were processed for SNP array using the manufacturer's recommendations (Affymetrix). The arrays were scanned on the Affymetrix Scanner 3000 7G. CEL files produced by Genotyping Console Version 4.0 software were imported into the Genomic Suite, Version 6.5 software (Partek; www.partek.com) for genotyping and copy number analyses. Both SNP and copy number variation probes were used in the analysis. Significantly different regions were determined using the Hidden Markov Model and Segmentation algorithms to detect copy number changes and homozygosity regions. As a normal reference population, we used the International HapMap project and individual references from the paired analysis of the fibroblast DNAs. Agilent Array-CGH technology (180K, n = 31; 1M, n = 1) was used in 32 BM samples DNAs following the manufacturer's recommendations (Agilent Technologies). The arrays were scanned using the SureScan High-Resolution Technology (Agilent Technologies), and the images were processed using Agilent Feature Extraction Version 10.7.3.1 software applying linear and lowest normalization methods and local background subtraction. Analysis was performed using the Agilent Genomic Workbench Version 6.0 software, with the help of the ADM-2 algorithm. Array-CGH data were also imported and analyzed into the Partek Genomic Suite, Version 6.5 software. For both the SNP array and array-CGH technologies, the final retained abnormalities were validated by visual analysis by 2 separate investigators (S.Q., J.S.) considering the size and log2 ratio of the abnormalities with respect to the individual background noise of each array at each particular chromosomal location, as we performed previously.17,18 Polymorphic copy number variations were excluded using the paired analysis of BM and fibroblast DNAs and using the Database of the Genomic Variants tracks in the University of California Santa Cruz Genome Browser or in the Agilent Genomic Workbench Version 6.0 software. Final validated data are indicated in the “Results,” tables, and figures. The University of California Santa Cruz Genome Browser was used for location of the genes on the human genome (www.genome.ucsc.edu). All original microarray data have been deposited in ArrayExpress under accession number E-MTAB-529.
Oncogenes and tumor suppressor gene sequence
Results
A highly recurrent pattern of chromosomal gains and losses in FA BM
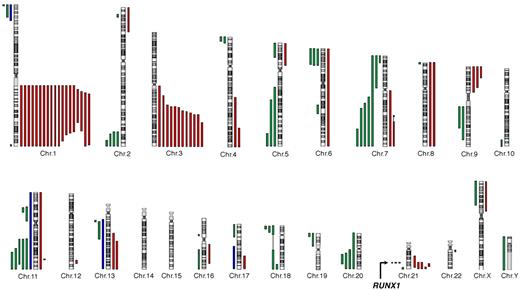
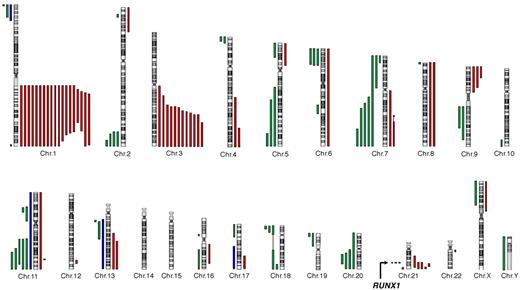
We analyzed BM samples from a series of 57 FA patients (supplemental Figure 1). All patients had both a conventional karyotype and a high-density genome-wide CGH/SNP arrays analyses. Somatic chromosomal gains and losses were detected in n = 35 patients of 57 (Table 2; complete data are shown in supplemental Table 1), with a highly recurrent profile (Figure 1). The most frequent abnormality was the gain of the long arm of chromosome 1 (1q+; n = 19 of 35 patients with abnormalities, 54.3%). The 1q gain was complete (n = 11) or interstitial (n = 8), the minimal region mapping from 1q23 to 1q32 (74.9 Mb) (Figure 1). Other recurrent abnormalities were the gain of the telomeric part of chromosome 3 (3q+, n = 12 of 35 patients with abnormalities, 34.3%), 21q abnormalities (n = 8, 22.9%), partial or complete deletion of chromosomes 7 (−7/7q, n = 5, 14.3%), 11q (11q−, n = 5, 14.3%), 20q (20q−, n = 4, 11.4%), 5q (5q−, n = 3, 8.6%), 9p gain (9p+, n = 3, 8.6%), and trisomy 8 (+8, n = 2, 5.7%). The 3q gain was telomeric in all cases, the 41.5-Mb minimal duplicated region mapping from 3q25 to 3q29 as described by Tonnies et al.11 This region included the EVI1 oncogene; break-apart FISH probes of the EVI1 locus detected 3 bicolor signals related to 3q+ when expected from the CGH/SNP arrays data but did not evidence any direct, balanced or unbalanced, translocation (n = 34 tested patients, data not shown).
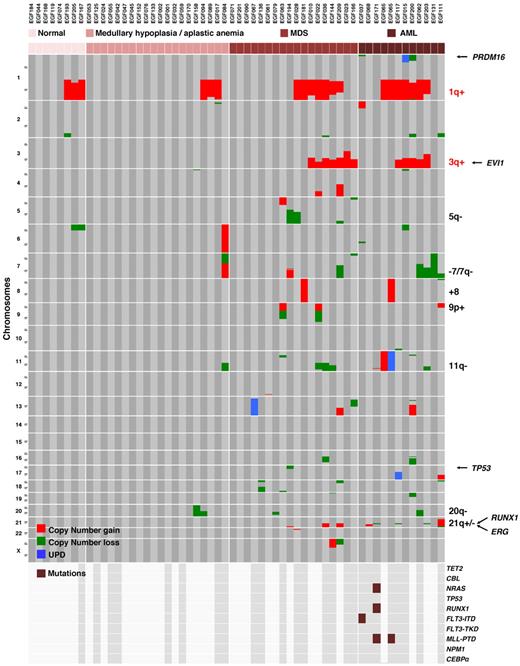
A recurrent pattern of somatic copy number abnormalities and UPD in FA BM cells. All acquired copy number and UPD regions found in the BM cells of 57 individuals with FA are shown. Green lines represent deletions; red lines, gains; and blue lines, regions of UPD (somatic copy-neutral loss of heterozygosity). Constitutional copy-neutral homozygosity regions are not shown. The RUNX1 gene location at 21q22 is shown.
A recurrent pattern of somatic copy number abnormalities and UPD in FA BM cells. All acquired copy number and UPD regions found in the BM cells of 57 individuals with FA are shown. Green lines represent deletions; red lines, gains; and blue lines, regions of UPD (somatic copy-neutral loss of heterozygosity). Constitutional copy-neutral homozygosity regions are not shown. The RUNX1 gene location at 21q22 is shown.
Frequent regions of homozygosity in FA patients are related to consanguinity but very rarely to acquired UPD
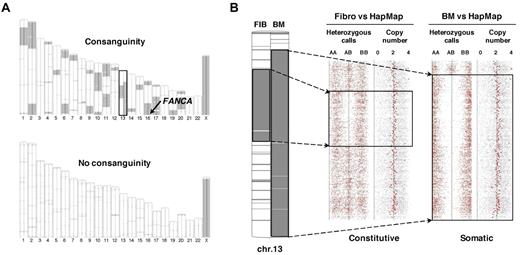
SNP array analysis using paired DNAs from BM and fibroblasts allows high-resolution detection of the acquired UPD (also called copy-neutral loss of heterozygosity) in addition to copy-number changes.21 SNP array analysis was performed in samples from 39 patients, including 36 with a paired BM + fibroblast analysis. In 4 patients only, a region of somatic UPD was present (Figure 1; supplemental Table 1), indicating that this type of genomic abnormality is rare in clonal evolution of FA. Noteworthy, a number of patients (n = 11) had highly frequent regions of constitutive homozygosity as shown by fibroblast versus HapMap SNP array analysis (Figure 2A). This feature was related to consanguinity, as expected from the familial history and homozygous constitutive FANC mutations (Table 1). In these cases (all FANCA), the FANCA locus at 16q24 was included in a region of constitutive homozygosity (Figure 2A). Noteworthy, in one patient, a large region of acquired interstitial UPD in chromosome 13q was detected in the BM cells and encompassed a shorter region of constitutive homozygosity, as demonstrated by the comparative paired analysis (Figure 2B).
SNP array analysis of paired BM and fibroblast DNAs distinguishes between constitutional homozygosity and acquired UPD. (A) Large chromosomal regions of constitutive homozygosity were detected by SNP array in the DNAs of a male FA patient with a degree of consanguinity (patient EGF067, top panel) but not in a male heterozygote composite FA patient (patient EGF074, bottom panel). Homozygosity regions were detected by comparison with a HapMap reference set; fibroblast and BM DNA analyses are shown on the left and on the right of each chromosome ideogram, respectively. The location of the FANCA gene at 16q24 is shown. Chromosome 13, which harbors both a constitutive homozygosity region in fibroblast (left) and a larger acquired UPD in the BM only (right), is boxed. (B) Enlargement of chromosome 13 data from patient EGF067. Heterozygous (AB) and homozygous (AA and BB) calls and copy number data are indicated. The short constitutive copy-neutral region of homozygosity in fibroblasts (left) and the larger acquired UPD (copy-neutral loss of heterozygosity) in BM (right) are boxed. Analysis was performed from Affymetrix SNP array data with the Partek software.
SNP array analysis of paired BM and fibroblast DNAs distinguishes between constitutional homozygosity and acquired UPD. (A) Large chromosomal regions of constitutive homozygosity were detected by SNP array in the DNAs of a male FA patient with a degree of consanguinity (patient EGF067, top panel) but not in a male heterozygote composite FA patient (patient EGF074, bottom panel). Homozygosity regions were detected by comparison with a HapMap reference set; fibroblast and BM DNA analyses are shown on the left and on the right of each chromosome ideogram, respectively. The location of the FANCA gene at 16q24 is shown. Chromosome 13, which harbors both a constitutive homozygosity region in fibroblast (left) and a larger acquired UPD in the BM only (right), is boxed. (B) Enlargement of chromosome 13 data from patient EGF067. Heterozygous (AB) and homozygous (AA and BB) calls and copy number data are indicated. The short constitutive copy-neutral region of homozygosity in fibroblasts (left) and the larger acquired UPD (copy-neutral loss of heterozygosity) in BM (right) are boxed. Analysis was performed from Affymetrix SNP array data with the Partek software.
Recurrent RUNX1/AML1 genomic abnormalities in MDS/AML
RUNX1/AML1 translocations or mutations were initially found by CGH/SNP arrays and gene sequencing analyses in n = 5 patients (supplemental Table 1). Therefore, we performed a systematic FISH analysis using RUNX1 locus break-apart probes in n = 34 patients of the series. This confirmed the array-based data by showing unbalanced translocations in 2 cases (EGF036 and EGF111) and a large deletion in 1 case (EGF203), whereas, as expected, the short deletion/mutations were undetectable by FISH (EGF117 and EGF171). Moreover, the break-apart FISH screen unrevealed an additional RUNX1 locus abnormality in a sixth FA patient (EGF201), which is a cryptic balanced translocation that had not been previously detected by conventional karyotype (20 mitoses without abnormalities) or by CGH/SNP arrays (supplemental Table 1).
Altogether, the RUNX1 abnormalities were as follows (Figure 3): 3 focused deletions and a 7-bp insertion, 2 unbalanced translocations, including a t(1;21)(p36;q22)/RUNX1-PRDM16 (evidenced by a RUNX1 FISH signal at 1p36 and a split of the PRDM16 probes), and in a sixth FA patient a balanced RUNX1 translocation. Apart from the patient with the RUNX1-PRDM16 translocation, insufficient material did not allow further investigation of the RUNX1 partner region in the 2 other translocations (only PRDM16 could be excluded by FISH in case EGF201). Importantly, all the 6 FA patients with RUNX1 genomic abnormalities had severe MDS or AML.
RUNX1/AML1 gene lesions in MDS/AML of several FA patients. (A) Overview of the chromosomal and molecular lesions detected on the RUNX1 gene locus at 21q22 in the BM cells of 6 FA patients with MDS/AML. (Top panel) The genomic position of the RUNX1 exons and of the FISH probes. (Bottom panel) Deletions and gains are represented in green and red, respectively. In patient EGF111, 2 unbalanced translocations involved chromosome 21, the most telomeric interrupting RUNX1 as indicated; a balanced translocation detected in a sixth patient (EGF201) using the RUNX1 flanking probes is not shown. (B) A 7-bp insertion in RUNX1 was found in the EGF171 patient in addition to the large deletion shown in panel A (blast cells 70%). (C) Break-apart and duplication of the 5′ telomeric RUNX1 probe (green) in patient EGF036 with a t(1;21)(p36;q22) (objective lens, original magnification × 63). A fusion PRDM16-RUNX1 was confirmed by combinations of RUNX1 and PRDM16 probes (not shown).
RUNX1/AML1 gene lesions in MDS/AML of several FA patients. (A) Overview of the chromosomal and molecular lesions detected on the RUNX1 gene locus at 21q22 in the BM cells of 6 FA patients with MDS/AML. (Top panel) The genomic position of the RUNX1 exons and of the FISH probes. (Bottom panel) Deletions and gains are represented in green and red, respectively. In patient EGF111, 2 unbalanced translocations involved chromosome 21, the most telomeric interrupting RUNX1 as indicated; a balanced translocation detected in a sixth patient (EGF201) using the RUNX1 flanking probes is not shown. (B) A 7-bp insertion in RUNX1 was found in the EGF171 patient in addition to the large deletion shown in panel A (blast cells 70%). (C) Break-apart and duplication of the 5′ telomeric RUNX1 probe (green) in patient EGF036 with a t(1;21)(p36;q22) (objective lens, original magnification × 63). A fusion PRDM16-RUNX1 was confirmed by combinations of RUNX1 and PRDM16 probes (not shown).
Rare mutations of common MDS/AML oncogenes and tumor suppressor genes in FA
In 25 patients with sufficient tumor DNA material, including 20 with a clonal population evidenced by CGH/SNP arrays (17 with MDS or AML), we searched for mutations of a set of cancer genes that are commonly targeted in MDS and/or AML, namely, TET2, CBL, NRAS, TP53, RUNX1, CEBPα, NPM1, FLT3-TKD, FLT3-ITD, and MLL-PTD.19,22-26 A few mutations were detected: MLL-PTD in 2 cases and FLT3-ITD, RUNX1, and NRAS mutations in one case each (supplemental Table 1). No mutation was detected by sequencing TP53, TET2, CBL, CEBPα, FLT3-TKD, and NPM1. In the 2 patients with MLL-PTD, the copy number gain within the MLL gene was also detected by CGH/SNP arrays, as expected (supplemental Table 1).
In an additional AML case (patient EGF068), we found a 4.4-Mb amplification at 21q22 by array-CGH, which included not the RUNX1 but the ERG oncogene (supplemental Figure 2). In another patient with an RAEB-1, we detected an unbalanced t(1;2)(p36;p21), leading to the translocation of the PRDM16/MEL gene to the ZFP36L2 locus as mapped by array-CGH and confirmed by PRDM16 FISH (patient EGF037, supplemental Figure 2). Such abnormalities have been previously reported in rare non-FA MDS, AML, or blast crisis of myeloproliferative disorder.27-29 Of note, the karyotype detected no chromosomal abnormality in patient EGF068, the ERG amplification as evidenced by array-CGH being cryptic and the sole abnormality found in this patient.
Chromosomal and genomic abnormalities, especially 3q+, 7/7q−, and RUNX1 abnormalities, but not 1q+ and 20q−, are associated with MDS/AML
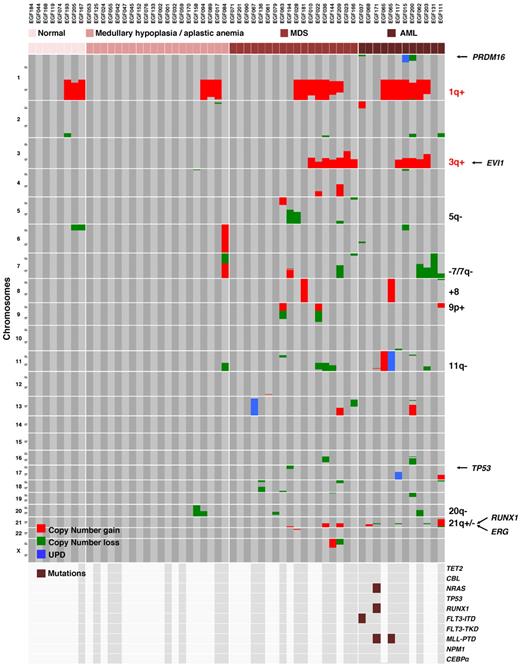
BM morphology of the FA patients of the present series ranged from normal FA morphology (n = 8), MH or AA (n = 20), MDS of various WHO 2008 subtypes (n = 18), to AML (n = 11). The majority of the chromosomal and genomic abnormalities were found in the 29 patients with MDS or AML (112 of 130 abnormalities, P < .000001; Table 2; Figure 4). An important exception to this feature was the frequent gain of chromosome 1q; indeed, 1q+ was found at all stages of FA BM evolution: normal (3 of 8 cases), MH/AA (3 of 20), MDS (7 of 18), and AML (6 of 11) (P value of normal/MH/AA vs MDS/AML nonsignificant, Table 2). A similar trend was found for 20q−, as expected from what is known in non-FA MDS.30 By contrast, gain of 3q, deletion 7/7q, and RUNX1 abnormalities were strictly found in the MDS/AML patients (Table 2; Figure 4). Specifically, 3q+ was found in 7 of 18 MDS (mainly high grade) and 5 of 11 AML; −7/7q in 1 of 18 MDS (this last being a RAEB-1) and 4 of 11 AML; and RUNX1 abnormalities in 2 of 18 MDS (both AREB-1) and 4 of 11 AML, demonstrating the strong association of these 3 abnormalities with high-grade MDS and blastic transformation (Table 2; Figure 4). Of note, an isochromosome 7q (7p−/7q+), found in one patient with aplastic anemia, was not included in the −7/7q category. Deletion of 5q was observed in 3 MDS cases, one of them also displaying the only deletion 17p of this series, and trisomy 8 in 2 patients (1 MDS and 1 AML, both with a 1q+). A 4.4-Mb amplification at 21q22, including ERG, was found as a unique abnormality in an AML case with a normal karyotype, and a translocation t(1;2) targeting PRDM16 was found in an AREB-1 (supplemental Figure 2).
Distribution of the chromosomal and molecular lesions in FA patients. Somatic copy-number gains, losses, and UPD are color-encoded and plotted vertically in chromosomal order for each patient. Samples were clustered horizontally according to the progression stage groups in the BM and then by visual inspection to cluster the abnormalities. The recurrent abnormalities and a selection of relevant genes are indicated on the right part of the figure. 1q+ and 3q+ are specific from FA and are indicated in red, the other abnormalities can be found in non-FA MDS/AML and are indicated in black. Oncogenes and tumor suppressor gene mutation screen data are shown on the lowest panel of the figure; the dark gray background and brown boxes indicate which samples were screen and presence of mutations, respectively.
Distribution of the chromosomal and molecular lesions in FA patients. Somatic copy-number gains, losses, and UPD are color-encoded and plotted vertically in chromosomal order for each patient. Samples were clustered horizontally according to the progression stage groups in the BM and then by visual inspection to cluster the abnormalities. The recurrent abnormalities and a selection of relevant genes are indicated on the right part of the figure. 1q+ and 3q+ are specific from FA and are indicated in red, the other abnormalities can be found in non-FA MDS/AML and are indicated in black. Oncogenes and tumor suppressor gene mutation screen data are shown on the lowest panel of the figure; the dark gray background and brown boxes indicate which samples were screen and presence of mutations, respectively.
Altogether, 27 of 35 patients with chromosomal/genomic abnormalities had MDS/AML, and this value raised to 27 of 28 patients by keeping only the abnormalities other than 1q+ and 20q−. We then analyzed the association of the recurrent chromosomal or molecular abnormalities. Frequent combinations were 1q+/3q+, 1q+/3q+/7q−, 1q+/3q+/RUNX1, 1q+/3q+/11q−/RUNX1, or even in one patient 1q+/3q+/7q−/11q−/RUNX1. Noteworthy, one patient with AML had no detectable gross chromosomal abnormality but the combination of RUNX1, NRAS, and MLL-PTD mutations, consistent with what was reported in non-FA patients,26 another one with AML had 1q+, a fusion ZFP36L2-PRDM16 and FLT3-ITD, and another one had 1q+,+8, and MLL-PTD with UPD11 (Figure 4; supplemental Table 1). These combinations highlight the multistep oncogenic pathways in MDS/AML of FA patients.
No reversion mutation was found in the MDS/AML in FA patients
Acquired genetic reversion of the FANC gene mutations was reported in blood lymphocytes31 but also in an AML cell line from an FA FANCD1/BRCA2-mutated patient,32 suggesting that this phenomenon might be frequent and play a role in FA-related MDS/AML oncogenesis. Constitutional FANC genes mutations in the present series were initially identified in the primary fibroblast DNAs for the majority of the cases (J.S., D.S.-L., unpublished data, September 2010). We resequenced these mutations in the BM DNA of 20 patients with sufficient DNA and a clonal population on CGH/SNP array data. No reversion event could be found in the present series, suggesting that FANC genetic reversion (somatic mosaicism) is not a common progression event in MDS/AML, at least in this cohort of FA patients mainly of the FA-A group.
Discussion
To evaluate the pattern of chromosomal and genomic abnormalities in FA and its association with the stages of BM progression, we used high-resolution chromosomal and molecular techniques on BM samples from a series of FA patients. This series was enriched in patients older than 18 years and in patients with morphologic or karyotypic abnormalities on the follow-up BM aspirate. Most patients (84.2%) were from the FA-A group, which is representative of French patients. We found a complex and highly recurrent pattern of chromosomal and genomic abnormalities. It included chromosome abnormalities previously reported in FA karyotypes,5,8-11 such as −7/7q, 3q+, and 1q+ and, for the first time, in FA, cryptic abnormalities of the RUNX1/AML1 gene at 21q, and rare mutations or translocations of other known oncogenes. The majority of the chromosomal abnormalities were the result of unbalanced translocations leading to gain or deletion of gross chromosomal regions, often telomeric (Figure 1). It is probable that these result from FA-related unresolved DNA damage during S phase, which ultimately leads to double-strand breaks and error-prone repair by nonhomologous end joining. Indeed, nonhomologous end joining is largely efficient in FA cells in contrast with homologous repair, which is impaired.4,33,34 Moreover, few somatic copy-neutral loss of heterozygosity (UPD) regions were observed (Figure 1; supplemental Table 1), suggesting that homologous repair is a rare mechanism of genetic progression in FA, in contrast to what is seen in non-FA MDS/AML patients.35,36 We also searched for genetic reversion events (somatic reversion of the constitutional FA gene mutation), a phenomenon that leads to somatic mosaicism in FA lymphocytes,31 considering what was found in an AML cell line of the D1/BRCA2 group.32 No FANCA reverse mutation was observed by resequencing the genomic DNA from the clonal BM cells in this series, suggesting that simple genetic reversion is not a frequent progression event in the majority of FA patients. Looking for more complex reversion mechanisms would need cellular testing of the BM clonal cells.
FA patients experience a high selective pressure to develop clonal MDS and AML during their teens or young adulthood. This is probably directly related to the cellular defects of FA cells (ie, genomic instability and excess of cell death) together with a possible intrinsic hematopoietic stem cell defect and related BM failure.13,37-40 With respect to aplasia and MDS/AML in non-FA patients,16,21,22,35,36 2 chromosomal abnormalities were specifically associated with FA patients, 1q+ and 3q+. The 1q minimal duplicated region was very large (74.9 Mb) and contained many possible candidate oncogenes. For 3q, an obvious candidate oncogene within the large 41.5-Mb minimal duplicated region was EVI1.41 A focused EVI1 amplification was reported previously in AML cell lines derived from an FA patient of the particular D1/BRCA2 downstream group.42 Here, break-apart FISH found frequent EVI1 duplication, as expected from the CGH/SNP array data, but no direct involvement in a fusion gene resulting from a translocation. Therefore, the potential role of EVI1 in the MDS/AMLs with 3q+ in typical FA-core patients remains elusive. The other recurrent chromosomal and genomic abnormalities we found can also be seen in non-FA patients with MDS/AML. Molecular oncogenic significance of the deletions 7q and 11q has not been elucidated to date, by contrast of the UPD7q and UPD11q, which were recently associated with EZH2 and CBL mutations, respectively.43-45 Importantly, we found targeted abnormalities of the RUNX1/AML1 oncogene in several FA MDS/AML patients (ie, unbalanced or balanced translocations, including PRDM16-RUNX1 fusion and inactivating mutations or focused deletions; Figure 3), reminiscent of RUNX1 oncogenic lesions in non-FA patients.25 The RUNX1 protein is part of the Core Binding Factor heterodimeric transcription factor, which regulates different levels of embryonic and adult hematopoiesis. A range of RUNX1 genomic abnormalities were reported in non-FA MDS and AML, which leads to the inactivation of this gene.22,25,26,46-48 The recurrent involvement of RUNX1 in FA MDS/AML is of high interest because this is the first clear implication of a known oncogene in the multistep BM progression in these patients, opening roads to figure out the oncogenic cellular pathways in FA. In addition, we found rare mutations of common oncogenes of non-FA MDS/AML, namely, NRAS mutation, FLT3-ITD and MLL-PTD, whereas no mutations of TET2, CBL, TP53, NPM1, and CEBPα were found in this cohort (Figure 4; supplemental Table 1). We also observed unique cases with abnormalities of the PRDM16/MEL or ERG oncogenes, as occasionally reported in non-FA patients.27-29,47,48 Combinations of the recurrent chromosomal or molecular abnormalities were frequent, with no abnormality being clearly mutually exclusive of the others (Figure 4; supplemental Table 1). Collectively, these data suggest avenues of multistep oncogenesis in the BM progression of FA, in which 1q+ would be a central, possibly initiating event, that can be found in the aplastic form of the disease, and 3q+, −7/7q, and RUNX1 abnormalities, among others, would lead to high-grade MDS or AML. In this figure, 1q+ might clonally rescue the BM failure of FA, partially or completely, but would not definitely protect against progression to MDS and leukemia. Serial studies of clonal evolution in humans or xenografts are needed to determine the precise order of the chromosomal/genomic events, and gene expression profiling should help analyze their target genes and related cellular advantage in an aplastic BM and during transformation.49 Interestingly, we found that the TP53 gene at 17p was not deleted or mutated in FA (except in a unique case with 5q−; Figures 1, 4), which was somewhat unexpected considering frequent mutation in non-FA patients with complex karyotype MDS/AML.50 This suggests that, in the specific FA background (constitutional genomic instability and excess of cell death), mechanisms other than TP53 genomic mutation/deletion are probably more proficient. It is tempting to speculate that 1q+ or 3q+ might be implicated.
MDS/AML is a frequent and severe occurrence in FA. It is therefore important to follow up the patients by regular BM examination with expert morphologic and karyotype analyses, to detect transformation before the onset of a high-grade MDS/AML.1,2,5,7 In 2 patients with “normal” BM in the present study, conventional karyotype appeared to be more sensitive than CGH/SNP arrays for the detection of discreet 1q+ subclones because of the possibility to observe individual cells and probably an advantage for mitosis in culture (supplemental Table 1). Systematic interphasic FISH screening using probes for chromosome 7q, 3q (for instance a probe EVI1), and break-apart RUNX1 might increase the sensitivity of early detection of transformed cells. Importantly, we experienced several MDS/AML cases with a normal karyotype but cryptic chromosomal or genomic abnormalities detected by FISH and/or array analysis (supplemental Table 1). Therefore, we now implement the cytogenetic analysis using high-density DNA arrays and RUNX1 FISH when MDS or leukemia is diagnosed in FA patients. This approach is also useful to determine a more precise characterization of the abnormal karyotypes and frequently highlights meaningful chromosomal/molecular lesions (Figures 1,Figure 2,Figure 3–4; supplemental Table 1; supplemental Figure 2). Of note, if an SNP array analysis is performed to search for somatic UPD regions (copy number-loss of heterozygosity), paired BM and constitutive DNAs (like skin fibroblast DNA) analysis allows one to rule out regions of constitutive homozygosity because of a degree of consanguinity (Figure 2). Finally, and as also suggested by others,8 when 1q+ and/or 3q+ are found in a child or young adult with a de novo MDS or secondary-type AML, we carefully investigate an underlying diagnosis of FA, considering that physical signs of FA can be absent or very discreet and that the therapeutic consequences would be major.
In conclusion, BM progression in FA is associated with a complex pattern of recurrent chromosomal abnormalities, some commonly found in non-FA MDS and secondary AML, such as −7/7q or RUNX1 abnormalities, and some others specific from FA, such as 1q+ and 3q+. A careful analysis by karyotype, FISH, and in case of MDS/AML, by high-density DNA arrays allows a precise description of these clonal abnormalities. The prognostic value of the various chromosomal/genomic abnormalities will have to be carefully evaluated on the long-term in large cohorts of FA patients with respect to the therapeutic options and clinical benefits. For instance, a sole clonal abnormality, such as 1q+, can be present in “normal” or non-MDS hypoplastic BM cells and, in our experience, it may persist for years without progression into MDS or AML (T.L., G.S., and J.S., unpublished data, September 2010). Finally, the comprehensive collection of chromosomal/genomic data of FA patients constitutes a basis to figure out the stepwise hematopoietic clonal evolution and oncogenesis in a background of genomic instability, excess of cell death and BM failure, with probable relevance not only in FA but also for non-FA patients with aplastic anemia or MDS/AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the participating patients and families and the many clinicians from the French pediatric, genetic, and/or hematologic centers who contributed to the present work through their patient care; the Association Française de la Maladie de Fanconi, the French Society of Hematology, and the Société Française d'Hémato-Immunologie Pédiatrique; Daniela Geromin at the Tumorotheque of Hospital Saint-Louis (Assistance Publique-Hopitaux de Paris); and Helen Walden for proofreading the manuscript.
Our center is supported by the French Government (Direction de l'Hospitalisation et de l'Organization des Soins) as Center de Référence Maladies Rares Aplasies médullaires constitutionnelles (coordination, G.S.), by the Réseau Institut National du Cancera des Maladies Cassantes de l'ADN (coordination, D.S.-L. and A. Sarasin), and by the French Cytogenetic Lab Network for chromosomal breakage syndromes (coordination, J. Couturier and J.S.). This work was supported in part by Diagnostic de la maladie de Fanconi (Programme Hospitalier de Recherche Clinique national grant AOM05066), the Agence Nationale de le Recherche, Program Du gène à la physiopathologie; des maladies rares ausc maladies communes (GENOPAT; ANR-08-GENO-013-03), the Institut National du Cancer, Direction de l'Hospitalisation et de l'Organisation des Soins (North-West and Ile-de-France canceropoles), and DREAM (09-76-13). R.C. has a fellowship from the Association pour la Recherche sur le Cancer.
Authorship
Contribution: S.Q. performed array experiments and bioinformatic analysis and wrote the paper; W.C. performed cytogenetic analysis and wrote the paper; R.C., M.-P.P., N.V., and J.L. performed biologic experiments; C.P., R.P., V.R., J.-H.D., P.S., M.M., G.M., A.B., F.S., E.G., and T.L. handled patient clinical care and follow-up; C.D.E. and D.S.-L. performed constitutional FANC mutation analysis; O.N. and C.P. performed oncogene mutation analysis; G.S. handled patient clinical care and follow-up and designed the study; and J.S. designed the study, performed biologic and bioinformatic analyses, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean Soulier, Team Genome and Cancer, Hematology Laboratory and Inserm U944, University Paris 7, Denis Diderot, Saint-Louis Hospital, 1 Av Claude Vellefaux, 75010 Paris, France; e-mail: jean.soulier@sls.aphp.fr.
References
Author notes
S.Q. and W.C. contributed equally to this study.