Abstract
Histone deacetylase inhibitor (HDACI)–induced thrombocytopenia (TCP) is a major dose-limiting toxicity of this new class of drugs. Using preclinical models to study the molecular and biologic events that underpin this effect of HDACI, we found that C57BL/6 mice treated with both the HDAC1/2-selective HDACI romidepsin and the pan-HDACI panobinostat developed significant TCP. HDACI-induced TCP was not due to myelosuppression or reduced platelet lifespan, but to decreased platelet release from megakaryocytes. Cultured primary murine megakaryocytes showed reductions in proplatelet extensions after HDACI exposure and a dose-dependent increase in the phosphorylation of myosin light chain 2 (MLC2). Phosphorylation of MLC to phospho-MLC (pMLC) and subsequent proplatelet formation in megakaryocytes is regulated by the Rho-GTPase proteins Rac1, CDC42, and RhoA. Primary mouse megakaryocytes and the human megakaryoblastic cell line Meg-01 showed reductions in Rac1, CDC42, and RhoA protein levels after treatment with HDACIs. We were able to overcome HDACI-induced TCP by administering the mouse-specific thrombopoietin (TPO) mimetic AMP-4, which improved platelet numbers to levels similar to untreated controls. Our report provides the first detailed account of the molecular and biologic processes involved in HDACI-mediated TCP. Moreover, our preclinical studies provide evidence that dose-limiting TCP induced by HDACIs may be circumvented using a TPO mimetic.
Introduction
Covalent posttranslational modifications to specific sites within histone proteins, including acetylation, methylation, and phosphorylation, are able to affect gene transcription in cells.1 Increased acetylation of histones is associated with open DNA and increased transcription, whereas deacetylation is associated with transcriptional repression.2 Disruption of this balance is associated with cancer onset and progression.3 The histone deacetylase (HDAC) enzymes control the structural conformation of chromatin via deacetylation of core nucleosomal histones. To date, a total of 18 HDACs have been described and are divided into 4 general classes. Class I HDACs are thought to be located within the cell nucleus only, whereas class II and class IV HDACs shuttle between the cell cytoplasm and the nucleus. Class III HDACs comprise the NAD+-dependent sirtuin family proteins.4
HDAC inhibitors (HDACIs) are structurally diverse antineoplastic agents distinguished both by their chemical structure and by their target specificity.5 HDACIs induce chromatin remodeling and altered gene expression, and the function of nonhistone proteins may also be affected by direct acetylation.6,7 Panobinostat is a cinnamic hydroxamic acid with inhibitory effects against all class I, II, and IV HDAC enzymes and marked antitumor activity across a broad range of hematologic cancers, including Hodgkin lymphoma.8-10 Romidepsin is a bicyclic tetrapeptide that preferentially interacts with class I enzymes, and has activity in cutaneous T-cell lymphoma,11 peripheral T-cell lymphoma,12,13 and multiple myeloma.14 Romidepsin (ISTODAX) was approved by the U.S. Food and Drug Administration (FDA) in 2009 for the treatment of cutaneous T-cell lymphoma in patients who have received at least one prior systemic therapy.
Thrombocytopenia (TCP) is a common side effect of conventional chemotherapy and is the most common dose-limiting hematologic toxicity of HDACIs, threatening to compromise combination treatment strategies.10,15 Both panobinostat and romidepsin and the FDA-approved HDACI vorinostat (Zolinza) have induced TCP in clinical studies.8,10,11,15 Indeed, in a recent report of single-agent panobinostat in relapsed Hodgkin lymphoma, the incidence of grade 3 and 4 TCP was 79%.10 The mechanism by which this occurs has not been determined.
Megakaryocytes give rise to platelets in a process involving massive reorganization of the cytoskeleton and cytoplasmic organelles. Membranous cytoplasmic projections known as proplatelets emanate from mature megakaryocytes, with microtubule sliding allowing the elongation of extensions and organelle transportation.16,17 Platelets are thought to bud from the tips of proplatelets. Once shed into the circulation, the life span of platelets is regulated by the Bcl-2 family proteins Bcl-xL and Bak.18 Prosurvival Bcl-xL restrains pro-death Bak to maintain platelet viability. It is thought that within each individual platelet, Bcl-xL levels decrease relative to Bak over time until a threshold is reached and Bak becomes activated, resulting in platelet apoptosis and clearance from the circulation. Genetic mutations in the gene encoding Bcl-xL reduce platelet life span.18 Pharmacologic inhibition with the BH3-mimetic compound ABT-737 triggers platelet apoptosis and TCP in vivo, with marked reductions in platelet number occurring within 2 hours of administration.18-20 These effects are associated with a compensatory increase in thrombopoietin (TPO), the major cytokine regulator of megakaryocyte proliferation and differentiation, and subsequent enhanced platelet production. Given the ability of HDACIs to induce apoptosis in a variety of tumors and cell lines, it was considered possible that direct platelet or megakaryocyte apoptosis played some role in HDACI-induced TCP. However, the complex nature of platelet formation dictates that TCP induced by HDACIs could also potentially occur at multiple levels in the platelet-production process.
Agents with the potential to ameliorate drug-induced TCP include both pegylated human megakaryocyte growth and development factor and recombinant human TPO, which have demonstrated modest benefit in chemotherapy-induced TCP.21,22 Further clinical development of these agents was halted some years ago when cross-reacting antibodies against endogenous TPO caused immune TCP in several patients.23-25 Second-generation thrombopoietic agents without homology to TPO have subsequently been developed and proven successful in clinical trials of chronic idiopathic TCP and hepatitis,26,27 and this has led to both drugs gaining approval from the FDA.
These TPO mimetics have clinical activity even in settings in which TPO levels are already elevated. One of these molecules, romiplostim (AMG-531), consists of 2 identical peptides, each containing multiple linked TPO receptor–activating sequences attached to 2 covalently bound Fc fragments to prolong its circulating half-life. AMP-4 is a molecule with an identical “warhead” to AMG-531, but with a murine Fc receptor to reduce immunogenicity in mice.
In the present study, we used a murine model to recapitulate HDACI-induced TCP using the pan-HDACI panobinostat and the HDAC isoform-selective HDACI romidepsin. Using murine megakaryocytes and the human megakaryoblastic cell line Meg-01, we demonstrate that HDACIs inhibit platelet production concomitant with an increase in pMLC and reduced expression of the Rho family proteins RhoA, CDC42, and Rac1. We also show that in this model the TPO mimetic AMP-4 effectively ameliorated HDACI-induced TCP.
Methods
Reagents and cells
Panobinostat (Novartis Pharmaceuticals), romidepsin (Celgene), ABT-737 (Abbott Laboratories), AMP-4 (Amgen), N-hydroxysuccinimidobiotin (NHS-biotin; Sigma), and thiazole orange (Sigma) were provided or purchased as indicated. Murine TPO was synthesized at the Walter and Eliza Hall Institute (Parkville, Australia). Anti–P21-activated kinase 1 (anti-PAK1), anti–phospho-myosin light chain 2 (anti-pMLC2), and anti-MLC2 were purchased from Cell Signaling Technology; anti-Rac1 and anti-CDC42 were from Becton Dickinson; and anti-RhoA was from Santa Cruz Biotechnology. WR PAK18 was purchased from EMD Chemicals; avidin and biotin solutions were purchased from Dako; and a tyramide signal amplification biotin kit was purchased from Perkin Elmer. Meg-01 cells were purchased from DSMZ and cultured in 10% RPMI 1640 supplemented with 10% fetal calf serum, penicillin/streptomycin, and l-glutamine. Murine megakaryocytes were derived from fetal liver cells harvested from E13.5-14.5 pregnant C57BL/6 mice and plated out at 5 × 105 cells/mL in StemPro-34 medium (Invitrogen) supplemented with murine TPO (100 ng/mL) at 37°C with 5% CO2 for 3-5 days.
Mice
All animal studies were approved by the Peter McCallum Cancer Center Animal Experiment Ethics Committee. C57BL/6 mice 8-12 weeks of age were purchased from the Walter and Eliza Hall Institute and injected with either panobinostat 10 mg/kg or romidepsin 1 mg/kg intraperitoneally (IP) daily, either continuously or on an alternate weekly schedule. After confirmation of TCP with both drugs on the continuous daily IP schedule, AMP-4 (20 μg/kg) was administered IP in combination with both drugs, with one dose given on the same day that HDACI treatment was started (day 0) and another on day 6. For the Bak−/−Bax−/− experiments, irradiated wild-type C57BL/6 mice were reconstituted with Bak−/−Bax−/− fetal liver cells, as described previously.28 Briefly, wild-type or Bak−/−Bax+/− C57BL/6-CD45.2 mice were intercrossed and day-13.5 pregnant mice were killed, embryos removed, and fetal livers dissected. Genomic DNA was extracted from embryonic tissue and subjected to polymerase chain reaction amplification to determine genotype. Fetal-liver cell suspensions were prepared in phosphate-buffered saline (PBS), and 2 × 106 cells were injected into lethally irradiated C57BL/6-CD45.1 mice. To prevent infections, the transplanted animals were provided with water containing neomycin (Sigma). After stable reconstitution of their hematopoietic system (8 weeks later), donor contribution to peripheral blood leukocytes was analyzed by flow cytometry. Only mice showing 90%-98% engraftment were used in the experiments.28 C-mpl–knockout mice were a gift from Prof Warren Alexander (Walter and Eliza Hall Institute). For all experiments, at least 15 mice per cohort were used, with 3 mice eye-bled at each time point, and experiments were repeated at least 3 times.
Platelet clearance analysis and reticulated platelet staining
Mice were injected intravenously twice, 1 hour apart, with 600 μg of NHS-biotin in PBS and 10% dimethyl sulfoxide. At various time points, peripheral blood was isolated from the tail, and 1 μL was washed twice in PBS and 10mM EDTA (ethylenediaminetetraacetic acid). Platelets were stained with phycoerythrin-conjugated rat anti–CD41 (Becton Dickinson) and allophycocyanin-conjugated streptavidin (APC-A) (Becton Dickinson) for 30 minutes on ice. Samples were washed again and incubated with 0.125μg/mL of thiazole orange, which has increased uptake in high-RNA–containing platelets, staining the younger, reticulated platelet fraction, for 90 minutes. Flow cytometry was then performed on an LSR flow cytometer (Becton Dickinson). By plotting the number of biotinylated platelets against time, an estimate of the life span was obtained by linear extrapolation, while the number of thiazole orange–positive, nonbiotinylated platelets provided an estimate of new platelet production. All experiments were performed at least 3 times.
TPO analysis
After collection of blood from treated mice, TPO levels were measured by enzyme-linked immunosorbent assay using the RayBio TPO ELISA kit (RayBiotech) according to the manufacturer's instructions.
Proplatelet formation assay
Fetal liver cells were harvested from E13.5-14.5 pregnant C57BL/6 mice, and plated out at 5 × 105cells/mL in StemPro-34 medium (Invitrogen) supplemented with murine TPO (100 ng/mL) at 37°C with 5% CO2 for 3 days. Cells were subjected to a 3% (3 mL) and a 1.5% (3 mL) bovine serum albumin (BSA) gradient at 1g for 40 minutes at room temperature. Megakaryocytes were harvested from the lowest 800 μL to 1 mL and plated out at 1000 cells/100 μL in 96-well plates for 3 days with 100 ng/mL of TPO in combination with panobinostat or romidepsin in duplicate. Proplatelet-displaying megakaryocytes were defined as cells exhibiting one or more filament-like protrusions at least the diameter of the cell, counted under an inverted microscope, and the percentage of megakaryocytes with proplatelets was calculated. All experiments were performed at least 3 times.
Western blot analysis
Platelets were isolated from blood of mice by eye bleeding into 0.1 volume of Aster Jandl anticoagulant (85mM sodium citrate dihydrate, 69mM citric acid anhydrase, and 10mM glucose). Mouse platelet–rich plasma was obtained by centrifugation at 125g for 8 minutes. Platelets were washed by 2 sequential centrifugations at 860g for 5 minutes in wash buffer (140mM NaCl, 5mM KCl, 12mM sodium citrate, 10mM glucose, and 12.5mM sucrose, pH 6.0), and resuspended in resuspension buffer (10mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 140mM NaCl, 3mM KCl, 0.5mM MgCl2 hexahydrate, 0.5mM NaHCO3, and 10mM glucose, pH 7.4). C57BL/6 fetal liver cells were plated out with TPO (100 ng/mL) and treated with 10nM panobinostat or 10nM romidepsin on day 3. On day 5, the cells underwent BSA gradient separation, as described in “Proplatelet formation assay,” and megakaryocytes were isolated and washed 3 times in PBS. Meg-01 cells were treated with 20nM panobinostat or romidepsin for 24 hours and then lysed with Triton X lysis buffer (20mM Tris-pH 7.4, 135mM NaCl, 1.5mM MgCl2, 1mM EGTA [ethyleneglycoltetraacetic acid], 10% glycerol, and 1% Triton X-100) with protease and phosphatase inhibitors. Samples were boiled for 5 minutes in loading buffer, and equal amounts of extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Protein was transferred to nitrocellulose membranes and blocked for 60 minutes at room temperature in PBS with 5% BSA. Membranes were incubated with polyclonal rabbit anti–pMLC2 immunoglobulin G (IgG) at a dilution of 1/1000, polyclonal rabbit anti–MLC2 IgG at a dilution of 1/1000, polyclonal rabbit anti–PAK1 IgG at a dilution of 1/1000 (Cell Signaling Technology); monoclonal mouse anti–Rac1 IgG at a dilution of 1/500 and monoclonal mouse anti–CDC42 IgG at a dilution of 1/500 (Becton Dickinson); and mouse monoclonal anti–RhoA IgG at a dilution of 1/500 and goat polyclonal anti–ROCK1 IgG at a dilution of 1/500 (Santa Cruz Biotechnology). Primary antibodies were detected with secondary antibodies conjugated with horseradish peroxidase, and filters were developed with enhanced chemiluminescence (Amersham). All experiments were performed at least 3 times.
Fluorescence microscopy
Fetal liver cell-derived megakaryocytes were isolated as described in “Proplatelet formation assay” after treatment for 48 hours with 1nM panobinostat or 10nM romidepsin. Meg-01 cells were treated with 20nM panobinostat or 20nM romidepsin for 24 hours. Cells were then centrifuged onto slides coated with poly-l-lysine, fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton X-100 for 5 minutes, and then incubated with anti-pMLC2 (Cell Signaling Technology) and anti-tubulin for 1 hour at room temperature. After washing, the cells were incubated with the appropriate secondary antibodies conjugated with Alexa Fluor 488 or 543. Cells were coverslipped with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) containing ProLong Gold Antifade reagent (Invitrogen) for nucleus staining. Cells were examined under a BX-51 microscope (Olympus) equipped with a 60× water objective. All experiments were performed at least 3 times.
Immunohistochemistry
Immunohistochemical staining was performed using the tyramide signal amplification biotin system kit (Perkin-Elmer). After de-waxing, slides underwent heat antigen retrieval by placing them in 10mM sodium citrate, pH 6.0, in a pressure cooker for 3 minutes at 125°C. After cooling and washing in PBS, slides were incubated in a humidified chamber in avidin reagent for 10 minutes, washed in PBS, incubated in biotin reagent for 10 minutes, and washed again in PBS. Free aldehydes were blocked by incubation with 0.37 g/100 mL of glycine for 5 minutes, and then blocked in TNB buffer (0.1M Tris-HCl, pH 7.5, 0.15M NaCl, and 0.5% blocking reagent [supplied in the kit]). Primary pMLC antibody was applied for 1 hour before slides were washed in TNT wash buffer (0.1M Tris-HCl, pH 7.5, 0.15M NaCl, and 0.05% Tween). Biotinylated secondary anti–rabbit IgG was applied for 30 minutes, and after washing in TNT buffer, slides were incubated with streptavidin-horseradish peroxidase for 30 minutes. After further washing, slides were incubated with biotinyl tyramide plus fluorescein amplification reagent for 6 minutes. After washing in PBS, slides were coverslipped and cells were examined under a BX-51 microscope equipped with a 20× objective. Fluorescent intensity of staining of megakaryocytes was analyzed using Metamorph v7.63 software (Molecular Devices).
Retroviral transduction and transfection
Meg-01 cells expressing constitutively active (Q61L) or dominant-negative (T17N) Rac1 constructs (kind gifts from Dr Kathy Jastrzebski, Peter MacCallum Cancer Centre, Melbourne, Australia) were engineered by retroviral transduction. Retrovirus-containing supernatant was produced by transfecting 293T packaging cells with murine stem cell virus, internal ribosome entry site Cherry-red and an amphotropic helper plasmid using standard calcium phosphate–transfection methods. Viral supernatant was used to transduce Meg-01 cells. Seventy-two hours after transduction, Cherry-red–positive cells were isolated by flow cytometry–mediated cell sorting and cultured. After expansion of cells, Western blotting for pMLC was performed as described in “Western blot analysis” on cells that were at least 90% Cherry-red positive.
Results
HDACI-induced TCP in C57BL/6 mice
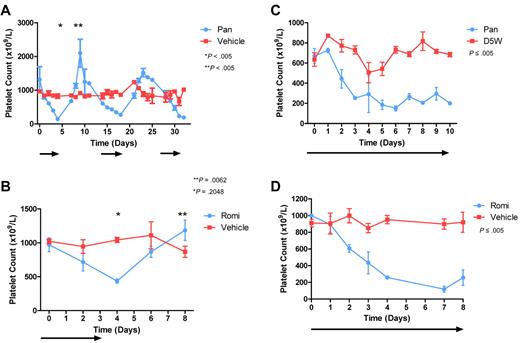
We have previously demonstrated that both panobinostat and romidepsin can be delivered to C57BL/6 mice at doses capable of inducing tumor-cell apoptosis, mediating a positive therapeutic effect.29,30 Treatment of C57BL/6 mice with panobinostat on an intermittent dosing schedule resulted in a reduction in platelet number, with a platelet nadir occurring around day 5 after commencement of treatment (Figure 1A). Cessation of panobinostat treatment resulted in a marked rebound thrombocytosis, with counts eventually normalizing after 1 week (Figure 1A). Repeat administration and cessation of panobinostat beyond this point caused an identical pattern of TCP and rebound thrombocytosis (Figure 1A). Treatment of C57BL/6 mice with romidepsin for 4 continuous days also resulted in TCP that resolved after cessation of treatment and, as with panobinostat treatment, rebound thrombocytosis was observed once treatment was discontinued (Figure 1B). Continued treatment of mice with either panobinostat or romidepsin resulted in sustained TCP and a plateau in platelet number (Figure 1C-D). The highest tolerated dose of each HDACI reduced platelet numbers to approximately 25% of normal, with higher doses causing excessive nonhematologic toxicity. Splenectomy was performed on C57BL/6 mice and, after a 1-month recovery period, the experiment was repeated on the splenectomized mice to exclude platelet pooling in the spleen as the cause of platelet count reduction (data not shown).
HDACIs reduce platelet number in mice. Cohorts of C57BL/6 mice were treated with (A) alternate weekly daily IP panobinostat 10 mg/kg (Pan) or vehicle or (B) 4 days of romidepsin 1 mg/kg (Romi) or vehicle (treatment schedules indicated by arrows). At the indicated time points, platelet counts were taken from 3 mice in each group. Platelet number in each HDACI-treated group decreased with treatment, and on drug cessation a rebound thrombocytosis occurred. Error bars represent SEM. Results were compared using the 2-tailed unpaired t tests. Cohorts of C57BL/6 mice were treated with (C) continuous daily IP panobinostat 10 mg/kg or vehicle or (D) continuous daily IP romidepsin 1 mg/kg or vehicle (treatment schedules indicated by arrows). At the indicated time points, platelet counts were taken from 3 mice in each group. Platelet number in each HDACI-treated group decreased with treatment and with continued dosing remained in a plateau. Error bars represent standard error of the mean. Differences in groups were calculated using linear regression analysis.
HDACIs reduce platelet number in mice. Cohorts of C57BL/6 mice were treated with (A) alternate weekly daily IP panobinostat 10 mg/kg (Pan) or vehicle or (B) 4 days of romidepsin 1 mg/kg (Romi) or vehicle (treatment schedules indicated by arrows). At the indicated time points, platelet counts were taken from 3 mice in each group. Platelet number in each HDACI-treated group decreased with treatment, and on drug cessation a rebound thrombocytosis occurred. Error bars represent SEM. Results were compared using the 2-tailed unpaired t tests. Cohorts of C57BL/6 mice were treated with (C) continuous daily IP panobinostat 10 mg/kg or vehicle or (D) continuous daily IP romidepsin 1 mg/kg or vehicle (treatment schedules indicated by arrows). At the indicated time points, platelet counts were taken from 3 mice in each group. Platelet number in each HDACI-treated group decreased with treatment and with continued dosing remained in a plateau. Error bars represent standard error of the mean. Differences in groups were calculated using linear regression analysis.
HDACI-induced TCP is due to a reduction in platelet production and not to direct platelet apoptosis
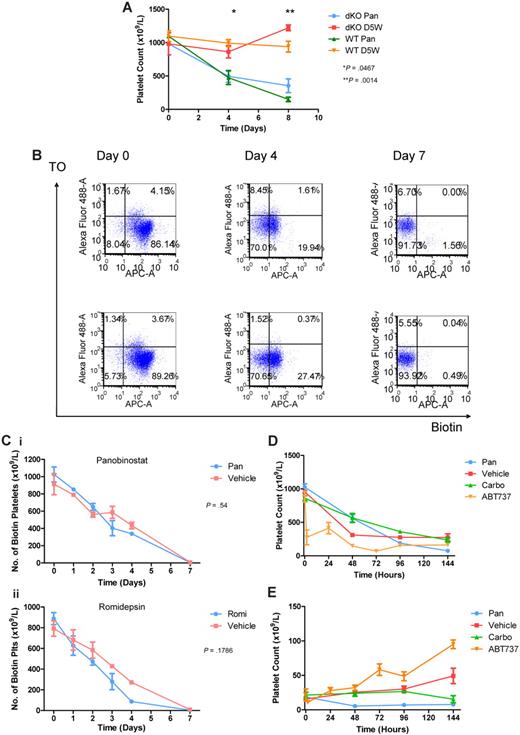
Platelet survival in the circulation has recently been shown to depend on the Bcl-2 family protein Bcl-xL and its ability to restrain the apoptotic program that circumscribes platelet life span.18 Pharmacologic inhibition of Bcl-xL with a BH3-mimetic compound triggers platelet apoptosis and TCP.18,20 This can be ameliorated by genetic deletion of the killer proteins Bak and Bax. Platelets lacking Bak and Bax are resistant to ABT-737, and Bak−/−Bax+/− mice do not develop TCP when treated with the drug.18,28 HDACI can increase the expression of proapoptotic genes, including Bak, and decrease the expression of prosurvival genes such as Bcl-xL.5 We therefore examined whether HDACIs induce TCP by directly activating platelet apoptosis. Lethally irradiated C57BL/6 mice were injected with wild-type or Bax−/−Bak−/− fetal liver cells, and 8 weeks after reconstitution were treated with panobinostat. HDACI administration caused TCP with similar kinetics and platelet nadir in mice reconstituted with wild-type or Bak−/−Bax−/− cells (Figure 2A).
HDACIs have no effect on platelet apoptosis or half-life. (A) Cohorts of C57BL/6 Bax−/−Bak−/− (dKO) and wild-type (WT) mice were treated with daily IP panobinostat 10 mg/kg (Pan) or vehicle. At the indicated time points, platelet counts were taken from 3 mice in each group. Platelet number in both HDACI-treated groups decreased with treatment, indicating that TCP is independent of apoptosis. Error bars represent SEM. P values reflect results between Bax−/−Bak−/− mice treated with panobinostat and vehicle at the indicated time points using 2-tailed unpaired t tests. (B) Flow cytometric analysis of circulating platelets for surface biotin and thiazole orange in C57BL/6 mice. Cohorts of mice were injected with 600μg of NHS-biotin and then treated with either daily IP panobinostat 10 mg/kg (top row) or vehicle (bottom row). Platelets were gated by side and forward scatter and CD41 staining. Platelets were then analyzed for uptake of thiazole orange (Alexa Fluor 488) and surface biotin (APC-A), allowing the calculation of biotinylated platelet number and new platelet fractions. (C) Mice injected with NHS-biotin and treated with (i) panobinostat 10 mg/kg or vehicle or (ii) romidepsin 1 mg/kg (Romi) IP daily or vehicle underwent flow cytometric analysis as in panel B, and also had a matched, automated full blood examination. The absolute biotinylated platelet count for each mouse was calculated and plotted. Error bars represent SEM. Results were compared using linear regression analysis. (D) C57BL/6 mice injected with NHS-biotin and treated with daily IP panobinostat 10 mg/kg or vehicle, a carboplatin 100 mg/kg single dose, or daily IP ABT-737 75 mg/kg underwent flow cytometric analysis as in panel B, and also had a matched, automated full blood examination taken. The absolute biotinylated platelet count for each mouse was calculated and plotted. Error bars represent SEM. (E) C57BL/6 mice injected with NHS-biotin and treated with daily IP panobinostat 10 mg/kg or vehicle, a carboplatin 100 mg/kg single dose, or daily IP ABT-737 75 mg/kg underwent flow cytometric analysis as above and also had a matched, automated full blood examination taken. The nonbiotinylated reticulated platelet fraction was calculated and plotted for each mouse. Error bars represent SEM.
HDACIs have no effect on platelet apoptosis or half-life. (A) Cohorts of C57BL/6 Bax−/−Bak−/− (dKO) and wild-type (WT) mice were treated with daily IP panobinostat 10 mg/kg (Pan) or vehicle. At the indicated time points, platelet counts were taken from 3 mice in each group. Platelet number in both HDACI-treated groups decreased with treatment, indicating that TCP is independent of apoptosis. Error bars represent SEM. P values reflect results between Bax−/−Bak−/− mice treated with panobinostat and vehicle at the indicated time points using 2-tailed unpaired t tests. (B) Flow cytometric analysis of circulating platelets for surface biotin and thiazole orange in C57BL/6 mice. Cohorts of mice were injected with 600μg of NHS-biotin and then treated with either daily IP panobinostat 10 mg/kg (top row) or vehicle (bottom row). Platelets were gated by side and forward scatter and CD41 staining. Platelets were then analyzed for uptake of thiazole orange (Alexa Fluor 488) and surface biotin (APC-A), allowing the calculation of biotinylated platelet number and new platelet fractions. (C) Mice injected with NHS-biotin and treated with (i) panobinostat 10 mg/kg or vehicle or (ii) romidepsin 1 mg/kg (Romi) IP daily or vehicle underwent flow cytometric analysis as in panel B, and also had a matched, automated full blood examination. The absolute biotinylated platelet count for each mouse was calculated and plotted. Error bars represent SEM. Results were compared using linear regression analysis. (D) C57BL/6 mice injected with NHS-biotin and treated with daily IP panobinostat 10 mg/kg or vehicle, a carboplatin 100 mg/kg single dose, or daily IP ABT-737 75 mg/kg underwent flow cytometric analysis as in panel B, and also had a matched, automated full blood examination taken. The absolute biotinylated platelet count for each mouse was calculated and plotted. Error bars represent SEM. (E) C57BL/6 mice injected with NHS-biotin and treated with daily IP panobinostat 10 mg/kg or vehicle, a carboplatin 100 mg/kg single dose, or daily IP ABT-737 75 mg/kg underwent flow cytometric analysis as above and also had a matched, automated full blood examination taken. The nonbiotinylated reticulated platelet fraction was calculated and plotted for each mouse. Error bars represent SEM.
To further confirm that direct platelet apoptosis was not the cause of HDACI-induced TCP, we assessed the murine platelet life span in mice treated with HDACIs. To assess the life span of platelets, we injected mice with intravenous NHS-biotin, allowing the biotinylation of circulating platelets. These mice were then treated for 7 days with panobinostat 10 mg/kg IP, romidepsin 1 mg/kg IP daily, or vehicle alone, and the number of biotinylated platelets in the peripheral blood compared with vehicle-treated mice was determined. The decrease in labeled platelets over time remained similar to vehicle-treated mice compared with panobinostat- or romidepsin-treated mice (Figures 2B-C). In contrast, and consistent with a previous report,18 the number of biotinylated platelets was rapidly reduced after treatment of mice with ABT-737, with a 50% reduction in platelet number seen 2 hours after administration of the compound (Figure 2D). Carboplatin, a chemotherapeutic agent capable of inducing TCP by megakaryocyte ablation,31 did not affect platelet life span (Figure 2D). These data indicate that changes in platelet life span are unlikely to explain HDACI-induced TCP.
To assess platelet production, C57BL/6 mice were treated with panobinostat, carboplatin, or ABT-737, and the RNA-containing platelet fraction (ie, new platelets) was determined by staining with thiazole orange (Figure 2E). The number of new, reticulated platelets in vehicle-treated mice remained relatively constant over the 6-day time span of the experiment. In contrast, treatment with panobinostat resulted in a dramatic decrease in the absolute number of reticulated platelets. The platelet count remained well below baseline levels throughout the course of the experiment. Consistent with previous experiments,18 ABT-737 caused a substantial increase in the production of new platelets as a compensatory response to the rapid induction of platelet apoptosis. Treatment of mice with carboplatin did not significantly affect new platelet production for the first 4 days of treatment; however, absolute reticulated platelet numbers were significantly reduced 6 days after the commencement of treatment. These data indicate that ABT-737, carboplatin, and panobinostat mediate TCP via different mechanisms. We hypothesized that HDACI-induced TCP was due to inadequate platelet production or poor platelet release from the megakaryocyte, rather than to myeloablation or direct platelet apoptosis, as was seen with carboplatin and ABT-737, respectively.
HDACIs cause megakaryocytic hyperplasia but reduce proplatelet numbers
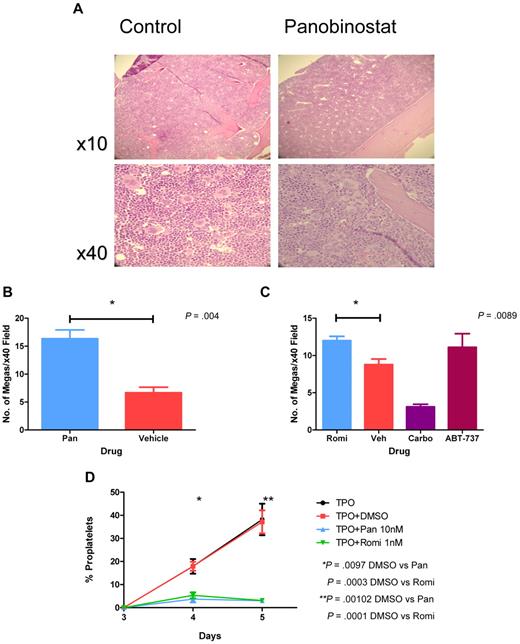
Examination of bone marrow sections of mice treated for 4 days with panobinostat or romidepsin revealed that both HDACIs induced a significant increase in megakaryocyte number, whereas carboplatin resulted in a significant reduction (Figure 3A-C). Megakaryocytic hyperplasia induced by HDACIs was associated with an increase in serum TPO levels (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). When panobinostat or romidepsin was administered to c-Mpl knockout mice lacking the TPO receptor,32 there was no increase in the number of megakaryocytes in the bone marrow. Furthermore although c-Mpl–knockout mice had the expected baseline platelet count of 10% of normal,32 HDACI treatment induced a further decrease in platelet numbers without a rebound in platelet numbers on drug cessation. (supplemental Figure 2A-B). These data indicate that HDACIs do not inhibit the production of megakaryocytes, and suggest instead that the process of platelet shedding by megakaryocytes is impaired. We therefore assessed the ability of primary mouse megakaryocytes to form proplatelets. Both panobinostat and romidepsin significantly inhibited proplatelet formation without an increase in megakaryocyte death, as determined by visual inspection, compared with vehicle controls (Figure 3D).
HDACIs increase megakaryocyte numbers but reduce proplatelet extensions. (A) Representative images of hematoxylin- and eosin-stained bone marrow from mice after treatment with daily IP panobinostat 10 mg/kg or vehicle for 4 days, demonstrating megakaryocytic hyperplasia in panobinostat-treated mice. (B) C57BL/6 mice were treated with daily IP panobinostat 10 mg/kg (Pan) or vehicle for 4 days. Bone marrow was harvested for histology, and the number of megakaryocytes seen in at least 10 × 40 high-powered microscopy fields were counted and plotted. Error bars represent SEM. Results were compared using the 2-tailed unpaired t test. (C) C57BL/6 mice were treated with daily IP romidepsin 1 mg/kg (Romi) or vehicle for 4 days, daily IP ABT-737 75 mg/kg for 4 days, or a carboplatin 100 mg/kg single dose IP on day 2. Shown are bone marrow megakaryocyte numbers in mice treated with daily IP romidepsin 1 mg/kg or vehicle, a carboplatin 100 mg/kg single dose, or daily IP ABT-737 75 mg/kg. Bone marrow was harvested for histology and the number of megakaryocytes seen in at least 10 × 40 high-powered microscopy fields were counted and plotted. Error bars represent standard error of the mean. Results were compared using the 2-tailed unpaired t test. (D) Murine fetal liver cells were cultured with 100 ng/mL of TPO for 3 days, after which time large megakaryocytes were collected using a 3%/1.5% BSA gradient. Megakaryocytes were plated out with 100 ng/mL of TPO alone or in the presence of 10nM panobinostat, 1nM romidepsin, or dimethyl sulfoxide in triplicate. The proportion of megakaryocytes elaborating proplatelets after treatment was counted for the next 3 days, with at least 500 cells counted for each sample. Error bars represent SEM. Results were compared using the 2-tailed unpaired t test.
HDACIs increase megakaryocyte numbers but reduce proplatelet extensions. (A) Representative images of hematoxylin- and eosin-stained bone marrow from mice after treatment with daily IP panobinostat 10 mg/kg or vehicle for 4 days, demonstrating megakaryocytic hyperplasia in panobinostat-treated mice. (B) C57BL/6 mice were treated with daily IP panobinostat 10 mg/kg (Pan) or vehicle for 4 days. Bone marrow was harvested for histology, and the number of megakaryocytes seen in at least 10 × 40 high-powered microscopy fields were counted and plotted. Error bars represent SEM. Results were compared using the 2-tailed unpaired t test. (C) C57BL/6 mice were treated with daily IP romidepsin 1 mg/kg (Romi) or vehicle for 4 days, daily IP ABT-737 75 mg/kg for 4 days, or a carboplatin 100 mg/kg single dose IP on day 2. Shown are bone marrow megakaryocyte numbers in mice treated with daily IP romidepsin 1 mg/kg or vehicle, a carboplatin 100 mg/kg single dose, or daily IP ABT-737 75 mg/kg. Bone marrow was harvested for histology and the number of megakaryocytes seen in at least 10 × 40 high-powered microscopy fields were counted and plotted. Error bars represent standard error of the mean. Results were compared using the 2-tailed unpaired t test. (D) Murine fetal liver cells were cultured with 100 ng/mL of TPO for 3 days, after which time large megakaryocytes were collected using a 3%/1.5% BSA gradient. Megakaryocytes were plated out with 100 ng/mL of TPO alone or in the presence of 10nM panobinostat, 1nM romidepsin, or dimethyl sulfoxide in triplicate. The proportion of megakaryocytes elaborating proplatelets after treatment was counted for the next 3 days, with at least 500 cells counted for each sample. Error bars represent SEM. Results were compared using the 2-tailed unpaired t test.
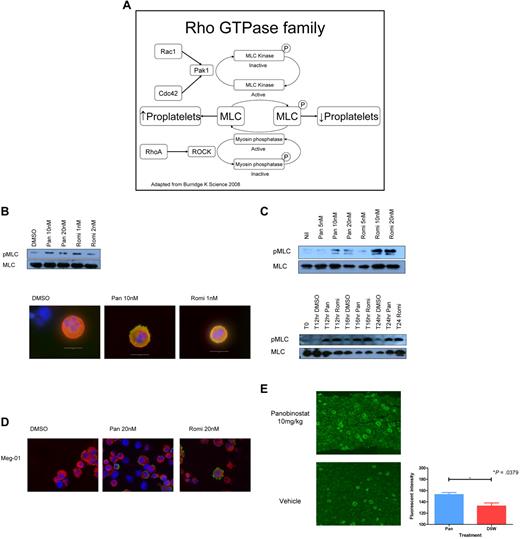
HDACIs increase phosphorylation of the regulatory light chain of myosin IIb and decrease expression of Rho/Rac family members
Recent reports have suggested that proplatelet formation may be inhibited by increased phosphorylation of the regulatory light chain of MLC IIb. This effect is thought to be regulated by RhoA-mediated activation of ROCK, which is thought to cause both direct phosphorylation and inhibition of MLC phosphatase.33 In other cell types, the Rho-GTPase family members CDC42 and Rac1 are thought to oppose this by inducing the activation of the serine/threonine kinase PAK1, which inhibits MLC kinase, with a subsequent decrease in pMLC levels (Figure 4A). Fetal liver–derived megakaryocytes treated for 48 hours with panobinostat or romidepsin showed an increase in pMLC as assessed by Western blot and immunofluorescence (Figure 4B). Given these results, we investigated the response of the human megakaryoblastic cell line Meg-01 to panobinostat and romidepsin. Both drugs were able to induce a dose- and time-dependent increase in pMLC, with HDACI-induced phosphorylation of MLC seen as little as 12 hours after exposure to the compounds (Figure 4C). Hyperphosphorylation of MLC after HDACI treatment of Meg-01 cells was also observed by immunofluorescence (Figure 4D). Finally, we demonstrated that this phenomenon occurs in an in vivo setting using immunohistochemistry to stain bone marrow trephines of mice. Specific staining of pMLC increased in the megakaryocytes of mice treated with HDACIs (Figure 4E)
HDACIs increase MLC phosphorylation in megakaryocytes. (A) Schema of the Rho/Rac/CDC42 pathway. (B) Western blot performed on fetal liver cell–derived megakaryocytes treated for 48 hours with increasing doses of panobinostat (Pan) and romidepsin (Romi) showing an increase in pMLC. Immunofluorescent staining performed on fetal liver cell–derived megakaryocytes treated for 48 hours with increasing doses of panobinostat and romidepsin demonstrating an increase in pMLC. Green indicates pMLC, red is tubulin, and blue is the nucleus stained with DAPI. (C) Western blot performed on Meg-01 cells treated for 24 hours with increasing doses of panobinostat and romidepsin showing an increase in pMLC levels, and on Meg-01 cells treated at 0, 12, 16, and 24 hours with 20nM panobinostat and 20nM romidepsin, showing increased pMLC from 12 hours on. (D) Immunofluorescent staining performed on Meg-01 cells treated for 24 hours with increasing doses of panobinostat and romidepsin, demonstrating an increase in pMLC. Green indicates pMLC, red is tubulin, and blue is the nucleus stained with DAPI. (E) Immunohistochemical staining of trephine sections of mice treated with panobinostat 10 mg/kg or vehicle for pMLC. The quantification of the fluorescent intensity of staining of mice treated with panobinostat 10 mg/kg or vehicle for pMLC was calculated using Metamorph v7.63 software. Megakaryocytes were isolated using a threshold for intensity of staining and manual elimination of other staining cells. At least 5 × 20 fields were examined per trephine.
HDACIs increase MLC phosphorylation in megakaryocytes. (A) Schema of the Rho/Rac/CDC42 pathway. (B) Western blot performed on fetal liver cell–derived megakaryocytes treated for 48 hours with increasing doses of panobinostat (Pan) and romidepsin (Romi) showing an increase in pMLC. Immunofluorescent staining performed on fetal liver cell–derived megakaryocytes treated for 48 hours with increasing doses of panobinostat and romidepsin demonstrating an increase in pMLC. Green indicates pMLC, red is tubulin, and blue is the nucleus stained with DAPI. (C) Western blot performed on Meg-01 cells treated for 24 hours with increasing doses of panobinostat and romidepsin showing an increase in pMLC levels, and on Meg-01 cells treated at 0, 12, 16, and 24 hours with 20nM panobinostat and 20nM romidepsin, showing increased pMLC from 12 hours on. (D) Immunofluorescent staining performed on Meg-01 cells treated for 24 hours with increasing doses of panobinostat and romidepsin, demonstrating an increase in pMLC. Green indicates pMLC, red is tubulin, and blue is the nucleus stained with DAPI. (E) Immunohistochemical staining of trephine sections of mice treated with panobinostat 10 mg/kg or vehicle for pMLC. The quantification of the fluorescent intensity of staining of mice treated with panobinostat 10 mg/kg or vehicle for pMLC was calculated using Metamorph v7.63 software. Megakaryocytes were isolated using a threshold for intensity of staining and manual elimination of other staining cells. At least 5 × 20 fields were examined per trephine.
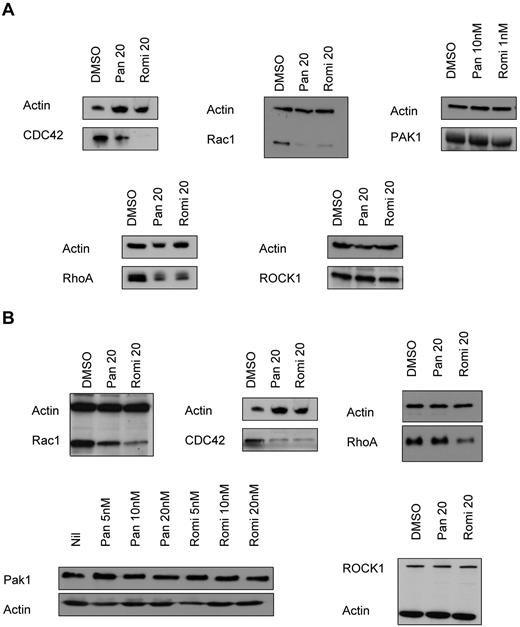
We next performed Western blot analyses to determine whether changes in expression or posttranslational modification of Rho/Rac family members might underlie the reduction in proplatelet extensions after HDACI treatment. Fetal liver–derived murine megakaryocytes treated with panobinostat or romidepsin showed a substantial decrease in the expression of Rac1 and CDC42 compared with vehicle-treated cells, but there was little or no change in the expression of PAK1. Both romidepsin and panobinostat caused a reduction in RhoA levels but no change in ROCK1 levels (Figure 5A). Consistent with the results seen in primary mouse megakaryocytes, treatment of Meg-01 cells with panobinostat or romidepsin resulted in a decrease in Rac1 and CDC42 but no change in expression of PAK1. Treatment of Meg-01 cells with romidepsin but not panobinostat decreased the expression of RhoA, whereas no changes in ROCK1 with either compound were observed (Figure 5B). These data demonstrate that treatment of mouse or human megakaryocytes with panobinostat or romidepsin consistently causes a reduction in Rac1, CDC42, and RhoA levels. In contrast, no changes in the Rac1/CDC42 substrate PAK1 nor the RhoA target protein ROCK1 were observed. We then went on to phenocopy these results using both pharmacologic and genetic approaches. The specific PAK1 inhibitor WR PAK18 induced an increase in pMLC levels (Figure 6A). The use of Meg-01 cells transduced with constitutively active and dominant-negative forms of Rac1 resulted in a reduction and elevation in pMLC levels, respectively, compared with the empty vector (Figure 6B). These results confirm that inhibition of PAK1 by HDACIs is important to pMLC status, and that the reduction in Rac1 levels may play an important role in HDACI-induced TCP.
HDACIs reduce the levels of Rho-GTPase family members in murine fetal liver–derived megakaryocytes and the human megakaryoblastic cell line Meg-01. (A) Western blot performed on fetal liver cell–derived megakaryocytes treated for 48 hours with 10nM panobinostat (Pan) and 1nM romidepsin (Romi) showing a reduction in CDC42, Rac1, and RhoA, but no change in PAK1 or ROCK1 levels. (B) Western blot performed on Meg-01 cells treated for 24 hours with 20nM panobinostat and 20nM romidepsin showing a reduction in CDC42, Rac1, and RhoA, but no change in PAK1 or ROCK1 levels.
HDACIs reduce the levels of Rho-GTPase family members in murine fetal liver–derived megakaryocytes and the human megakaryoblastic cell line Meg-01. (A) Western blot performed on fetal liver cell–derived megakaryocytes treated for 48 hours with 10nM panobinostat (Pan) and 1nM romidepsin (Romi) showing a reduction in CDC42, Rac1, and RhoA, but no change in PAK1 or ROCK1 levels. (B) Western blot performed on Meg-01 cells treated for 24 hours with 20nM panobinostat and 20nM romidepsin showing a reduction in CDC42, Rac1, and RhoA, but no change in PAK1 or ROCK1 levels.
Pak1 and RAC1 inhibition phenocopy the effects of HDACIs on pMLC levels. (A) Western blot performed on Meg-01 cells treated for 24 hours with 10μM of the PAK1 inhibitor WR PAK18 showing an increase in pMLC levels. (B) Western blot performed on Meg-01 cells transduced with a constitutively activate (CA Rac1) or dominant-negative (DN Rac1) Rac1, showing a decrease and increase, respectively, in pMLC levels compared with empty vector (murine stem cell virus [MSCV]) alone.
Pak1 and RAC1 inhibition phenocopy the effects of HDACIs on pMLC levels. (A) Western blot performed on Meg-01 cells treated for 24 hours with 10μM of the PAK1 inhibitor WR PAK18 showing an increase in pMLC levels. (B) Western blot performed on Meg-01 cells transduced with a constitutively activate (CA Rac1) or dominant-negative (DN Rac1) Rac1, showing a decrease and increase, respectively, in pMLC levels compared with empty vector (murine stem cell virus [MSCV]) alone.
HDACI-induced TCP in mice can be ameliorated by a TPO mimetic
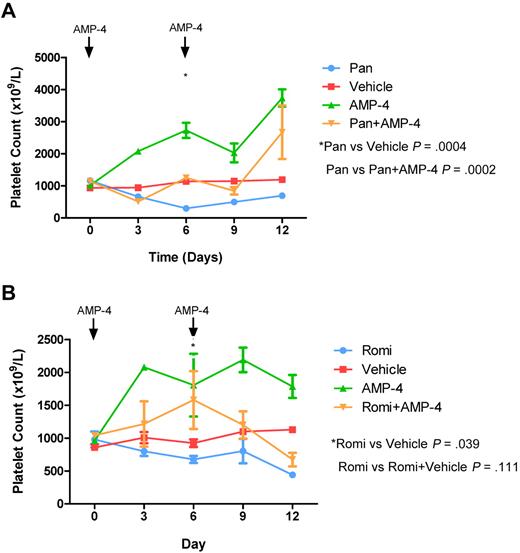
AMP-4 is a TPO mimetic identical to romiplostim except for the presence of a murine rather than a human Fc receptor. In wild-type C57BL/6 mice, a single IP dose of AMP-4 resulted in a marked thrombocytosis that peaked at 4-5 days and normalized by 9-10 days (supplemental Figure 3). Consistent with our previous data, treatment of mice with panobinostat caused sustained TCP over a 12-day period, and this effect could be ameliorated by cotreatment with AMP-4 at day 0 and day 6 (Figure 7A). Similarly, cotreatment of mice with AMP-4 circumvented romidepsin-induced TCP (Figure 7B). These data provide the first evidence of a treatment regimen that overcomes HDACI-induced TCP, a major dose-limiting toxicity for this class of anticancer therapy.
A TPO mimetic is effective in ameliorating HDACI-induced TCP. Cohorts of C57BL/6 mice were treated with (A) daily IP panobinostat (Pan) 10 mg/kg or (B) daily IP romidepsin (Romi) 1 mg/kg, with vehicle IP daily, AMP-4 20 μg/kg on days 3 and 6, or the combination of HDACI and AMP-4. At the indicated time points, platelet counts were assessed from 3 mice in each group. AMP-4 was able to ameliorate HDACI-induced TCP. Error bars represent SEM. Platelet counts of mice treated with HDACI alone and HDACI in combination with AMP-4 were compared using a 2-tailed unpaired t test.
A TPO mimetic is effective in ameliorating HDACI-induced TCP. Cohorts of C57BL/6 mice were treated with (A) daily IP panobinostat (Pan) 10 mg/kg or (B) daily IP romidepsin (Romi) 1 mg/kg, with vehicle IP daily, AMP-4 20 μg/kg on days 3 and 6, or the combination of HDACI and AMP-4. At the indicated time points, platelet counts were assessed from 3 mice in each group. AMP-4 was able to ameliorate HDACI-induced TCP. Error bars represent SEM. Platelet counts of mice treated with HDACI alone and HDACI in combination with AMP-4 were compared using a 2-tailed unpaired t test.
Discussion
The clinical utility of HDACIs has predominantly been as a single-agent therapy for relapsed/refractory hematologic malignancies.34 However, as the indications for these drugs become broader, it can be anticipated that they will be used earlier in the treatment paradigm and in combination with other effective agents, such as chemotherapeutics, monoclonal antibodies, and other small-molecule inhibitors, and indeed such strategies are already under way.14,35 TCP is the predominant hematologic toxicity of this class of drugs, and may compromise combination strategies. Given the broad spectrum of activity of these drugs against cancers, defining the pathophysiology behind and attempting to circumvent HDACI-induced TCP will likely allow for their increased utilization. In the present study, we have demonstrated that administration of either the pan-HDACI panobinostat or the class I–selective HDACI romidepsin in C57BL/6 mice induced a dose-dependent reduction in platelet number, recapitulating the human scenario. There was a significant increase in megakaryocyte number commensurate with treatment, and subsequent drug cessation resulted in a rebound thrombocytosis. The use of c-Mpl–knockout mice demonstrated that the megakaryocytosis and the rebound phenomenon were secondary to the increase in TPO levels that occurs on treatment. Our results are consistent with observations made by Giver et al, who reported similar reductions in platelet number with the administration of panobinostat to BALB/c mice, along with an increase in TPO levels and megakaryocyte number.36 Another group also reported that oral administration of a novel HDACI, FR235225, to Lewis rats over 1 week resulted in a dose-dependent TCP and an increase in megakaryocyte number.37 This group went on to demonstrate the effects of this and 2 other novel HDACIs not used in clinical practice on a human erythroleukemic cell line. Transcriptional repression and a decrease in protein expression of a key regulator of hematopoiesis, GATA-1, occurred, but not with all of the HDACIs used. This was postulated to potentially explain HDACI-induced TCP.37
We sought to identify the biologic and molecular processes that underlie HDACI-induced TCP. Given that HDACIs have been shown to alter the levels of the pro- and antiapoptotic genes Bak and Bcl-xL, respectively,5 and given the known role of these proteins in regulating platelet life span,18,20 we assessed the effect of HDACIs on platelet life span. Using a platelet half-life assay to assess existing (old) platelets in conjunction with a reticulated platelet assay to analyze new platelets, we demonstrated that HDACIs did not shorten platelet life span but did reduce the production of new platelets. We confirmed that HDACIs did not affect the levels of Bcl-xL or Bak in platelets harvested from treated mice by Western blot (data not shown).
The effect of HDACIs on platelet production was analyzed using primary mouse megakaryocytes that demonstrated decreased proplatelet production after HDACI treatment. This decrease in proplatelet formation was associated with an increase in pMLC, a result also seen in a human megakaryoblastic cell line. MLC phosphorylation is required for dynamic actin and myosin to cause contractile forces for cellular processes, including cytokinesis and cell migration, and was reported to be associated with decreased proplatelet formation in human megakaryocytes.33 MLC phosphorylation is regulated by Rho via its effector kinase ROCK by directly causing phosphorylation and inhibiting myosin phosphatase.38,39 MLC phosphorylation is also regulated by MLC kinase.40,41 Others have demonstrated that cell spreading and adhesion of BHK-21 cells is due to PAK1 being able to phosphorylate MLC kinase and consequently inhibit its activity.42 Another study has shown romidepsin to specifically reduce the kinase activity of PAK1 in breast cancer cells without reducing the total protein level.43 Given this background, and with evidence of increased pMLC occurring with HDACI treatment after 12 hours in our models, we investigated the Rho family as the likely prime drivers behind HDACI-induced TCP.
Rho GTPases switch from active GTP-bound forms to inactive GDP-bound forms, a state regulated by guanine nucleotide exchange factors, GTP-ase activating proteins, and guanine nucleotide–dissociation inhibitors.44 Total PAK1 levels were unchanged with HDACI treatment, but total protein levels of CDC42 and Rac1 were reduced after 24 hours of treatment with panobinostat and romidepsin. We therefore posit a model whereby PAK1 activity is reduced after HDACI treatment because of down-regulation of CDC42 and Rac1 total protein levels, resulting in an increase in pMLC and reduced proplatelet formation. The reduction in RhoA levels that was seen after treatment with romidepsin would be expected to cause a corresponding decrease in pMLC by reducing ROCK-mediated MLC phosphorylation. However, we hypothesize that the sum of HDACI effects on the Rho/Rac/CDC42 pathway results in a new rheostat being set, with a net increase in pMLC and reduced proplatelet formation in megakaryocytes.
In addition to providing novel insight into the biologic and molecular process involved in HDACI-induced TCP, we used a TPO mimetic to overcome the dose-limiting toxicity of HDACIs in a mouse model. Such an approach could conceivably be applied in the clinical setting. Although somewhat counterintuitive, TPO mimetics have already been found to be useful in disease scenarios in which both TPO levels and megakaryocyte numbers are elevated, such as chronic idiopathic TCP. Therefore, despite the high TPO levels seen after HDACI therapy, the use of supraphysiologic levels may be a rational way to overcome HDACI-induced TCP.
Both isotype-selective and pan-HDACIs were able to mediate near-identical effects on platelet production, increase pMLC, and reduce Rho-family proteins. This implies that class I HDACs are responsible for the effects on Rho-family kinase levels, either by a transcriptionally mediated event or, less likely, by posttranslational modifications of cytoplasmic proteins. Our work may also explain the TCP and megakaryocytic dysplasia associated with the widely used antiepileptic drug valproic acid, which is also known to function as an HDACI. The implications of our work extend beyond TCP alone. Increased pMLC levels in the smooth muscle of the gut may account for other prominent side effects seen with this class of drugs, such as diarrhea. The Rho family of proteins are ubiquitous, crucial to cellular migration and to pseudopodial and filopodial formation in both normal and malignant cells, and are implicated in metastasis,45 an area in which HDACIs may prove useful. One could also provide further rationale for the use of HDACIs for tumors particularly reliant on the Rho/Rac/CDC42/PAK1 pathways, such as neurofibromatosis.46
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Novartis, Celgene, Abbott Laboratories, and Amgen for supplying panobinostat, romidepsin, ABT-737, and AMP-4, respectively.
This work was supported by the National Health and Medical Research Council of Australia (fellowships to R.W.J., C.J., and B.T.K. and program and project grants 516725 and 575535), by the Australian Rotary Health, by the Sylvia and Charles Viertel Foundation (fellowship to B.T.K.), by the Leukemia & Lymphoma Society (fellowship to E.C.J.), and by Melbourne University.
Authorship
Contribution: M.J.B., B.P.M., N.M., C.J., E.C.J., and K.J.H. conducted the experiments and analyzed the data; and M.J.B., S.J.H., B.T.K., H.M.P., and R.W.J. designed the research and wrote the paper.
Conflict-of-interest disclosure: S.J.H. has received research funding from Novartis and Cellgene; H.M.P. has been on advisory boards for and received research grants from both Novartis and Cellgene; and R.W.J. has received research funding from Novartis. The remaining authors declare no competing financial interests.
Correspondence: H. Miles Prince, Department of Haematology, Peter MacCallum Cancer Centre, St Andrews Pl, East Melbourne, 3002, Victoria, Australia; e-mail: miles.prince@petermac.org; or Ricky W. Johnstone, Cancer Therapeutics Program, Gene Regulation Laboratory, St Andrews Pl, East Melbourne, 3002, Victoria, Australia; e-mail: ricky.johnstone@petermac.org.

![Figure 6. Pak1 and RAC1 inhibition phenocopy the effects of HDACIs on pMLC levels. (A) Western blot performed on Meg-01 cells treated for 24 hours with 10μM of the PAK1 inhibitor WR PAK18 showing an increase in pMLC levels. (B) Western blot performed on Meg-01 cells transduced with a constitutively activate (CA Rac1) or dominant-negative (DN Rac1) Rac1, showing a decrease and increase, respectively, in pMLC levels compared with empty vector (murine stem cell virus [MSCV]) alone.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/13/10.1182_blood-2010-11-318055/4/m_zh89991168620006.jpeg?Expires=1770030522&Signature=KNb8s9zaxrg9jMLu8ANpIwAmRsVFGKB6l1scZ3TsjUuSDnzLMSw7H50GKmu10feg03w4E1TuPAyDLXsolsKEA6rdTdIxJZWsL9vCgvBdBwad0s8ZW~KZgLDkrQcIudqGjJceMLohIefAyMLejn87q0ne2bxtvcxWsCE2yGFRUdhFrl9IZIKCCn2e9EpzxJrAjzM26vBzHpHuIj9F8d5qicoVUekz6n3IusjXTojc9MvsMbpUSug~wfebsXa8lVtdaGbZrQ1dJkWg6abnjgzP1wwgYdcbH4ZxWdJfn5zEkL5b0-HysFK0hHcIc8sc9rbOj2ig25g7hWusOIg6ckG-eA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)