Abstract
Abstract 234
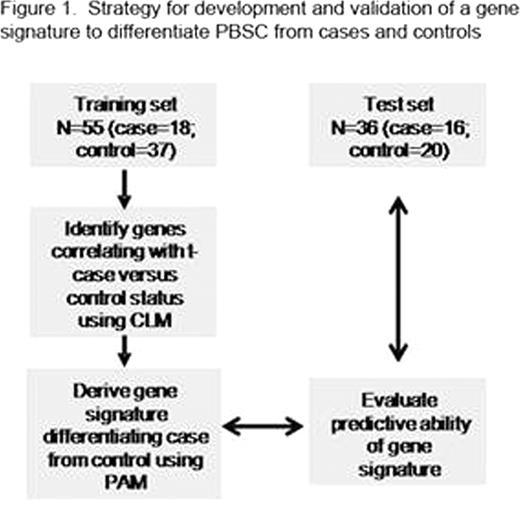
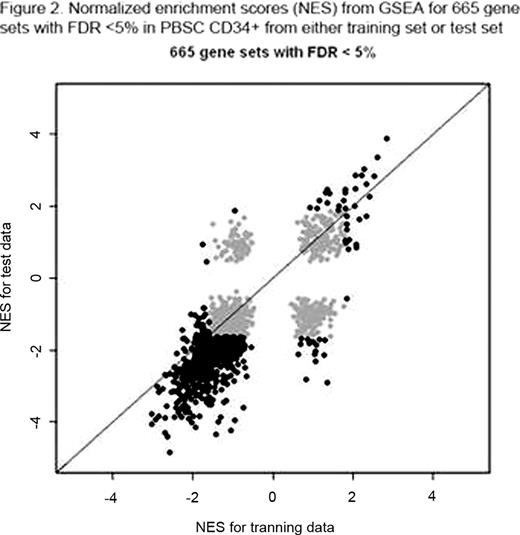
Therapy-related myelodysplasia or acute myeloid leukemia (t-MDS/AML) is a lethal complication of cancer treatment. Study of t-MDS/AML offers a unique opportunity to understand leukemogenesis since known genotoxic exposures can be temporally and causally related to genetic changes associated with development of leukemia. Although development of t-MDS/AML is associated with known genotoxic exposures, its pathogenesis is not well understood, and methods to predict risk of development of t-MDS/AML in individual cancer survivors are not available. To better understand the pathogenetic mechanisms underlying development of t-MDS/AML we performed microarray analysis of gene expression in patients who developed t-MDS/AML after autologous hematopoietic cell transplantation (aHCT) for Hodgkin lymphoma (HL) or non-Hodgkin lymphoma (NHL) and controls that did not develop t-MDS/AML after aHCT. Peripheral blood stem cell (PBSC) samples obtained pre-aHCT from patients who subsequently developed t-MDS post-aHCT (cases) and controls matched for primary diagnosis, age, race/ethnicity, and time since aHCT were studied. In a training set of 18 t-MDS/AML cases and 37 controls, CD34+ cells were selected from PBSC samples using flow cytometry, and gene expression evaluated using Affymetrix HG U133 plus 2.0 Arrays. Differences in gene expression in CD34+ cells from cases and controls were analyzed using conditional logistic model. Significant differences in gene expression were seen in PBSC obtained pre-aHCT from patients who later developed t-MDS/AML compared to controls.(Blood, 2009; 114: 677) PBSC obtained pre-aHCT from patients who subsequently t-MDS/AML after aHCT showed significant downregulation of gene sets related to mitochondria and oxidative phosphorylation, ribosomes, aminoacyl-tRNA biosynthesis, amino acid metabolism, cell cycle regulation, and hematopoietic differentiation. G-protein coupled receptors, hematopoietic regulation, and cell adhesion related genes were upregulated in PBSC from cases. There was reduced expression of genes with binding motifs for the transcription factor NRF2, which regulates oxidative stress and drug detoxification. We then sought to identify a smaller PBSC gene signature that would identify NHL and HL patients at the pre-aHCT timepoint at high risk for developing t- MDS/AML after aHCT (Figure 1). A cross-validated 38-gene classifier was derived from the training set using prediction analysis of microarray (PAM). This gene classifier was applied to an independent test set of PBSC obtained pre-HCT from 16 patients who developed t-MDS/AML after aHCT for NHL or HL, and 20 matched controls that did not develop t-MDS/AML. Application of the 38-gene signature to the test set correctly classified 19 of the 20 subjects (95%) who did not subsequently develop t-MDS/AML, and 14 of the 16 subjects (87.5%) who did develop t-MDS/AML, with significant correlation between predicted and true disease status (P<0.001). These results indicate that the gene expression profile of hematopoietic cells pre-aHCT can identify patients at high risk for t-MDS/AML post-aHCT. GSEA analysis revealed extensive overlap of up and down-regulated gene sets in t-MDS/AML cases in the training and test sets (Figure 2). Gene expression changes related to mitochondria, metabolism, cell cycle regulation and hematopoietic progenitors that were observed in the training set were validated in the test set. These results indicate that genetic programs associated with t-MDS/AML are perturbed long before disease onset, and that PBSC gene signatures can accurately identify patients at high risk of developing this complication.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2010 by The American Society of Hematology
2010