Abstract
The stem cell source for autologous transplantation has shifted from bone marrow to peripheral blood (PB). We previously showed that relapse incidence in patients with acute myelocytic leukemia autografted in first remission (CR1) was greater with PB than bone marrow, and a poorer outcome was associated with a shorter CR1 to PB transplantation interval (≤ 80 days). Leukemic and normal progenitors are CD34+ and can be concomitantly mobilized; we assessed whether an association exists between the infused CD34+ cell dose and outcome. The infused CD34+ cell doses were available for 772 patients autografted more than 80 days after CR1 and were categorized by percentiles. We selected the highest quintile (> 7.16 × 106/kg) as the cutoff point. By multivariate analysis, relapse was more probable in patients who received the highest dose (hazard ratio = 1.48; 95% confidence interval, 1.12-1.95; P = .005), and leukemia-free survival was worse (hazard ratio = 0.72; 95% confidence interval, 0.55-0.93; P = .01). In conclusion, in patients autografted in first remission, relapse was higher and leukemia-free survival lower for those who received the highest CD34+ PB doses.
Introduction
Over the past 30 years, autologous hematopoietic stem cell transplantation (ASCT) has been widely used to consolidate remission in patients with acute myelocytic leukemia (AML).1-8 However, the modalities of ASCT have evolved in the past 15 years, and one of the most important modifications has been the transition of the stem cell source from bone marrow (BM) to peripheral blood (PB) obtained by leukaphereses.9-11 This shift has facilitated the procedure for both patients and medical personnel, and it has considerably reduced the cost of the procedure because of the accelerated engraftment. However, this shift may also have negative consequences, such as the recruitment of tumor cells in the graft by the mobilization procedure, which may increase relapse. Indeed, we recently determined that relapse was higher and leukemia-free survival (LFS) was lower in patients who received autografts from PB than in those who received BM autografts in a European Group for Bone and Marrow Transplantation (EBMT) survey.12 This finding was independent of the length of the interval from the first remission (CR1) to transplantation, but patients who received PB more than 80 days after CR1 responded significantly better than those transplanted earlier.
Current mobilization techniques used to prepare for leukapheresis and constitution of the PB autograft aim to collect the highest number of CD34+ cells with a minimum goal of more than 5 × 106/kg. This goal is to ensure safe engraftment and possibly reduce the incidence of nonrelapse mortality (NRM). However, leukemic as well as normal progenitors express the CD34+ antigen. A previous European Organisation for Research and Treatment of Cancer (EORTC) AML-10 study analyzed the stem cell mobilizing capacity in patients who received autografts in CR1 and reported a correlation with relapse.13
In this study, we investigated the impact of the infused dose of CD34+ PB cells on the treatment outcome in patients with AML using the EBMT Acute Leukemia Database. We selected patients who received transplantations more than 80 days after CR1 as our study population because relapse in those who received transplantations earlier (resulting from less in vivo purging) was previously reported to be unacceptably high.12
Methods
Patients and case reports
From January 1994 to December 2007, 4154 patients with de novo non-M3 AML who received PB autografts were registered in the EBMT database; however, extensive data (Med-B forms) were available for only 1631 patients. Of these patients, 1262 received transplantations more than 80 days after CR1, and the infused CD34+ cell doses (measured after thawing) were available for 772 patients. Data on the methods of counting CD34 were not reported by the transplantation centers.
The median age of the patient population was 47 years (range, 18-77 years), and median follow-up was 30 months (range, 1-168 months). The data were anonymous. The study was approved by the Acute Leukemia Working Party of the EBMT and followed EBMT study guidelines.
Endpoints
LFS was defined as the time from the transplantation to relapse or death, whichever occurs first. To evaluate the relapse incidence, patients who died either of direct toxicity resulting from the procedure or of any other cause not related to leukemia were censored. The NRM was defined as the time to nonrelapse death. Patients were censored either at the time of relapse or at the date of last contact. For all comparisons, all patients with a follow-up of more than 3 years after transplantation were censored at this time.
Statistics
Cumulative incidence curves were used for relapse and NRM in a competing risk setting because death and relapse were in competition. Probabilities of LFS were calculated using the Kaplan-Meier estimate, and the log-rank test was used for univariate comparisons. Variables related to patients, disease, and transplantations were compared using the χ2 test for categorical variables and the Kruskal-Wallis or median test for continuous variables. All P values were 2-sided with the type I error rate fixed at .05. Factors associated with a P value less than .20 by univariate analysis were included in the final model.
For all prognostic analyses, continuous variables were categorized; each variable was divided into 5 categories at approximately the 20th, 40th, 60th, and 80th percentiles. If the relative event rates (ratio of the observed number of events to the expected number of events in a category with the assumption of no variation across categories) in 2 or more adjacent categories (and mean times to the event) were not substantially different, the categories were merged. If a linear trend was observed in the relative event rates, the variable was used as a continuous factor. Otherwise, the median was used as a cutoff point.14 Patient-, disease-, and transplantation-related variables of both groups (according to the CD34 cell doses) were compared, using the χ2 statistic for categorical and the Mann-Whitney test for continuous variables. All P values are 2-sided with type I error rate fixed at .05.
Potential prognostic factors and factors differing in distribution between the 2 groups with a P value less than .05 were included in the final model. Associations of patient and graft characteristics with outcomes were evaluated using multivariate analyses with Cox proportional hazards and reported with hazard ratio (HR) and 95% confidence interval (CI). To test for the possible effect of the medical center on LFS, we introduced a random effect or frailty for each center into the model.15-17 Statistical analyses were performed using the SPSS Version 18 and Splus Version 8.1 software packages.
Results
Overall outcome
The 3-year LFS for the 772 patients with available CD34+ doses infused was 46% (± 2%), and this result was identical to the LFS for the overall population of 1262 patients who received transplantations more than 80 days after CR1. In these 772 patients, relapse occurred in 46% (± 2%), and NRM was 7.9% (± 1%).
Doses of infused CD34+ cells
Univariate analyses.
The doses of infused CD34+ cells were divided into 5 categories as described previously. For the 5 CD34+ cell dose groups, which were less than 2.5 × 106/kg, 2.5 to 3.46 × 106/kg, 3.46 to 4.7 × 106/kg, 4.7 to 7.16 × 106/kg, and more than 7.16 × 106/kg, 3-year LFS was 45% (± 5%), 53% (± 4%), 47% (± 4%), 49% (± 4%), and 35% (± 4%), respectively, and 3-year relapse was 46% (± 5%), 39% (± 4%), 45% (± 4%), 46% (± 4%), and 57% (± 4%), respectively.
The likelihood of the Cox model did not decrease significantly when the first, second, third, and fourth categories were successively merged. However, the likelihood of the Cox model did decrease significantly when the fifth category was merged. Thus, we identified the fifth percentile (> 7.16 × 106/kg) as the cutoff point for relapse. Table 1 provides the characteristics of patients with AML who received doses of CD34+ cells either more than or equal to 7.16 × 106/kg (referred to as the high-dose group) or less than 7.16 × 106/kg (the low-dose group). The high-dose group had significantly higher white blood cell counts at initial diagnosis and received transplantations a few days earlier. There were no other differences, and cytogenetic characteristics were evenly distributed between the 2 groups.
Table 2 describes the number of chemotherapy courses and mobilization procedures in the 2 groups. There were no differences in the number of patients reaching CR1 with 1 or more than 1 chemotherapy induction course or the number of chemotherapy consolidation courses given before PB collection. Regarding mobilization, almost all patients received granulocyte colony-stimulating factor (G-CSF), including 94% in the low-dose group and 96% in the high-dose group. The number of patients mobilized without G-CSF (chemotherapy only) was therefore too small to allow any comparisons. Among patients who received G-CSF, 52% received G-CSF alone, 19% received G-CSF plus cytosine arabinoside (Ara-C), and 29% received G-CSF plus other chemotherapy without Ara-C. These mobilization regimens were evenly distributed among the 2 groups (P = .39).
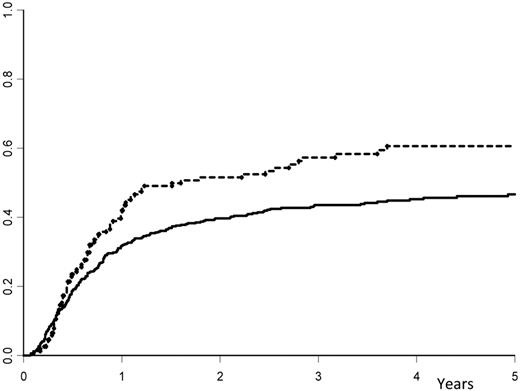
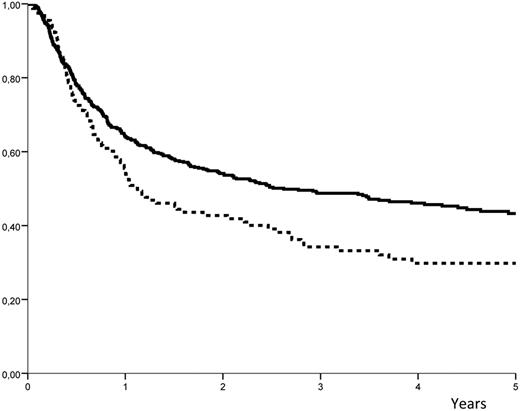
Relapse occurred in 57% (± 4%) of the high-dose group versus 44% plus or minus 2% in the low-dose group (P = .008; Figure 1). The LFS was 35% (± 4%) in the high-dose group versus 49% (± 2%) in the low-dose group (P = .007; Figure 2). The NRM was similar for the 2 groups, with 7.7% (± 1%) in the high-dose group versus 8.5% (± 2%) in the low-dose group (P = .85). When the same analyses were performed except that the infused cell doses were expressed in nucleated cells/kg, there was no association (and no linear trend) with the outcome. The LFS at 3 years was 42% (± 4%) in patients who received doses less than or equal to 7.5 × 108 cells/kg (median value) and 47% (± 3%) in those who received higher doses (P = .69).
Cumulative incidence of relapse. The cumulative occurrence of relapse after autologous hematopoietic stem cell transplantation with peripheral blood stem cells: solid line indicates dose of infused CD34+ cells ≤ 7.16 × 106 cells/kg; and hyphenated line, dose of infused CD34+ cells > 7.16 × 106 cells/kg.
Cumulative incidence of relapse. The cumulative occurrence of relapse after autologous hematopoietic stem cell transplantation with peripheral blood stem cells: solid line indicates dose of infused CD34+ cells ≤ 7.16 × 106 cells/kg; and hyphenated line, dose of infused CD34+ cells > 7.16 × 106 cells/kg.
Probability of LFS. The probability of leukemia-free survival (LFS) after ASCT with peripheral blood stem cells: solid line indicates dose of infused CD34+ cells ≤ 7.16 × 106 cells/kg; and hyphenated line, dose of infused CD34+ cells > 7.16 × 106 cells/kg.
Probability of LFS. The probability of leukemia-free survival (LFS) after ASCT with peripheral blood stem cells: solid line indicates dose of infused CD34+ cells ≤ 7.16 × 106 cells/kg; and hyphenated line, dose of infused CD34+ cells > 7.16 × 106 cells/kg.
Multivariate analysis.
Regarding the infused dose of CD34+ cells, using the Cox model adjusted for all potential prognostic factors and factors differing in the patient distribution according to CD34 cell doses, the HRs of the first 4 quintiles were 1.28, 1.42, 1.39, and 1.26 for LFS and 0.72, 0.63, 0.66, and 0.84 for relapse incidence using the fifth quintile as the reference group (HR = 1). Table 3 provides the results of the multivariate analyses used to identify independent prognostic factors The highest dose versus all others was associated with more relapses (HR = 1.46; 95% CI, 1.08-1.98, P = .013) and worse LFS (HR = 0.73; 95% CI, 0.55-0.96; P = .027). As expected, good cytogenetics were favorable with less relapses (HR = 0.35; 95% CI, 0.21-0.58; P = .001) and better LFS (HR = 2.86, 95% CI, 1.79-4.55, P < .001). Achievement of complete remission with more than 1 course of induction chemotherapy (slow remitters) was an adverse factor with more relapses (HR = 1.96; 95% CI, 1.37-2.79; P < .001), higher NRM (HR = 2.27; 95% CI, 1.07-4.82; P = .03), and worse LFS (HR = 0.50; 95% CI, 0.36-0.68; P < .001). The pretransplantation regimen (total body irradiation vs other modalities) was not associated with treatment outcomes. The number of consolidation courses before PB collection did not influence relapse, and LFS but was associated with increased NRM (HR = 1.96; 95% CI, 1.02-3.77; P = .04). None of the outcome parameters was influenced by the variable of medical center.
Discussion
ASCT was initially designed in the late 1970s to consolidate remission in patients with AML who had no compatible siblings or were too old for allogeneic transplantation.18 Until the mid-1990s, the only source of hematopoietic stem cells was BM. From 1982 until 1995, patients with AML in complete remission received autologous BM transplantation, and medical teams developed several techniques for purging the marrow autograft of residual tumor cells that could increase the risk of relapse. These techniques are referred to as “in vitro purging.”19 Many studies provided evidence in favor of in vitro purging of marrow; these studies included retrospective comparisons of patients who were autografted with purged and unpurged marrow.20-22 Additional studies demonstrated that infused leukemic clones may contribute to relapse.23 However, definitive proof that in vitro purging improved the clinical outcome was not established because there were no data from prospective randomized trials. Furthermore, autologous BM transplantation with cyclophosphamide derivative-mediated purging was associated with slow engraftment and required prolonged red blood cell and platelet transfusion support.22,24,25
Beginning in 1985, PB collected at the time of recovery from chemotherapy-induced aplasia was shown to contain a large number of hematopoietic progenitors that reconstituted hematopoiesis.26,27 This was true for PB that was mobilized with hematopoietic growth factors after chemotherapy or in steady state. PB rapidly became the predominant source for stem cells for a number of factors. The greatest advantage was that leukapheresis for PB stem cell collection was easier to perform than marrow collection, which required general anesthesia at that time. In addition, autologous transplantation with PB resulted in more rapid engraftment than transplantation with BM. Currently, PB is the preferred source of stem cells in more than 85% of the patients who are autografted for AML.10
The initial hope with PB was that the level of tumor contamination would be less than that with BM, or even potentially nonexistent. However, this has not been substantiated, and several early reports cautioned against an increased risk of relapse after PB infusion, suggesting that this modality was associated with mobilization of tumor cells and massive contamination of the autograft.28-30 This suggestion was supported by studies showing that AML progenitors expressed hemopoietic growth factor receptors on their surfaces31 and by mobilization studies in patients with solid tumors that reported concomitant mobilization of normal and tumor progenitors.32
In a previous EBMT-based study, we compared relapse and LFS in patients with AML in CR1 who received autografts from BM or PB.12 We initially observed that, among patients who received PB, those who received transplantations fewer than 80 days after entering remission had a worse outcome. To investigate this observation further, we compared 3 groups of patients: those who received BM autografts, those who received PB autografts early (≤ 80 days), and those who received PB autografts late (> 80 days). In a multivariate analysis that adjusted for differences between the groups and medical centers, relapse was more frequent for both the early PB group (56% ± 3%) and the late PB group (46% ± 2%), compared with the BM group (39% ± 2%). This translated into significantly worse LFS for the early PB group (36% ± 3%) and a trend toward worse LFS for the late PB group (46% ± 2%), compared with the BM group (52% ± 2%). We postulated that more frequent relapse and worse LFS might reflect the mobilization of tumor cells with PB and insufficient in vivo purging in those patients given transplantations earlier. Although our major conclusion was that BM should be the preferred stem cell source, we also suggested that late, rather than early, transplantation should be the rule for PB transplantation.
The design of the present study was based on those findings. Because leukemic as well as normal progenitors express the CD34+ antigen, we were interested in determining whether the infused dose of PB CD34+ cells affected the outcome. We selected patients who received late PB transplantations as our patient study population because early PB transplantation was associated with the highest relapse and is not recommended. Interestingly, comparing the doses of CD34+ cells infused in early and late PB transplantation, we determined that patients who received autografts early also received higher CD34+ cell doses than those who received transplantations later (data not shown).
Our study not unexpectedly confirmed the important impact of the 2 most recognized favorable prognostic factors, that is, good cytogenetics and achievement of CR1 with only 1 course of induction chemotherapy, thereby defining the so-called “rapid remitters.” Importantly, our results clearly indicate that the dose of CD34+ cells infused also affected the outcome. In patients with AML in CR1 who received PB autografts, the highest infused doses of CD34+ cells (ie, > 7.16 × 106/kg) were associated with more frequent relapse and worse LFS than lower doses of CD34+ cells. Interestingly, the number of consolidation courses before PB collection had no influence on relapse.
These observations are consistent with the results of a previous study from the EORTC group, which assessed the CD34+ cell-mobilizing capacity of G-CSF consolidation courses in 342 patients with AML in CR1 who received PB autografts as part of the AML-10 protocol. Patients with the highest mobilizing capacity had a worse prognosis because of more frequent relapse.13 In the EORTC study, the highest yield of CD34+ cells from a single apheresis was adopted as a surrogate marker for mobilizing capacity. The 342 patients were categorized into 4 groups: (1) no harvest receiving BM (n = 76), (2) low yield (< 1 × 106 CD34+/kg; n = 50), (3) intermediate yield (1-6.9 × 106 CD34+ cells/kg; n = 128), and (4) high yield (≥ 7 × 106 CD34+ cells/kg; n = 88). Interestingly, the high-yield groups in the EORTC study and in our study (> 7.16 × 106/kg) exhibited similar survival and relapse. The 3-year disease-free survival was 46.7% in the no-harvest group, 65.0% in the low-yield group, 50.4% in the intermediate-yield group, and 26.9% in the high-yield group (P < .001). Relapse occurred in 47.5% of the no-harvest group, 30.1% of the low-yield group, 43.1% in the intermediate-yield group, and 71.9% in the high-yield group (P < .001). A multivariate Cox proportional hazards model showed that the CD34+ yield was the most important independent prognostic variable (P = .005). The EORTC study differed from ours in that it focused on the leukapheresis yields (collections), whereas the present EBMT study takes into account the CD34+ cell doses actually infused into the patients and consists of a larger patient population from more contributing centers. One of the limitations of our study is that the methods of counting CD34 were not reported by the transplantation centers. A survey made in 1999 by the European Working Group on Clinical Cell Analysis has attempted to standardize CD34+ stem cell enumeration across 24 clinical sites. This survey33 showed that the use of a common standardized protocol and targeted training significantly reduced intralaboratory and interlaboratory CD34+ cell count variation. Therefore, the EBMT has established a quality assessment of hematopoietic stem cell grafts committee to assess the quality of hematopoietic stem cell preparations.34
In the past 20 years, several modifications have been made to the original procedures for autografting patients with AML, including: (1) the disappearance of in vitro marrow purging, which had been used by many medical teams; (2) the shift from BM to PB as a source of stem cells; (3) the use of G-CSF for mobilization despite the early concern that leukemic clones could also be stimulated; and (4) the use of chemotherapy only rather than total body irradiation during the pretransplantation regimen. From our previous study comparing BM with PB12 and the present study demonstrating the negative impact of infusing higher numbers of CD34+ cells, we conclude that new, more stringent guidelines should be established when autografting patients with AML with PB. There must be caution against performing early transplantations and using high CD34+ cell counts.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
Paper preparation and submission followed internal EBMT regulations.
Authorship
Contribution: N.-C.G., M.L., and V.R. designed the study; N.-C.G. wrote the paper; M.L. did the statistical work; N.-C.G., M.L., and V.R. prepared the last version for submission; and all authors contributed patients to the study, reviewed the manuscript, and made modifications.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the European Cooperative Group for Blood and Marrow Transplantation appears in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
The current affiliation for J.R. is CRLCC Institut Bergonié, Bordeaux, France.
Correspondence: Norbert-Claude Gorin, Hopital Saint-Antoine, 184 rue du Faubourg Saint-Antoine, 75012 Paris, France; e-mail: Norbert-claude.gorin@sat.aphp.fr.