Abstract
Antiphospholipid syndrome (APS) is an autoimmune disease characterized by arterial and venous thrombosis, recurrent abortions, and antiphospholipid antibodies (aPL). However, it is possible to find patients with clinical signs of APS who persistently test negative for aPL (seronegative APS, or SN-APS). The aim of this study was to identify new antigenic target(s) of autoantibodies in APS patients, which may also be recognized in SN-APS. We tested sera from patients with SN-APS with a proteomic approach by analyzing endothelial cell-surface membrane proteins. Sera from SN-APS patients revealed 2 reactive spots corresponding to vimentin, a protein that is shown to bind cardiolipin in vitro. Antivimentin/cardiolipin antibodies were tested in 29 SN-APS patients, 40 APS patients, 30 patients with systemic lupus erythematosus, 30 with rheumatoid arthritis, 30 with venous or arterial thrombosis, and 32 healthy control patients. We observed that not only a large proportion of SN-APS patients but also almost all the APS patients displayed the presence of antivimentin/cardiolipin antibodies. To verify the possible pathogenic role of these autoantibodies, we demonstrated that affinity-purified antivimentin/cardiolipin antibodies induced interleukin receptor-associated kinase phosphorylation and nuclear factor-κB activation in endothelial cells. Our results prompt to identify vimentin as a “new” cofactor for aPL, which may represent a useful tool mainly in SN-APS patients.
Introduction
Antiphospholipid syndrome (APS) is an autoimmune disease characterized by arterial and venous thrombosis, by recurrent abortions or fetal loss, and is associated with circulating antiphospholipid antibodies (aPLs).1,2 A diagnosis of APS requires the combination of at least one clinical and one laboratory criterion.3,4 Anticardiolipin (aCL) and anti-β2 glycoprotein-I (anti-β2GPI) antibodies, detected by the use of enzyme-linked immunosorbent assay (ELISA), and the lupus anticoagulant (LA), detected by clotting assays, are the recommended tests to test for aPLs.5,6 Indeed, aPLs represent a heterogeneous family of antibodies that react with serum phospholipid-binding plasma proteins, among which β2GPI represents the main protein cofactor.7,8 In addition, protein S,9,10 protein C,11 prothrombin,12 annexin V,13 or annexin II14 also have been demonstrated as antigenic targets for these autoantibodies.
Nevertheless, new antigenic targets for aPL in APS have been proposed. In particular, it has been described that antibodies directed to the lyso(bis)phosphatidic acid (ie, anti-LBPA) may represent a marker of APS.15,16 Moreover, we demonstrated the possibility of detecting aPL by immunostaining on thin-layer chromatography (TLC) plates.17
However, in daily clinical practice, it is possible to find patients with clinical signs suggestive of APS but who are persistently negative for the routinely used aCL, anti-β2GPI, and LA tests. Therefore, it was recently proposed for these cases the term “seronegative APS” (SN-APS).18,19 Although it is known that the routine screening tests (aCL and/or LA) might miss some cases, careful differential diagnosis and repeat testing are mandatory before the diagnosis of SN-APS can be made.6
The aim of this study was to identify possible new antigenic target(s) of autoantibodies in these SN-APS patients who presented clinical signs of APS but were repeatedly negative for the conventional used aCL, anti-β2GPI, and LA. Because aPLs are able to trigger a signal transduction pathway in endothelial cells, leading to interleukin receptor-associated kinase (IRAK) phosphorylation and nuclear factor kappa B (NF-κB) activation,20-22 our primary aim in this study was to seek and characterize endothelial molecules specifically recognized by serum autoantibodies in patients with APS and with the so-called SN-APS. Thus, we decided to use an autoantibody-based screening method, a proteomic approach that uses endothelial cell-surface membrane proteins, which represents a potent tool for the identification of target antigens.
By this approach, sera from SN-APS patients revealed 2 strongly reactive spots that we identified as vimentin, a protein that is shown to bind cardiolipin in vitro. Using ELISA, we analyzed the presence of serum autoantibodies specific to the complex vimentin/cardiolipin, and we observed that not only a large proportion of SN-APS patients, but also almost all the APS patients, displayed the presence of these antibodies. To verify the possible pathogenic role of these autoantibodies, we demonstrated that immunoglobulin G (IgG) specific for the vimentin/cardiolipin complex from the sera of SN-APS patients induced IRAK phosphorylation and NF-κB activation in endothelial cells.
Methods
Patients
This study included carefully selected 29 consecutive patients, 20 who visited the Lupus Clinic at Saint Thomas' Hospital of London and 9 who visited the Rheumatology Unit at “Sapienza” University of Rome. All the patients were women with multiple autoimmune manifestations. They presented clinical features that were consistent with a diagnosis of APS but who were negative (at least 2 times in samples taken 12 weeks apart) for conventional aCL, anti-β2GPI, and LA tests. All experiments involving patients were approved by the Istituto Superiore di Sanità ethical committee. Clinical manifestations included venous and/or arterial thrombosis and pregnancy morbidity as stated in the classification criteria to definite APS.4 All patients showed normal screening for other causes of thrombophilia, such as anti–thrombin III, protein C and protein S deficiency, hyperhomocysteinemia, factor V Leiden, and prothrombin mutations. For each patient, 2 serum samples taken at least 12 weeks apart were studied.
In addition, we analyzed 40 patients who were diagnosed according to the classification criteria as definitely having APS (ie, they were positive for aCL),1 Thirty patients with systemic lupus erythematosus (SLE), 30 patients with rheumatoid arthritis (RA), and 30 randomly patients with venous or arterial thrombosis. A group of 32 healthy white control patients matched for age and sex also were included in the study. After we obtained informed consent from each patient in accordance with the Declaration of Helsinki, we performed a venous blood draw. Sera were stored at −20°C until the assay.
ELISA for anti-β2GPI, anti–annexin V, and antiprothrombin
aCL and anti-β2GPI ELISA kits were obtained from Inova Diagnostics Inc. ELISA was performed according to manufacturer's instructions. Anti–annexin V and antiprothrombin were performed as previously described.12,13 A positive control and several normal human sera were run in the same assay to confirm the specificity of the results.
LA test
LA was studied in 2 coagulation systems, a dilute-sensitized activated partial thromboplastin time and a dilute Russell viper venom time, followed by a confirmation test, by the use of reagents and instrumentation from the Hemoliance Instrumentation Laboratory.
Proteomic assay of endothelial cell-surface membrane proteins
Endothelial cells (ie, immortalized hybridoma cell line EAhy92623 ) were grown to 60%-70% confluence and seeded at 5 × 106 well on glass coverslips. Cell-surface membrane proteins were purified from endothelial cells by use of the Pierce Cell Surface Protein Isolation Kit according to the manufacturer's instructions with slight modifications (Pierce). In brief, 1 × 107 cells were incubated in 1 mL of Sulfo-NHS-SS-Biotin, a cleavable biotinylation reagent. After the biotinylation step, we washed the cells twice with Tris-buffered saline. The cells were subjected to sonication, and the biotinylated proteins were incubated with Immobilized NeutrAvidin Gel (Pierce). After extensive lavage of the gel (9 times), the proteins were eluted according to the protocol and loaded into 2-dimensional electrophoresis (2DE). Isoelectrofocusing was performed on 7-cm immobilized pH gradient strips (range, pH 3-10) by use of the IPGphor Isoelectric Focusing System (Amersham Pharmacia Biotech). The second dimension was performed on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) system after equilibrating the strips for 10 minutes in SDS Equilibration buffer containing 50mM Tris/HCl (pH 8.8), 6M urea, 30% glycerol, 2% SDS, 2% dithiothreitol (DTT), and 2.5% iodoacetamide. Gels were then stained by colloidal Coomassie blue (Sigma-Aldrich) or used for Western blot analysis alternatively incubated with human sera diluted 1:50. Peroxidase-conjugated goat anti–human IgGs (Bio-Rad) were used as second antibodies, and the reactions were developed with 3-3′ diaminobenzidine (Sigma-Aldrich).
Colloidal Coomassie blue–stained spots corresponding to those identified by immunoblotting were excised from gels and destained by washing them twice with 50% acetonitrile (ACN) in 5mM ammonium bicarbonate. Mass spectrometry was performed by Nurex service (Nurex srl). Gel pieces were dried for 30 minutes in ACN. Dried pieces of gel were subjected to protein digestion by trypsin. Mass spectrometry analysis were performed with a matrix-assisted laser desorption/ionization (MALDI) micro MX (Micromass), equipped with a delayed extraction unit, according to the tuning procedures suggested by the manufacturer. Sample was loaded onto MALDI target by the use of 2 μL of the tryptic digest mixed 1:1 with a solution of α-cyano-4-hydroxycinnamic acid (10 mg/mL in 40% ACN, 0.1% vol/vol trifluoroacetic acid). A peak list was generated with Proteinlynx Data Preparation by use of the following parameters: external calibration with lock mass with mass 2465.1989 Da of adrenocorticotropic hormone, background subtract type adaptive combining all scans, and the performance of deisotoping with a threshold of 5%. The aforementioned peak list was used in Mascot with a Swiss-Prot database. Search settings allowed one missed cleavage with the trypsin enzyme selected, oxidation of methionine as potential variable modification, carboamidomethyl of cysteine as fixed modifications, peptide tolerance of 100 ppm, and taxa human.
Vimentin/cardiolipin complex
Cardiolipin (50 μg/mL; Sigma-Aldrich) in methanol was evaporated under nitrogen and then was resuspended with human recombinant vimentin (5 μg/mL; R&D Systems) in 0.05μM NaHCO3 buffer, pH 9.5. The association between vimentin and cardiolipin in the complexes was tested by coimmunoprecipitation experiments.
Vimentin/cardiolipin binding assay
Vimentin/cardiolipin complexes were resuspended in a buffer containing 20mM Tris-HCl, pH 7.5; 0.15M NaCl; 1mM ethylenediaminetetraacetic acid (EDTA); 0.02% NaN3; and 10mM NaF. The mixtures were incubated with 10 μg of goat polyclonal antivimentin (R&D Systems) per milligram of protein and rocked for 2 hours at 4°C. At the end of the incubation, protein A-Sepharose (Sigma-Aldrich) was added, and the mixture was rocked at 4°C for an additional 1 hour. As a negative control, immunoprecipitation was performed with an irrelevant goat IgG (Sigma-Aldrich). A major portion of the immunoprecipitate was subjected to phospholipid extraction according to the method of Folch et al24 and separated by high-performance TLC (HPTLC) in a single dimension by the use of a solvent system of chloroform/methanol/acetic acid/water (100:75:7:4, vol/vol/vol/vol). Phospholipids were stained by exposure to iodide vapors and also immunostained with the purified human aCL IgG as previously reported.17
In parallel experiments, a mixture of cardiolipin (and, as a control, phosphatidylcholine, phosphatidylserine, or 20/80 phosphatidylserine/phosphatidylcholine), including 1% (wt/wt) [3H]1-palmitoyl-2-[11-[4-trifluoromethyldiazirinyl]unde-canoyl]-sn-glycero-3-phosphorylcholine in chloroform, was evaporated under nitrogen, according to Perides et al.25 In brief, 10 μg of vimentin was incubated with 20 μg of phospholipid vesicles at room temperature for 10 minutes. The reaction mixture was irradiated with ultraviolet light and subjected in SDS-PAGE. Bands were cut out and the gel slices incubated in 1 mL of toluene-350 (Packard Instrument Co) for 24 hours at 37°C, 10 mL of toluene-based scintillation mixture containing 0.005% (wt/vol) 1.4-bis[2-(5-phenyl)-oxazolyl]benzol, and 0.416% (wt/vol) 2.5-diphenyloxazol was added. After the mixtures had been incubated for another 24 hours at 37°C, their radioactivity was measured in a Packard Tri-Carb 460CD liquid scintillation spectrometer.
Detection of antivimentin/cardiolipin complex antibodies by ELISA
Antivimentin/cardiolipin complex antibodies were detected by a slight modification of ELISA method previously reported.26 Polystyrene plates (96-well) were coated and incubated overnight at 4°C with 100 μL/well of cardiolipin (50 μg/mL; Sigma-Aldrich) in methanol and then with 100 μL/well of human recombinant vimentin (5 μg/mL; R&D Systems) in 0.05μM NaHCO3 buffer, pH 9.5. Coated plates were incubated overnight at 4°C and then washed 3 times with phosphate-buffered saline containing 0.1% Tween 20 (PBS-T). Plates were blocked for 2 hours at room temperature with 100 μL of 1% bovine serum albumin (BSA) in PBS. After washing 3 times with PBS-T, the wells were incubated, for 1 hour at room temperature, with 100 μL of patient sera, diluted 1:100 in the blocking buffer. Each serum was analyzed in triplicate. Goat polyclonal antivimentin (R&D Systems) was used as a positive control.
After 3 washes with PBS-T, the plates were incubated for 1 hour at room temperature with horseradish peroxidase-conjugated antibodies, either goat anti–human IgG or rabbit anti–goat IgG (Sigma-Aldrich) were diluted in 1% BSA in PBS. The plates were washed 3 times with PBS-T, the bound peroxidase was then revealed with 100 μL of O-phenylenediamine dihydrochloride, and color development was stopped with H2SO4 0.2M for 5 minutes. Absorbance was measured at 492 nm in a microplate reader. Data were presented as the mean optical density (OD) corrected for background (wells without coated antigen). Thirty-two normal human sera were also tested, and a cut-off value was established at a mean of optical density (OD) ± 3 SD of normal human sera. Parallel experiments were performed in which all the procedures were identical without coated cardiolipin/vimentin complex. Virtually no reactivity was detected in all the samples (data not shown). Antivimentin antibodies also were detected by the use of human recombinant vimentin (5 μg/mL; R&D Systems) as an antigen by ELISA.
Purification of specific autoantibodies from patients' sera
The purification of antibodies was performed as previously described.27 In brief, vimentin/cardiolipin complex or, as a control, vimentin alone (R&D Systems), was spotted onto a nitrocellulose filter and incubated with the sera from patients with APS. To purify antibodies specific for the vimentin/cardiolipin complex, we used sera from patients who were IgG positive to the complex but negative to vimentin in ELISA. After washing with PBS-T, the antibodies were eluted with glycine 100mM, pH 2.5, and mixed for 10 minutes. The eluted antibodies were immediately neutralized with 1M Tris-HCl, pH 8. Antibodies from a preparation of intravenous immunoglobulin, precipitated by saturated ammonium sulfate solution, were used as a control.
In vitro exposure of endothelial cells to affinity-purified antivimentin/cardiolipin antibodies from SN-APS patients
For in vitro studies, human umbilical vein endothelial cells (HUVECs; PromoCell) were maintained in PromoCell Growth Medium containing endothelial cell growth medium kit at 37°C in a humified 5% CO2 atmosphere. Experiments were performed in cells grown to 60%-70% confluence. HUVECs were incubated with affinity-purified antivimentin/cardiolipin antibodies (200 μg/mL), according to Raschi et al,20 with affinity purified antivimentin antibodies (200 μg/mL), with normal human IgG fractions (NHS-IgG, 200 μg/mL), or with lipopolysaccharide (LPS; 100 ng/mL) as a positive control. All the materials contained less the 0.00025 ng of endotoxin/μg protein, as determined by the Limulus amebocyte lysate test, performed at Associates of Cape Cod.
Preparation of cell extracts
Unstimulated or stimulated HUVEC cells with affinity-purified antivimentin/cardiolipin antibodies, purified antivimentin antibodies, NHS-IgG fractions, or LPS were incubated for 45 minutes at 37°C, in 5% CO2. After treatment the medium was removed, cells placed on ice, washed once in PBS, and scraped in PBS. For the preparation of whole-cell extracts, cells were resuspended in lysis buffer (20mM 4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid [HEPES], pH 7.2; 1% Nonidet P-40; 10% glycerol; 50mM NaF; and 1mM Na3VO4, including protease inhibitors (Sigma-Aldrich). DNA was sheared by brief sonication, and soluble proteins were recovered after centrifugation of lysates at 15 000g for 15 minutes at 4°C. Nuclear extracts were prepared as described previously.28 In brief, cells were resuspended in buffer A (20mM HEPES, pH 7.9; 20 mM KCl; 3.0 mM MgCl2; 0.3 mM Na3VO4; and freshly added 200μM leupeptin, 10μM E64, 300μM phenylmethanesulfonylfluoride, 0.5 μg/mL pepstatin, 5mM DTT, and 0.1% Nonidet P-40) and vortexed. After 30 minutes on ice, cells were centrifuged for 30 minutes at 10 000g at 4°C. Pellets were resuspended in buffer B (40mM HEPES, pH 7.9; 0.84M NaCl; 0.4mM EDTA; 50% glycerol; 0.3mM Na3VO4; and freshly added 200μM leupeptin, 10μM E64, 300μM phenylmethanesulfonylfluoride, 0.5 μg/mL pepstatin, and 5mM DTT), and vortexed. After 1 hour on ice, nuclear extracts were cleared at 10 000g for 1 hour at 4°C, and supernatants were transferred to new vials. Protein content was determined by Bradford assay by the use of BSA as a standard (Bio-Rad). Samples were frozen at−80°C.
Western blot analysis of phospho-IRAK1 and phospho-NF-κB
Equal amounts of whole or nuclear extracts proteins (from unstimulated or stimulated HUVECs with affinity-purified antivimentin/cardiolipin antibodies, purified antivimentin antibodies, NHS-IgG fraction, or LPS) were separated in 7.5% SDS-PAGE under unreducing conditions. The proteins were electrophoretically transferred onto nitrocellulose membrane (Bio-Rad) and then, after blocking with PBS, containing 1% albumin, probed with polyclonal antiphospho-IRAK1 (Cell Signaling, Inc) or polyclonal antiphospho–NF-κB p65 (Cell Signaling, Inc). Bound antibodies were visualized with HRP-conjugated anti–rabbit IgG (Sigma-Aldrich), and immunoreactivity was assessed by the chemiluminescence reaction with the ECL Western blotting system (Amersham Pharmacia Biotech). As a control for nonspecific reactivity, parallel SDS-PAGE gels were blotted as described by the use of an anti–rabbit IgG (Sigma-Aldrich). As a control for loading and purity of preparation, phospho-NF-κB p65-blotted membranes were stripped and reprobed with polyclonal anti–histone H1 antibodies (Upstate). IRAK1-blotted membranes were stripped and reprobed with polyclonal antiactin antibodies (Sigma-Aldrich).
Results
Demographic and clinical characteristics of SN-APS patients
All patients included in this group were white women with a mean age of 47.4 years (range, 23-82 years) and a mean disease duration of 9 years (range,1-57 years). Clinical characteristics of patients are summarized in Table 1. The prevalence of clinical manifestations of APS in our cohort of patients was 17 of 29 for vascular thrombosis (10 venous, 6 arterial, and 1 venous and arterial thrombosis) and 16 of 29 for pregnancy morbidity. All the patients were previously screened for conventional antiphospholipid tests (aCL by ELISA and LA),4 as well as for anti-β2GPI, anti–annexin V, and antiprothrombin and repeatedly were found to be negative.
Vimentin is identified as an endothelial protein cofactor in SN-APS patients
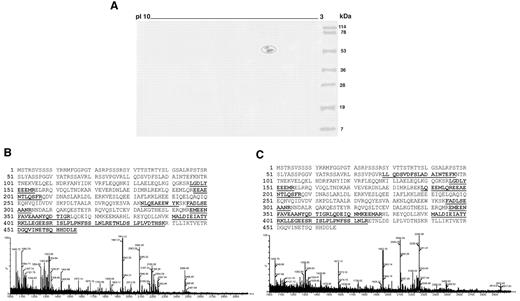
To identify a possible cofactor protein in these patients' sera, endothelial cell-surface membrane proteins separated by 2DE were transferred onto nitrocellulose membrane and analyzed with serum from 2 SN-APS patients (Figure 1A). The 2 spots identified, which revealed a molecular weight of 54 and 57 kDa, were excised from 2DE gel, digested with trypsin, and then analyzed by MALDI time-of-flight mass spectrometry. The detected peptide masses were searched against Swiss-Prot database protein. Results of the database search revealed vimentin as significant candidate protein of the 2 spots that therefore represented 2 distinct isoforms of the same protein (Figure 1B,C). The cell-surface expression of vimentin on endothelial cells was confirmed by fluorescence-activated cell-sorting analysis (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Identification of vimentin as an endothelial protein cofactor in SN-APS patients. (A) Endothelial cell-surface membrane proteins separated by 2DE were transferred onto nitrocellulose membrane and analyzed by immunoblotting with serum from 2 APS-seronegative patients. Two spots, with a molecular weights of 54 and 57 kDa, strongly reactive with serum IgG were identified (circled). (B, C) The 2 spots identified were excised from 2DE gel, digested with trypsin, and then analyzed by MALDI time-of-flight mass spectrometry. MS/MS spectra of trypting peptides matching 2 isoforms of vimentin. x-axis, m/z; y-axis, relative ion intensity. The matched amino acid sequences are bold underlined.
Identification of vimentin as an endothelial protein cofactor in SN-APS patients. (A) Endothelial cell-surface membrane proteins separated by 2DE were transferred onto nitrocellulose membrane and analyzed by immunoblotting with serum from 2 APS-seronegative patients. Two spots, with a molecular weights of 54 and 57 kDa, strongly reactive with serum IgG were identified (circled). (B, C) The 2 spots identified were excised from 2DE gel, digested with trypsin, and then analyzed by MALDI time-of-flight mass spectrometry. MS/MS spectra of trypting peptides matching 2 isoforms of vimentin. x-axis, m/z; y-axis, relative ion intensity. The matched amino acid sequences are bold underlined.
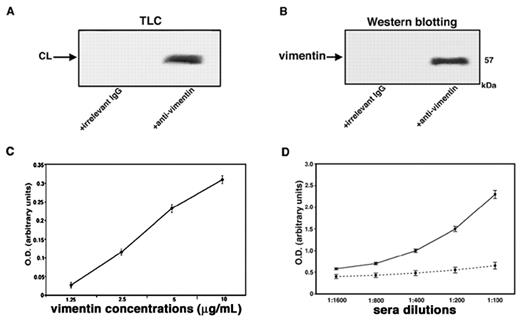
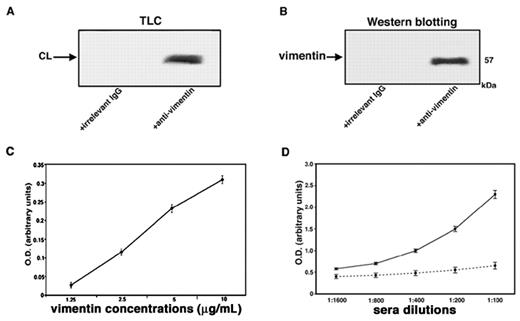
Evidence for vimentin/cardiolipin binding in vitro
A strict in vitro association between vimentin and cardiolipin was demonstrated by coimmunoprecipitation experiments. Indeed, these experiments showed that both phospholipid and protein were present in vimentin/cardiolipin complexes. Cardiolipin was detected in the immunoprecipitate by HPTLC analysis (Figure 2A); vimentin was detected by Western blot after SDS-PAGE gel electrophoresis (Figure 2B). As a control for the specificity of the immunoprecipitation, an irrelevant goat IgG was used. Next, we demonstrated that increasing concentrations of vimentin were associated dose dependently with coated cardiolipin, as revealed by antivimentin binding (Figure 2C). The reactivity of serum antibodies to vimentin/cardiolipin complex was shown to be dose dependent and was completely prevented by previous adsorption with the complex (Figure 2D).
Evidence of vimentin/cardiolipin binding. (A) Vimentin/cardiolipin complexes were resuspended in a buffer containing 20mM Tris-HCl, pH 7.5; 0.15M NaCl; 1mM EDTA; 0.02% NaN3; and 10mM NaF. The mixtures were immunoprecipitated with polyclonal antivimentin. The immunoprecipitates were subjected to phospholipid extraction and analyzed by monodimensional HPTLC analysis and then stained by exposure to iodide vapors. As a negative control, immunoprecipitation was performed with an irrelevant goat IgG. (B) Vimentin/cardiolipin complexes were resuspended in a buffer containing 20mM Tris-HCl, pH 7.5; 0.15M NaCl; 1mM EDTA; 0.02% NaN3; and 10mM NaF. The unextracted precipitates were separated by SDS-PAGE and probed with the antivimentin Ab. As a negative control, immunoprecipitation was performed with an irrelevant goat IgG. (C) Scalar doses of vimentin in 0.05μM NaHCO3 buffer, pH 9.5, from 10 μg/mL to 1.25 μg/mL, were incubated with 50 μg/mL of cardiolipin in methanol. After washes with PBS-T and blocking in PBS containing 3% BSA, plates were incubated with goat polyclonal antibodies against vimentin. (D) Sera of SN-APS patients with detectable levels of antivimentin/cardiolipin antibodies (diluted 1:100, 1:200, 1:400, 1:800, 1:1600) were tested by the use of ELISA with vimentin/cardiolipin complex. As a control of binding specificity sera were previously adsorbed with vimentin/cardiolipin (incubation, 2:1 vol/vol, with 3 mg/mL cardiolipin micelles containing 3 μg/mL vimentin for 1 hour at 37°C and then overnight at 4°C. The mixture was centrifuged at 27 000g for 15 minutes at 4°C). The supernatants were kept as adsorbed sera and tested by ELISA.
Evidence of vimentin/cardiolipin binding. (A) Vimentin/cardiolipin complexes were resuspended in a buffer containing 20mM Tris-HCl, pH 7.5; 0.15M NaCl; 1mM EDTA; 0.02% NaN3; and 10mM NaF. The mixtures were immunoprecipitated with polyclonal antivimentin. The immunoprecipitates were subjected to phospholipid extraction and analyzed by monodimensional HPTLC analysis and then stained by exposure to iodide vapors. As a negative control, immunoprecipitation was performed with an irrelevant goat IgG. (B) Vimentin/cardiolipin complexes were resuspended in a buffer containing 20mM Tris-HCl, pH 7.5; 0.15M NaCl; 1mM EDTA; 0.02% NaN3; and 10mM NaF. The unextracted precipitates were separated by SDS-PAGE and probed with the antivimentin Ab. As a negative control, immunoprecipitation was performed with an irrelevant goat IgG. (C) Scalar doses of vimentin in 0.05μM NaHCO3 buffer, pH 9.5, from 10 μg/mL to 1.25 μg/mL, were incubated with 50 μg/mL of cardiolipin in methanol. After washes with PBS-T and blocking in PBS containing 3% BSA, plates were incubated with goat polyclonal antibodies against vimentin. (D) Sera of SN-APS patients with detectable levels of antivimentin/cardiolipin antibodies (diluted 1:100, 1:200, 1:400, 1:800, 1:1600) were tested by the use of ELISA with vimentin/cardiolipin complex. As a control of binding specificity sera were previously adsorbed with vimentin/cardiolipin (incubation, 2:1 vol/vol, with 3 mg/mL cardiolipin micelles containing 3 μg/mL vimentin for 1 hour at 37°C and then overnight at 4°C. The mixture was centrifuged at 27 000g for 15 minutes at 4°C). The supernatants were kept as adsorbed sera and tested by ELISA.
The analysis of labeling of vimentin with [3H]1-palmitoyl-2-[11-[4-trifluoromethyldiazirinyl]unde-canoyl]-sn-glycero-3-phosphorylcholine in the presence of cardiolipin compared with other types of phospholipids vesicles revealed that the radioactivity bound to vimentin was 4310 ± 128 cpm for cardiolipin, 460 ± 14 cpm for phosphatidylcholine, 2170 ± 65 cpm for phosphatidylserine, and 2520 ± 72 cpm for 20/80 phosphatidylserine/phosphatidylcholine mixture. These findings demonstrate that cardiolipin shows a stronger interaction with vimentin compared with phosphatidylcholine and phosphatidylserine.
Detection of antibodies to vimentin/cardiolipin
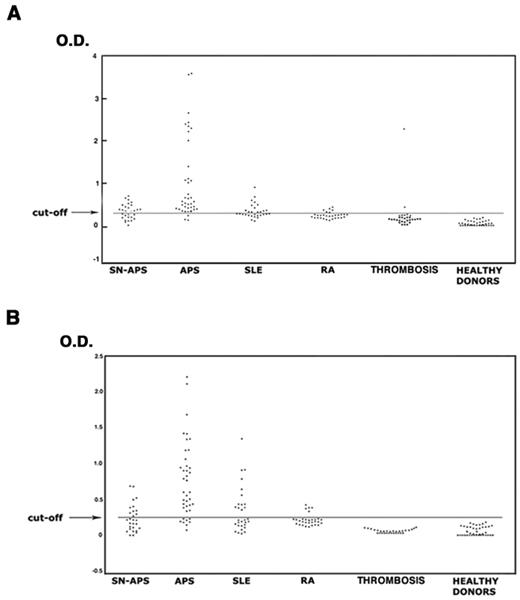
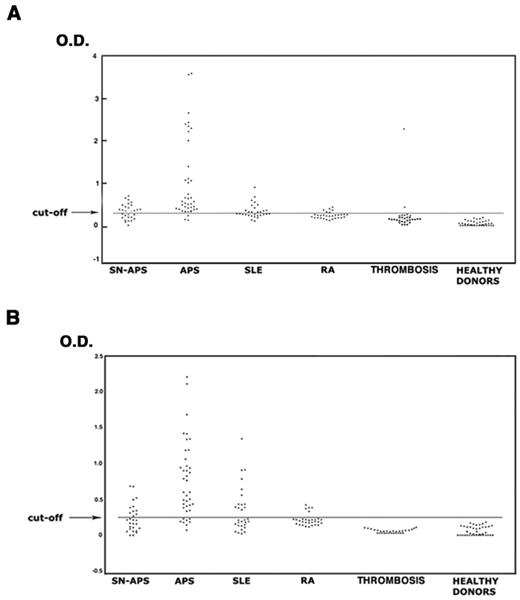
The analysis of the sera under testing showed that 37 of 40 patients with APS (92.5%), 16 of 29 with SN-APS (55.2%), 13 of 30 with SLE (43.3%), 5 of 30 with RA (16.7%), 2 of 30 (6.7%) with venous or arterial thrombosis, and none of healthy subjects displayed IgG antivimentin/cardiolipin antibodies. The analysis of IgM antivimentin/cardiolipin antibodies revealed similar results (Table 2). The occurrence of both IgG and IgM antibodies was significantly greater in patients with APS, SN-APS, and SLE compared with healthy donors (P < .0001). Moreover, the occurrence in APS patients also was significantly greater compared with SLE, RA, and patients with venous or arterial thrombosis (P < .0001). OD of the sera under test are reported in Figure 3. The test, which was performed with a second sample obtained at least 12 weeks after the first, confirmed the same result in all APS and SN-APS patients. No significant correlation was found between IgG or IgM antivimentin/cardiolipin antibodies and clinical manifestations in SN-APS patients (Table 3). Furthermore, no significant correlation was found between IgG or IgM antivimentin/cardiolipin antibodies and thrombotic risk factors (see Table 1).
Antivimentin/cardiolipin complex antibodies in patients and healthy subjects. The sera of patients with APS, SN-APS, SLE, RA, venous or arterial thrombosis, and of healthy subjects were analyzed by ELISA for the detection of IgG and IgM antivimentin/cardiolipin antibodies. The occurrence of both IgG (A) and IgM (B) antibodies was significantly greater in patients with APS, SN-APS, and SLE compared with healthy donors (P < .0001). Moreover, the occurrence in APS patients was also significantly greater compared with SLE and RA (P < .0001).
Antivimentin/cardiolipin complex antibodies in patients and healthy subjects. The sera of patients with APS, SN-APS, SLE, RA, venous or arterial thrombosis, and of healthy subjects were analyzed by ELISA for the detection of IgG and IgM antivimentin/cardiolipin antibodies. The occurrence of both IgG (A) and IgM (B) antibodies was significantly greater in patients with APS, SN-APS, and SLE compared with healthy donors (P < .0001). Moreover, the occurrence in APS patients was also significantly greater compared with SLE and RA (P < .0001).
Of note, only 26% of total APS patients showed IgG and 23.1% showed IgM to vimentin, with OD significantly lower compared with OD detected with the use of vimentin/cardiolipin complex (P < .001; supplemental Table 1). This finding suggests a role for vimentin/cardiolipin complex in the in vitro binding of the antibodies.
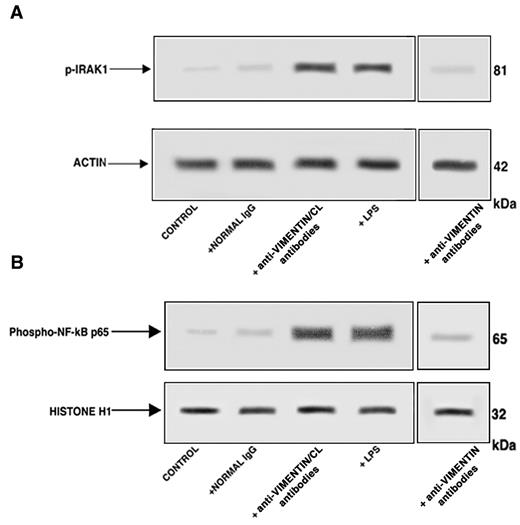
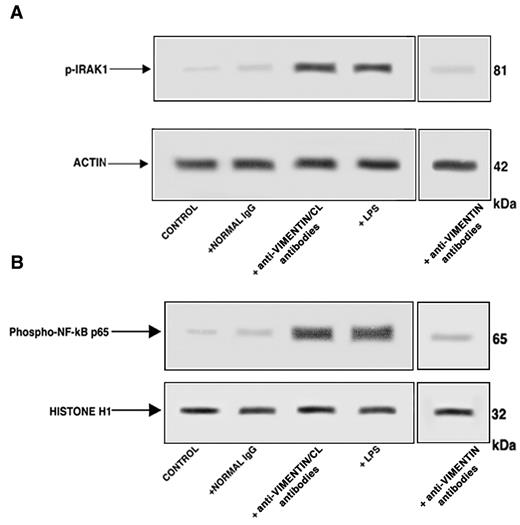
Affinity-purified antivimentin/cardiolipin antibodies from SN-APS induce IRAK1 phosphorylation and NF-κB activation in endothelial cells
Western blot analysis of cell lysates showed that affinity-purified antivimentin/cardiolipin antibodies, as well as LPS, induced IRAK1 phosphorylation, as revealed by antiphospho-IRAK1 antibodies reactivity (Figure 4A). On the contrary, cells stimulated with purified antivimentin antibodies or with normal human IgG virtually did not show antiphospho-IRAK1 reactivity. Cell lysates obtained by the use of non-IRAK1–specific IgG yielded no reactivity (data not shown).
Affinity-purified antivimentin/cardiolipin antibodies from SN-APS induce IRAK1 phosphorylation and activate NF-κB. Endothelial cells were stimulated with affinity-purified antivimentin/cardiolipin antibodies (200 μg/mL), NHS-IgG fraction (200 μg/mL), LPS (100 ng/mL), and affinity-purified antivimentin antibodies (200 μg/mL) for 45 minutes at 37°C, in 5% CO2 and cellular extracts were obtained. (A) Phosphorylated levels of IRAK1 (p-IRAK1) were analyzed in whole-cell extracts by Western blotting with antiphospho-IRAK1 antibodies; for control, the blotted membranes were stripped and reprobed with antiactin antibodies. Bound antibodies were visualized with horseradish peroxidase-conjugated IgG and immunoreactivity was assessed by ECL. One example representative of the patients with antivimentin/cardiolipin antibodies. (B) NF-κB activation was analyzed in nuclear cell extracts by Western blot with antiphospho–NF-κB p65 Ser antibodies; for a control, the blotted membranes were stripped and reprobed with anti–histone H1 antibodies. Bound antibodies were visualized with horseradish peroxidase-conjugated IgG, and immunoreactivity was assessed by ECL. One example representative of the patients with antivimentin/cardiolipin antibodies is shown.
Affinity-purified antivimentin/cardiolipin antibodies from SN-APS induce IRAK1 phosphorylation and activate NF-κB. Endothelial cells were stimulated with affinity-purified antivimentin/cardiolipin antibodies (200 μg/mL), NHS-IgG fraction (200 μg/mL), LPS (100 ng/mL), and affinity-purified antivimentin antibodies (200 μg/mL) for 45 minutes at 37°C, in 5% CO2 and cellular extracts were obtained. (A) Phosphorylated levels of IRAK1 (p-IRAK1) were analyzed in whole-cell extracts by Western blotting with antiphospho-IRAK1 antibodies; for control, the blotted membranes were stripped and reprobed with antiactin antibodies. Bound antibodies were visualized with horseradish peroxidase-conjugated IgG and immunoreactivity was assessed by ECL. One example representative of the patients with antivimentin/cardiolipin antibodies. (B) NF-κB activation was analyzed in nuclear cell extracts by Western blot with antiphospho–NF-κB p65 Ser antibodies; for a control, the blotted membranes were stripped and reprobed with anti–histone H1 antibodies. Bound antibodies were visualized with horseradish peroxidase-conjugated IgG, and immunoreactivity was assessed by ECL. One example representative of the patients with antivimentin/cardiolipin antibodies is shown.
Because IRAK phosphorylation leads to NF-κB activation,20 we investigated the effects of affinity purified antivimentin/cardiolipin antibodies on p65 NF-κB. Western blot analysis of nuclear extracts revealed that affinity-purified antivimentin/cardiolipin antibodies, as well as LPS, induced NF-κB phosphorylation, as revealed by antiphospho–NF-κB p65 Ser antibodies reactivity (Figure 4B). On the contrary, cells stimulated with purified antivimentin antibodies or with normal human IgG virtually did not show antiphospho–NF-κB p65 Ser reactivity. Cell lysates obtained with the use of non-NF-κB p65–specific IgG yielded no reactivity (data not shown). These findings indicate that IgG from SN-APS induce IRAK1 phosphorylation, with consequent NF-κB activation in endothelial cells.
Discussion
APS is a clinical entity characterized by a wide spectrum of aPL,29-31 including antibodies directed to cofactor proteins (mainly β2GPI, prothrombin, protein S, protein C, annexin V, annexin II), phospholipids-protein complexes, and/or pure phospholipids.7-14 However, a seronegative catastrophic APS has been recently described.32,33
In this study, we analyzed new possible antigenic target(s) of the antibodies that could be used to identify these patients. With this aim, we tested sera from patients with so-called seronegative APS by a proteomic approach by analyzing endothelial cell-surface membrane proteins. Using this analysis, we identified vimentin as a strongly immunoreactive autoantigen. Vimentin is a cytoskeleton intermediate filament protein ubiquitously expressed. Surface-expressed forms of vimentin have recently been discovered on several cell types, including apoptotic neutrophils and T cells,34,35 activated macrophages,36 platelets,37 vascular endothelial cells,38 brain microvascular endothelial cells,39 Sezary T cells,40 and skeletal muscle cells.41 The mechanism by which vimentin reaches the cell surface, which domains are exposed, and its function at the surface, remain unknown, However, vimentin and cardiolipin can interact at the surface of apoptotic cells to form an immunogenic particle.41
Antivimentin antibodies were detected in patients with SLE42 ; however, a role for these antibodies in the diagnosis of this disease has not been confirmed, and the detection of these antibodies is not included in the criteria consensus for the diagnosis.4 In our study, vimentin molecule was shown to be able to bind cardiolipin in vitro. Cardiolipin-vimentin binding may be attributable to electrostatic interaction between positive charged aminoacids of vimentin and negative charged of cardiolipin. Our findings on phospholipids-vimentin interaction revealed that cardiolipin shows a stronger interaction with vimentin compared with phosphatidylcholine and phosphatidylserine. These findings are consistent with previous observations of Perides et al,25 who analyzed the interaction of vimentin with different phospholipid bilayers.
It prompted us to identify vimentin/cardiolipin complex as a molecular target of the antibodies in patients with SN-APS. Interestingly, we observed that not only a large proportion of SN-APS patients, but also almost all the APS patients under testing, displayed the presence of antivimentin/cardiolipin complex antibodies. This finding suggests that vimentin may be considered a “new” antigenic cofactor for aPL in APS. This finding is not completely surprising because a significant correlation between antivimentin and aCL antibodies has been already reported.34 Moreover, their particular role in the pathogenesis of thrombotic events in autoimmune diseases has been described.43 In particular, Leong et al44 demonstrated that antivimentin antibodies lead to activation of platelets and leukocytes, as revealed by induced expression of P-selectin, fibrinogen, tissue factor, and formation of platelet-leukocyte conjugates via platelet-activating factor. Furthermore, platelet vimentin may regulate fibrinolysis in plasma and thrombus formation by binding platelet-derived vibronectin-plasminogen activator inhibitor complexes.37
Recently, it was demonstrated that aPL may exert its pathogenic role by triggering a signal transduction pathway involving Toll-like receptor 4, IRAK phosphorylation, NF-κB activation, and translocation with consequent release of proinflammatory and procoagulant factors by endothelial20-22 and/or monocytic cells.45 To verify the possible pathogenic role of autoantibodies in SN-APS patients, we verified whether affinity-purified antivimentin/cardiolipin antibodies from the sera of these patients were able to induce IRAK phosphorylation and NF-κB activation by endothelial cells. Our results confirmed this hypothesis.
How can vimentin/cardiolipin complex become antigenic? At present, this point remains unknown. However, the authors of previous studies reported a cell-surface expression of intermediate filament proteins, including vimentin, in thrombin-activated platelets as well as in apoptotic cells,46 suggesting that programmed cell death is a physiopathologic mechanism that may expose this antigen on the plasma membrane, as well as cardiolipin.46,47 The presence of autoantibodies in certain diseases may be caused by abnormal exposure of the autoantigen on apoptotic cells. In fact, caspase-dependent cleavage of vimentin, with consequent exposure of vimentin on the cell surface, is a necessary requisite for apoptosis.
Taken together, our results, obtained with both a proteomic and an immunologic approach, prompt to identify vimentin/cardiolipin as a “new” target of the APS. However, although the presence of these antibodies may be considered highly sensitive in these patients, it is not very specific because they were also detected in 43.3% of patients with SLE and in 16.6% of patients with RA. Detection of these antibodies may represent a useful tool mainly in those patients with clinical features suggestive of APS in which the classical tests for detection aPL result persistently negative.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.O., A.C., T.C., F.C, C.A., A.L., and P.M. performed research; T.G. and R.M. designed research and analyzed data; M.A.K. and G.R.V.H. selected the patients and performed clinical and laboratory analyses; and E.O., G.V., and M.S. designed research and wrote the paper.
Conflict-of-interest disclosure: A patent relating to the content of the manuscript is pending.
Correspondence: Prof Maurizio Sorice, Dipartimento di Medicina Sperimentale, “Sapienza” Università di Roma, Viale Regina Elena 324, Rome 00161, Italy; e-mail: maurizio.sorice@uniroma1.it.