Abstract
Chemokine CC motif receptor-like 2 (CCRL2) is a heptahelic transmembrane receptor that shows the highest degree of homology with CCR1, an inflammatory chemokine receptor. CCRL2 mRNA was rapidly (30 minutes) and transiently (2-4 hours) regulated during dendritic cell (DC) maturation. Protein expression paralleled RNA regulation. In vivo, CCRL2 was expressed by activated DC and macrophages, but not by eosinophils and T cells. CCRL2−/− mice showed normal recruitment of circulating DC into the lung, but a defective trafficking of antigen-loaded lung DC to mediastinal lymph nodes. This defect was associated to a reduction in lymph node cellularity and reduced priming of T helper cell 2 response. CCRL2−/− mice were protected in a model of ovalbumin-induced airway inflammation, with reduced leukocyte recruitment in the BAL (eosinophils and mononuclear cells) and reduced production of the T helper cell 2 cytokines, interleukin-4 and -5, and chemokines CCL11 and CCL17. The central role of CCRL2 deficiency in DC was supported by the fact that adoptive transfer of CCRL2−/− antigen-loaded DC in wild-type animals recapitulated the phenotype observed in knockout mice. These data show a nonredundant role of CCRL2 in lung DC trafficking and propose a role for this receptor in the control of excessive airway inflammatory responses.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells and key regulators of T-cell functions.1,2 In the airway epithelium, DCs form an extensive network, where they continuously sense environmental antigens. After antigen capture, lung DCs migrate to mediastinal lymph nodes, where antigens are presented to T cells. Activation of a T helper cell 2 (Th2)–skewed response by airway DCs is responsible for allergic immune responses in the lung.3,4
Chemokine receptors play a crucial role in the migration of maturing DCs to secondary lymphoid organs,5,6 and several studies have shown that this process is a crucial event for the appropriate activation of the immune response.7-10 DC migration to lymph nodes relies on the functional expression of CCR75,6 as well as other chemotactic receptors, such as CCR8 and BLT1.9-11 In addition, lung DC migration is also regulated by PD1, one of the 2 PGD2 receptors, and by the transcription factors PPRγ and Runx3.12-14 However, as a difference from DCs localized in other anatomical compartments, the homing of lung DCs to lymph nodes is independent of the action of MRP1, the LTC4 transporter that regulates CCR7 functions.11 This finding indicates that DC trafficking is regulated in a tissue-specific manner.
Chemokine CC motif receptor-like 2 (CCRL2), also known as L-CCR (lipopolysaccharide [LPS]–inducible CC chemokine-related gene and Eo1), is a heptahelic serpentine receptor that shares the highest homology with the chemokine receptors, CCR1 and CCR5. CCRL2 is structurally characterized by the presence of a noncanonical DRYLAIV motif. CCRL2 was originally identified in the mouse macrophage cell line RAW 264.715 and was recently reported to bind the chemotactic protein chemerin, though in the absence of any detectable intracellular signaling.16 CCRL2 expression at the mRNA level has been described in murine macrophages,15 glial cells, astrocytes, and microglia stimulated with LPS17,18 and in mast cells.16 CCRL2 was also reported to be up-regulated in lung macrophages and epithelial cells after in vivo sensitization.19 The human gene most closely related to CCRL2 is HCR with its 2 splicing variants, CRAM-A and CRAM-B.
Here, we describe that CCRL2 is rapidly induced during mouse DC maturation with a kinetics that precedes CCR7 induction. To evaluate the relevance of this receptor in DC biology, we generated CCRL2-deficient mice and used them in an established model of allergen-induced airway inflammation, in which DCs are known to play a crucial role.20 The results reported here highlight a nonredundant role for CCRL2 in the migration of lung DCs to regional lymph nodes and in the induction of Th2-oriented airway allergic inflammation. These results propose CCRL2 as a new potential target for therapeutic strategies aimed at controlling lung hypersensitivity.
Methods
Dendritic cells
Bone marrow–derived DCs were generated from CD34+ bone marrow cells and were characterized for antigen expression, pinocytic capacity, and chemotaxis, as previously described.21 Migration of lung DCs was evaluated in sensitized mice by the intratracheal injection of 80 μL of fluorescein isothiocyanate-ovalbumin (FITC-OVA; 10 mg/mL). CD11c+FITC+ DCs were counted by fluorescence-activated cell sorting (FACS) in mediastinal lymph nodes 8, 24, 48, and 72 hours later. For adoptive transfer, bone marrow-derived DCs were pulsed overnight with 100 μg/mL OVA, washed, and instilled intratracheally. In vitro chemotaxis assays were performed using micro Boyden chambers, as previously described.21
Generation of the CCRL2-deficient mice
A SalI-HindIII and a HindIII-BamHI fragment (6.5 and 3 kb, respectively) containing the CCRL2 gene, but lacking its open reading frame, were obtained and ligated to a PGK-neomycin resistance gene cassette. This construct was electroporated into R1 embryonic stem cells, and resistant clones were isolated and analyzed by Southern blots after KpnI digestion, using an external probe. One targeted clone was injected into C57BL/6 blastocysts, and resulting chimeras were mated with C57BL/6 mice (Charles River Breeding Laboratories) to obtain an outbred line carrying the mutated CCRL2 allele that was backcrossed in the C57BL/6 background for 10 generations. Age- and sex-matched littermates were used in all studies. Procedures involving animals and their care were conformed to institutional guidelines in compliance with national and international law and policies (European Economic Community Council Directive; National Institutes of Health, Guide for the Care and Use of Laboratory Animals.
Quantification of mRNA levels by Northern blot and real-time PCR
Total RNA was extracted with TRIzol reagent (Invitrogen). Northern blots were performed, as previously described.21 The ApaI-HincII fragment of CCRL2 cDNA was used as a probe. The CCR7 probe was obtained as previously described.21 For real-time polymerase chain reaction (PCR) analysis, total RNA samples were treated with DNAse I (Invitrogen) before reverse transcription (RT). Real-time quantitative PCR reactions were performed on an ABI PRISM 7700 Sequence Detector System (Applied Biosystems), using a SYBR Green PCR master mix (Applied Biosystems) and specific primers (supplemental Figure 5, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Gene expression was normalized to β-actin or 18S mRNA.
Generation of mAb to CCRL2
First, 107 X-rayed CCRL2/L1.2 cells in phosphate-buffered saline (PBS) were injected intraperitoneally (i.p.) every other week for 6 weeks into CCRL2-deficient mice. Three days after the last challenge, spleen cells were fused following conventional protocols. Hybridomas recognizing CCRL2/CHO-K and CCRL2/L1.2 transfectants, but not parental cells, were cloned and tested for specificity on CCRL2, CCRs, and CXCRs transfectants. One hybridoma (hybr 4, IgG2a) was selected for these studies, purified, and biotinylated.
FACS analysis
Cells were blocked with CD16/32 2.4G2, incubated with biotinylated anti-CCRL2 antibody (Ab); (3 μg/mL), and then with streptavidin-FITC or -phycoerythrin (PE); (Becton Dickinson). Directly conjugated Abs to CD3e (145-2C11), CD4 (A15.1.17), CD8α (53-6.7), CD11b (M1/70), Gr-1 (RB6-8C5), I-A-I-E (2G9), CD11c (HL3), CD80 (16-10A1), CD86 (GL1), and CD40 (3/23) from Becton Dickinson, CCR7, CD103 (2E7), Siglec H (440c) from eBioscience, and PDCA-1 (JF05-1C2.4.1) from Miltenyi Biotec were used for analysis of cell subpopulations. Staining was analyzed by a FACSCanto flow cytometer (Becton Dickinson), using CellQuest Pro 5.2.1 software. Flow cytometric analysis of lung single-cell suspensions was performed as previously described.22
Allergen-induced airway inflammation
Mice were sensitized with OVA (0.01 mg/mouse intraperitoneally) in 0.2 mL alum on days 0 and 12, exposed daily to OVA aerosol (5%; 20 minutes) from days 18 to 23, and killed 24 hours after the last aerosol. Airways were washed 3 times with 1 mL of PBS via a tracheal cannula. Differential cell counts were performed by using Diff-Quik–stained cytospins. Cells from lung tissue were obtained after 0.15 mg/mL collagenase (type D; Roche) and 25 μg/mL DNase (type I; Roche) incubation (37°C for 1 hour) as previously described.23 For histopathology, lungs were fixed in 10% normal buffered formalin. Then, 4-μm paraffin-embedded sections were stained with hematoxylin and eosin. For immunohistochemistry, sections were obtained from frozen lungs from 3 mice per experimental group. Immunostaining was performed using anti-CD11c (BD), anti-Siglec H (rat anti–mouse, kindly provided by Prof M. Colonna, Washington University, St Louis, MO) and anti-CD103 (eBioscience). Primary antibodies were revealed from using anti–hamster or –rat (Vector Labs) biotin-conjugated secondary antibodies, followed by streptavidin–horseradish peroxidase (HRP) (for CD11c and Siglec H) or streptavidin-PA (for CD103) and DAB (all from Dako) or Ferangi Blue (Biocare Medical).
Airway responsiveness was evaluated 24 hours after the last OVA challenge by measurements of lung resistance (RL) and dynamic compliance in anesthetized and tracheostomized mice in response to inhaled methacholine in a Buxco system (Buxco). Goblet cells were counted on periodic acid-Schiff (PAS)–stained lung sections, using an arbitrary scoring system, as previously described.24 Data are expressed as mean ± SEM, n = 14-15 mice/group. For in vitro antigen restimulation of lymphocytes, mediastinal lymph nodes were excised, 5 × 105 cells in 200 μL were seeded in U-bottomed plastic plates, and OVA was added at varying concentrations; plates were incubated at 37°C for 72 hours.
Cytokine and IgE amount evaluation
Cytokines and total IgE were measured by standard sandwich enzyme-linked immunosorbent assay (ELISA), from Pharmingen (interleukin [IL]-4, IL-5, interferon [INF]-γ, and IgE) and R&D Systems (IL-2, IL-13, CCL2, CCL5, CCL11, CCL17, and CCL22). OVA-specific IgE were detected by using anti–mouse IgE monoclonal antibody (mAb; Becton Dickinson) as the capture antibody and biotinylated OVA (EZ-Link Sulfo-NHS-LC-Biotinylation Kit; Pierce) as the detection reagent.
Statistical analysis
Statistic significance was calculated by Student t test, Mann-Whitney U test, and one-way analysis of variance (ANOVA), as appropriate. Differences were considered significant at P < .05.
Results
Regulation of CCRL2 expression in mouse DCs
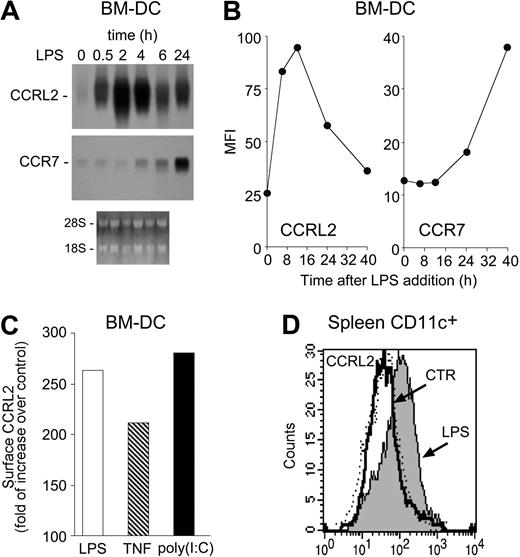
In a preliminary screening of orphan/uncharacterized putative chemotactic receptors (unpublished), CCRL2 was selected for its unique ability to be up-regulated during DC maturation. CCRL2 mRNA was barely detectable in resting bone marrow–derived myeloid DCs. LPS, a prototypic maturation factor for DCs, rapidly induced CCRL2 expression, starting at 30 minutes, reaching a peak at 2 hours, and decreasing thereafter (Figure 1A). As expected,21 CCR7 transcript levels were also increased by LPS, but with delayed kinetics, starting after 4 hours and reaching peak levels at 24 hours (Figure 1A). Regulation at the mRNA level was paralleled by protein expression, with peak levels detected at 12 hours that returned to nearly basal levels at 40 hours of stimulation. The evaluation of CCR7 membrane expression confirmed the delayed kinetics observed at the mRNA level (Figure 1B). Other inflammatory mediators, such as tumor necrosis factor α (TNFα) and poly(I:C), known to induce DC maturation, also up-regulated CCRL2 expression (Figure 1C). No constitutive CCRL2 membrane expression was detected in CD11c+ DCs purified from lymph nodes, spleen, thymus, bone marrow, and lung (data not shown). However, CCRL2 was readily induced in spleen CD11c+ DCs 6 hours after LPS intravenous administration (Figure 1D), a time frame compatible with the in vivo redistribution of activated DCs from the marginal zone to the T-cell areas.25
Induction of CCRL2 in maturing DCs. DCs were generated in vitro from CD34+ bone marrow precursors. (A) Total RNA was purified from immature DC (0 hours) or DC treated for different times with 100 ng/mL LPS and analyzed by Northern blot. Ethidium bromide staining is shown. (B) Kinetics of CCRL2 and CCR7 membrane expression in DC stimulated with 100 ng/mL LPS. The graphs show the mean fluorescence intensity (MFI) of cells labeled with CCRL2 and CCR7 mAbs. (C) FACS analysis of DCs treated with 100 ng/mL LPS, 20 ng/mL TNFα, or 25 μg/mL poly (I:C) for 12 hours. Data are expressed as folds of increase of CCRL2 labeling over vehicle-treated DC. (D) CCRL2 expression in spleen CD11c+ DCs isolated 6 hours after intravenous injection of 25 μg/mouse of LPS. Histograms represent CCRL2 expression in WT (dotted line) and CCRL2−/− mice (solid lines). Data are representative of 2-4 separate experiments.
Induction of CCRL2 in maturing DCs. DCs were generated in vitro from CD34+ bone marrow precursors. (A) Total RNA was purified from immature DC (0 hours) or DC treated for different times with 100 ng/mL LPS and analyzed by Northern blot. Ethidium bromide staining is shown. (B) Kinetics of CCRL2 and CCR7 membrane expression in DC stimulated with 100 ng/mL LPS. The graphs show the mean fluorescence intensity (MFI) of cells labeled with CCRL2 and CCR7 mAbs. (C) FACS analysis of DCs treated with 100 ng/mL LPS, 20 ng/mL TNFα, or 25 μg/mL poly (I:C) for 12 hours. Data are expressed as folds of increase of CCRL2 labeling over vehicle-treated DC. (D) CCRL2 expression in spleen CD11c+ DCs isolated 6 hours after intravenous injection of 25 μg/mouse of LPS. Histograms represent CCRL2 expression in WT (dotted line) and CCRL2−/− mice (solid lines). Data are representative of 2-4 separate experiments.
Evaluation of WT and CCRL2−/− mice in a model of OVA-induced airway hyperresponsiveness
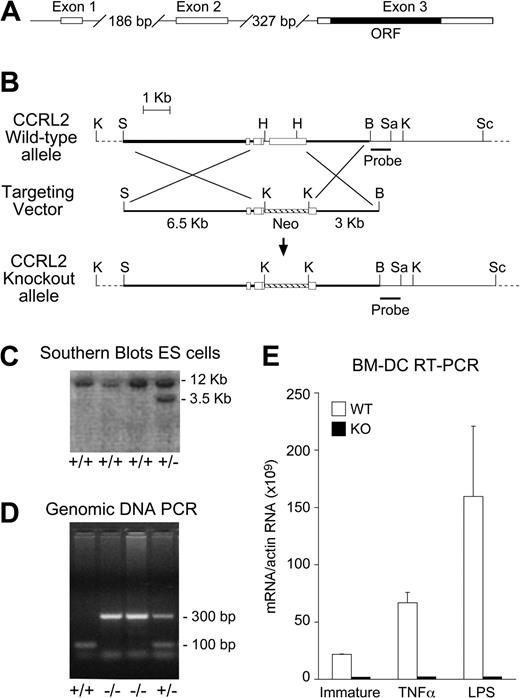
To directly evaluate CCRL2 biologic relevance, we generated CCRL2-deficient mice (CCRL2−/−) by homologous recombination techniques, as in Figure 2A and B. After electroporation of R1 embryonic stem cells, neomycin-resistant clones were analyzed by Southern blotting (Figure 2C). Mice were routinely genotyped by PCR with a set of 3 primers that detected the wild-type (WT) and the targeted alleles (Figure 2D). CCRL2 deficiency was assessed by real-time RT-PCR in bone marrow-derived DCs and tissues, using specific primers (Figure 2E and data not shown). CCRL2−/− mice developed normally to term and were fertile. No significant alterations were found after histologic and flow cytometric analysis of lymphoid organs and blood, when compared with age- and sex-matched WT control mice (not shown). CCRL2−/− mice had a normal lifespan and did not show an overt phenotype under steady-state conditions.
Generation of CCRL2 deficient mice. (A) Exon/intron structure of the CCRL2 gene. (B) Schematic representation of WT and KO alleles of the CCRL2 gene and targeting vector. Restriction sites: K, KpnI; S, SalI; H, HindIII; B, BamHI; Sa, SacI; Sc, ScaI. KpnI-digested genomic DNA fragments were detected by a specific probe. (C) Southern blot analysis of KpnI-digested genomic DNA from transfected embryonic stem cells hybridized with the CCRL2 probe generated a 12-kb WT restriction fragment and a 3.5-kb homologous recombinant restriction fragment. (D) PCR analysis of genomic DNA of CCRL2+/+ (lane 1), CCRL2−/− (lanes 2 and 3), and CCRL2+/− (lane 4) mice. (E) Total RNA was isolated from immature as well as TNF-α– and LPS-stimulated (24 hours) bone marrow DCs.
Generation of CCRL2 deficient mice. (A) Exon/intron structure of the CCRL2 gene. (B) Schematic representation of WT and KO alleles of the CCRL2 gene and targeting vector. Restriction sites: K, KpnI; S, SalI; H, HindIII; B, BamHI; Sa, SacI; Sc, ScaI. KpnI-digested genomic DNA fragments were detected by a specific probe. (C) Southern blot analysis of KpnI-digested genomic DNA from transfected embryonic stem cells hybridized with the CCRL2 probe generated a 12-kb WT restriction fragment and a 3.5-kb homologous recombinant restriction fragment. (D) PCR analysis of genomic DNA of CCRL2+/+ (lane 1), CCRL2−/− (lanes 2 and 3), and CCRL2+/− (lane 4) mice. (E) Total RNA was isolated from immature as well as TNF-α– and LPS-stimulated (24 hours) bone marrow DCs.
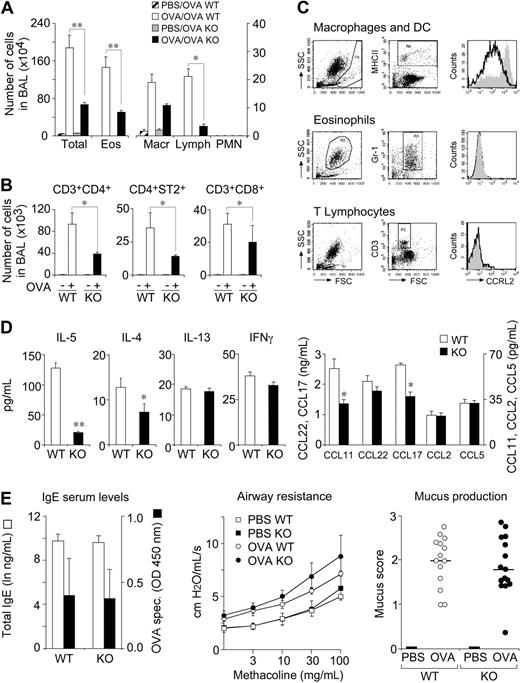
Lung DCs play a crucial role in the transport of antigens to mediastinal lymph nodes and in the induction of airway hypersensitivity.11,26 The role of CCRL2 was therefore investigated in a model of airway hypersensitivity. OVA-immunized CCRL2−/− mice, challenged by aerosol for 6 consecutive days, showed a dramatic reduction in the total number of leukocytes and, in particular, of eosinophils and lymphocyte/mononuclear cells, with respect to WT animals (Figure 3A). The decrease in T cells was evident for 3 main cell subsets, namely CD4+, CD8+, and ST2+CD3+/Th2 cells (Figure 3B). However, a possible direct role for CCRL2 in the recruitment of eosinophils and T cells into the airways was ruled out by the lack of expression of CCRL2 in these 2 cell types; conversely, BAL MHCII+ cells (macrophages and DCs) expressed CCRL2 (Figure 3C).
Role of CCRL2 in OVA-induced airway inflammation. WT and CCRL2−/− mice were sensitized and challenged with OVA by aerosol. BAL was collected 24 hours after the last aerosol. (A) Differential cell counts in BAL. (B) Evaluation of T-cell subsets in BAL. (C) CCRL2 membrane expression by MHCII+ cells, eosinophils, and T lymphocytes by FACS analysis. Histograms represent CCRL2 mAb staining of cells from WT (filled curves) and CCRL2−/− mice (open curves). (D) Cytokine and chemokine levels in BAL. (E) Total and OVA-specific IgE in serum (left panel) and bronchial hyperreactivity (AHR) to methacholine (middle panel) and mucus production (right panel). Data are mean ± SEM of 5 (A) or 3 (B-E) representative experiments with 6-12 mice per group. * P < .05; ** P < .01.
Role of CCRL2 in OVA-induced airway inflammation. WT and CCRL2−/− mice were sensitized and challenged with OVA by aerosol. BAL was collected 24 hours after the last aerosol. (A) Differential cell counts in BAL. (B) Evaluation of T-cell subsets in BAL. (C) CCRL2 membrane expression by MHCII+ cells, eosinophils, and T lymphocytes by FACS analysis. Histograms represent CCRL2 mAb staining of cells from WT (filled curves) and CCRL2−/− mice (open curves). (D) Cytokine and chemokine levels in BAL. (E) Total and OVA-specific IgE in serum (left panel) and bronchial hyperreactivity (AHR) to methacholine (middle panel) and mucus production (right panel). Data are mean ± SEM of 5 (A) or 3 (B-E) representative experiments with 6-12 mice per group. * P < .05; ** P < .01.
In parallel, the BAL of CCRL2−/− mice contained lower levels of the Th2 cytokines IL-4 and IL-5 and similar levels of IL-13 and the Th1 cytokine IFNγ (Figure 3D). Of note, although Th2 cells and eosinophils represent an important source for Th2 cytokines, IL-13 can also be secreted by lung smooth muscle cells.27 This may explain the different regulation of IL-13 versus IL-4 and IL-5 observed here. The alterations observed in the BAL were not paralleled by changes in the lung parenchyma, where normal degrees of infiltrating leukocytes (supplemental Figure 1A-B) and cytokine levels were detected (supplemental Figure 1C). To further examine the mechanisms associated with the defective leukocyte recruitment observed in CCRL2−/− mice, the levels of some relevant chemokines were evaluated. The eosinophil-attracting chemokine, CCL11/eotaxin, and the Th2-attracting chemokine CCL17/TARC levels were significantly decreased in BAL (Figure 3D), but not in lung tissue (supplemental Figure 1C). This finding is consistent with the decreased number of eosinophils and Th2 cells observed in the airway lumen. Conversely, no difference was observed in the expression of CCL22 (BAL and lung) and CCL11 and CCL17 in the lung. CCL2 and CCL5 levels were decreased in lung tissues of OVA-sensitized CCRL2−/− (supplemental Figure 1C), but not in the BAL (Figure 3D). In addition, OVA-sensitized CCRL2−/− mice showed normal levels of circulating total and OVA-specific IgE (Figure 3E). This finding is consistent with the ability of CCRL2−/− mice to mount a normal primary and secondary antibody response after intraperitoneal immunization with OVA/Alum or OVA/complete Freund's adjuvant (unpublished results). Finally, the typical features of asthma, namely, airway hyper-responsiveness (AHR) and mucus hypersecretion, were assessed in OVA-sensitized CCRL2−/− and WT mice. Both strains showed a significant increase in methacholine responsiveness evaluated as lung resistance (Figure 3E) and dynamic compliance (data not shown) that was, however, similar in the 2 mouse strains (Figure 3E). Similarly, OVA-sensitized CCRL2−/− and WT mice displayed a comparable number of goblet cells (Figure 3E).
Defective trafficking of pulmonary CCRL2−/− DCs to regional lymph nodes
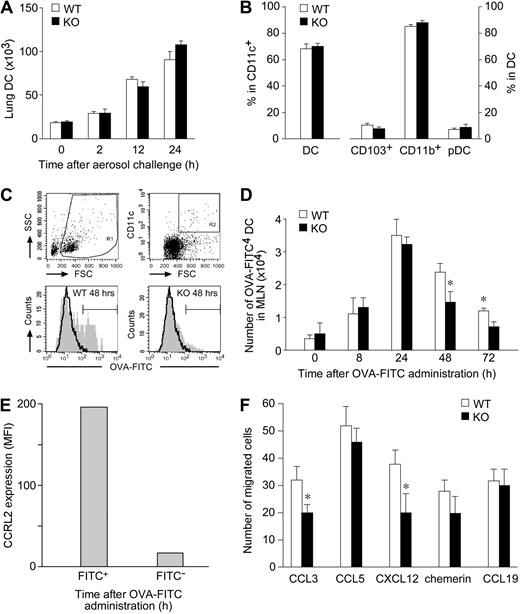
The migration of lung DCs from periphery to regional lymph nodes is a key step for the induction of immune response and tolerance.5,6,28 Therefore, lung DC trafficking was investigated in OVA-challenged CCRL2−/− mice. In agreement with previous reports,29 Figure 4A shows that the influx of DC into the lung was already detectable after 2 hours of OVA challenge and reached peak levels at 24 hours. No significant difference in the absolute number of DCs (Figure 4A) in their activation phenotype (ie, CD80, CD86, CD40, and MHCII expression; data not shown) was found between CCRL2−/− and WT mice at any of the time points investigated. Similarly, no difference was observed in the relative proportion of the 3 major lung DC subsets, namely, myeloid CD103+ and CD11b+ DC and plasmacytoid DC (Figure 4B). Furthermore, the in vivo distribution of DC subsets was investigated by immunohistochemistry of lung frozen tissue sections. CD103+ cells were mainly identified in the peribronchiolar space lining the conducting airways. Siglec H+ and CD11c+ cells were localized in the peribronchiolar space admixed to other immune cells as well as in the interstitial space of the lung parenchyma. CD11c stain was also observed in large cells located in the alveolar space, likely reflecting expression by alveolar macrophages. However, no difference was observed between WT and CCRL2−/− mice in the distribution of these DC subsets in either resting or OVA-stimulated conditions (supplemental Figure 2). To study the migration of airway DCs, OVA-sensitized animals were instilled intratracheally with a single administration of FITC-OVA, and CD11c+FITC+ DCs were enumerated in mediastinal draining lymph nodes.4,23 Figure 4C and D report that the migration of FITC+ DCs was already detectable after 8-hour stimulation and reached a peak at 24 hours, as previously reported.23 CCRL2−/− mice showed a statistically significant reduction in FITC+ DC trafficking to lymph nodes at 48 and 72 hours after antigen administration; of note, only FITC+ DCs expressed CCRL2 (Figure 4E). These results highlight a crucial role of CCRL2 in directing pulmonary DC migration to draining lymph nodes. To investigate the molecular basis for the defective migration of lung DCs, experiments were performed by using bone marrow–derived DCs. In vitro, DCs generated from CCRL2−/− and WT bone marrow progenitors were similar in terms of cell yield (not shown), expression of membrane markers, and costimulatory molecules, and in their ability to take up antigens (supplemental Figure 3A-B). These results postulate that CCRL2 deficiency does not interfere with DC development and maturation in vitro. CCRL2−/− DCs were also tested for the expression and function of chemotactic receptors. When evaluated at the mRNA level, CCRL2−/− DCs showed a normal expression of the chemokine receptors, CCR1, CCR2, CCR5, CXCR4, and CXCR6, and CCR7 (supplemental Figure 3C). Expression of CCR-3, -4, -6, -8, -9, CXCR-2, -3, -5, -6, -7, CX3CR1, and XCR1 was low in both WT and CCRL2−/− DCs (less than 500 mRNA molecules per 106 β-actin molecules) and was not modulated during maturation (data not shown). When tested in chemotaxis assays, immature CCRL2−/− DCs showed a normal in vitro migration to CCL5 and chemerin, and a slightly reduced response to CCL3 and CXCL12. Mature CCRL2−/− DCs were competent in migrating to CCL19, a CCR7 ligand (Figure 4F). Finally, skin-painting experiments did not reveal any alteration in the in vivo migration ability of endogenous CCRL2−/− DCs, and no alteration was seen in the migration to lymph nodes of in vitro generated carboxyfluorescein succinimidyl ester-labeled DCs (from WT and CCRL2 mice), when injected in the footpads of WT mice (data not shown). Altogether, these results exclude the possibility that CCRL2 deletion may be associated to a major alteration in the expression and function of DC chemotactic receptors.
Role of CCRL2 in lung DC trafficking. (A) Lung tissue and trachea were isolated from sensitized mice after one OVA-aerosol challenge. DCs were quantified by FACS analysis. Results are expressed as total number of DCs per mouse (mean ± SEM, n = 3; 6 mice/group). (B) The percentage of CD11c+/low autofluorescent DCs in total lung CD11c+ cells and DC subsets (CD103+, CD11b+, and Siglec H+/PDCA1+ pDCs) are presented. (C-D) OVA-sensitized mice received intratracheally 800 μg of OVA-FITC. (C) Representative analysis of WT and CCRL2−/− mediastinal lymph node cells obtained 48 hours after FITC-OVA administration. (D) Kinetics of FITC+ DCs in lymph nodes. Data are mean ± SEM, n = 3; 12-15 mice/group. *P < .05. (E) Surface expression of CCRL2 in lymph node FITC+ and FITC− WT DCs after OVA-FITC administration (n = 2; 4 mice/group). (F) Chemotaxis of bone marrow–derived DCs. One experiment representative of 6, each one performed with independent DC cultures. Results are at the net of basal migration (10 ± 3 and 8 ± 2 cells for WT and KO cells, respectively. *P < .05).
Role of CCRL2 in lung DC trafficking. (A) Lung tissue and trachea were isolated from sensitized mice after one OVA-aerosol challenge. DCs were quantified by FACS analysis. Results are expressed as total number of DCs per mouse (mean ± SEM, n = 3; 6 mice/group). (B) The percentage of CD11c+/low autofluorescent DCs in total lung CD11c+ cells and DC subsets (CD103+, CD11b+, and Siglec H+/PDCA1+ pDCs) are presented. (C-D) OVA-sensitized mice received intratracheally 800 μg of OVA-FITC. (C) Representative analysis of WT and CCRL2−/− mediastinal lymph node cells obtained 48 hours after FITC-OVA administration. (D) Kinetics of FITC+ DCs in lymph nodes. Data are mean ± SEM, n = 3; 12-15 mice/group. *P < .05. (E) Surface expression of CCRL2 in lymph node FITC+ and FITC− WT DCs after OVA-FITC administration (n = 2; 4 mice/group). (F) Chemotaxis of bone marrow–derived DCs. One experiment representative of 6, each one performed with independent DC cultures. Results are at the net of basal migration (10 ± 3 and 8 ± 2 cells for WT and KO cells, respectively. *P < .05).
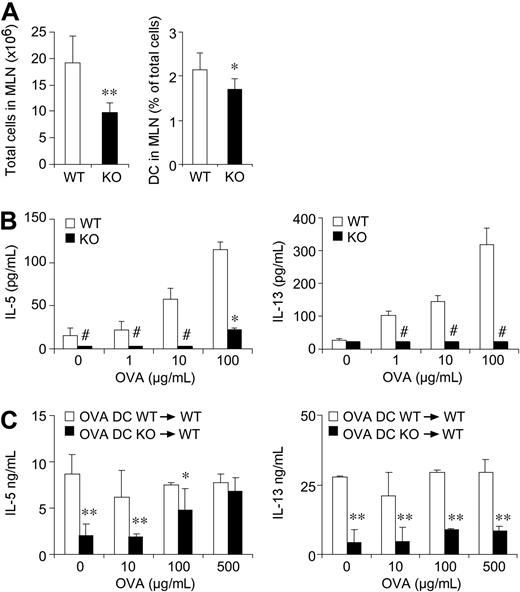
Defective lymphocyte priming in mediastinal lymph nodes of CCRL2−/− mice
The anti-OVA specific immune response was evaluated using cells collected from lung draining lymph nodes. For this purpose, lymph nodes were collected 24 hours after the last aerosol challenge and homogenized, and cell suspensions analyzed. Consistently with the reduced migration of antigen-loaded DCs,30 mediastinal lymph nodes from CCRL2−/− mice showed a 2-fold reduction in total cellularity and a significant reduction in the percentage of total CD11c+ cells (Figure 5A). Lymph node cell suspensions were then exposed to OVA, and Th1/Th2 cytokine levels were assessed in culture supernatants. As shown in Figure 5B, at the end of 4-day culture, WT cells secreted IL-5 and IL-13 at levels that were significantly higher than those detected using cells from CCRL2−/− lymph nodes. In the same experiments, IL-4 and IFNγ levels were below the assay detection limit.
Impaired production of Th2 cytokines in mediastinal lymph nodes of CCRL2−/− mice. Lymph nodes single-cell suspensions were obtained 24 hours after the last aerosol administration. (A) Total cells/mouse and percentage of DCs in gated CD11c+MHCII+ cells. (B) Lymph node cells were cultured (0.5 × 106 cells/0.2 mL/well) with OVA for 96 hours. (C) WT or CCRL2−/− OVA-pulsed bone marrow DCs (106 cells/mouse) were injected intratracheally into WT mice at days 18 and 20. Forty-eight hours later, lymph nodes were excised and cytokine production determined after ex vivo stimulation with OVA. Data are mean ± SEM of 10-20 mice/group from > 2 separate experiments. *P < .05; **P < .01 in comparison to WT mice. # indicates undetectable levels.
Impaired production of Th2 cytokines in mediastinal lymph nodes of CCRL2−/− mice. Lymph nodes single-cell suspensions were obtained 24 hours after the last aerosol administration. (A) Total cells/mouse and percentage of DCs in gated CD11c+MHCII+ cells. (B) Lymph node cells were cultured (0.5 × 106 cells/0.2 mL/well) with OVA for 96 hours. (C) WT or CCRL2−/− OVA-pulsed bone marrow DCs (106 cells/mouse) were injected intratracheally into WT mice at days 18 and 20. Forty-eight hours later, lymph nodes were excised and cytokine production determined after ex vivo stimulation with OVA. Data are mean ± SEM of 10-20 mice/group from > 2 separate experiments. *P < .05; **P < .01 in comparison to WT mice. # indicates undetectable levels.
To directly investigate the role of CCRL2 in the elicitation of the Th2 response in vivo, WT mice were sensitized with OVA at days 0 and 11, and at days 18 and 20, received the intratracheal injection of OVA-pulsed DCs generated from either WT or CCRL2−/− mice. Forty-eight hours after the last DC instillation, mediastinal lymph node cells were collected and cultured in vitro in the presence of OVA. As shown in Figure 5C, cells obtained from mice that had received WT DCs showed a strong Th2 response in terms of IL-5 and IL-13 production that was not further stimulated by the in vitro addition of OVA. The lack of response to OVA in vitro is possibly due to the high concentration of the antigen used to load DCs before adoptive transfer. On the contrary, lymph node cells from mice that had received CCRL2−/− DCs showed very modest response that could be elicited in a dose-dependent manner by the in vitro addition of OVA (Figure 5C). Cells from lymph nodes of mice instilled with un-pulsed WT or CCRL2−/− DCs did not produce IL-5 or IL-13 both under basal conditions and in response to OVA (not shown). The defective ability of CCRL2−/− DCs to prime a Th2 response could not be ascribed to an intrinsic defect of CCRL2−/− DCs, since they were fully able to induce T-cell proliferation in allostimulatory mixed leukocyte reactions (MLRs). Indeed, supplemental Figure 4A shows similar levels of carboxyfluorescein succinimidyl ester-labeled CD4+ cell proliferation when cultured with either allogenic WT or CCRL2−/− DCs. Similarly, CCRL2−/− DCs induced a normal degree of OT-II OVA-specific TCR transgenic CD4+ T-cell proliferation in vitro (supplemental Figure 4B). In agreement with these results, similar levels of cytokines (IL-2, IL-4, and IFN-γ) were detected in the supernatants of T-cell cultures performed with WT and CCRL2−/− DCs (supplemental Figure 4).
Discussion
The migration of lung DCs from periphery to mediastinal lymph nodes is a key step in the development of airway allergy and inflammation, including asthma.3,4,11,31 In this study, we report that the expression of the poorly characterized receptor, CCRL2, is rapidly induced during DC maturation, both in vitro and in vivo, with kinetics that precede CCR7 expression, the master lymph node homing chemokine receptor.5,6 To investigate the potential role of CCRL2 in DC biology, we have generated CCRL2-deficient mice and used them in an established Th2 model of OVA-induced airway inflammation, in which DC migration was shown to play a prominent role.3,6 In this experimental model, antigen-loaded DCs revealed an impaired capacity to traffic to regional lymph nodes and this defect was associated with reduced T-cell priming. CCL11 and CCL17 were also significantly reduced in the airways of CCRL2-deficient mice. CCL11 has been shown to be an important chemoattractant for eosinophils and Th2 cells, whereas CCL17 is a chemoattractant for Th2 cells.32 Therefore, the decreased production of these 2 cytokines likely accounts for the reduced number of these 2 cell types in the airway lumen of CCRL2−/− mice. Indeed, lung eosinophils and T cells do not express CCRL2, ruling out a direct involvement of this receptor in their recruitment. In the lung, CCL11 and CCL17 are produced by epithelial and endothelial cells, whereas smooth muscle cells and alveolar macrophages are responsible for the production of other chemokines, such as CCL2, CCL12, and CCL22.31,33 CCRL2 is expressed by bronchial epithelial cells and lung macrophages after OVA challenge.19 Therefore, it is possible that CCRL2 deficiency in these additional cell types might also contribute to the reduced airway inflammation observed in CCRL2−/− mice. On the contrary, the degree of leukocyte infiltration and the levels of CCL11 and CCL17 in lung tissue were not altered.
The levels of the Th2 cytokines, IL-4 and IL-5, were reduced in both the airway lumen and the lung interstitium, highlighting a defect of CCRL2−/− mice in generating Th2 responses. Consistently with these results, T-cell priming in mediastinal lymph nodes was almost abolished in CCRL2−/− mice. Since lung antigen-specific T-cell response is initiated in the lymph nodes, these results suggest a critical role for CCRL2 in the generation of local primary immune responses.
Indeed, adoptive transfer experiments of antigen-loaded CCRL2−/− DCs into WT animals support the concept that the defective Th2 priming in mediastinal lymph nodes was strictly associated to a defect in DC trafficking. On the other hand, the recruitment of circulating DCs to the lung of CCRL2−/− mice exposed to OVA was not different from that of WT animals. This result suggests that this receptor is not involved in the tissue recruitment of peripheral DCs. Bone marrow-derived DCs generated from CCRL2−/− mice were indistinguishable from WT mice in terms of antigen uptake, membrane phenotype, and antigen presentation capacity. Furthermore, CCRL2−/− DCs expressed normal mRNA levels of all the chemokine receptors investigated and normal in vitro and in vivo migration properties, excluding the possibility that CCRL2 deletion might be associated with a more general alteration of the chemotactic receptor functions. Finally, it should be noted that in vitro, DCs obtained from CCRL2−/− mice were fully competent in promoting the proliferation of allo- or syngeneic OT-II cells and in inducing Th2 cytokine production. Taken together, these results exclude an intrinsic defect of CCRL2−/− DCs to promote T-cell activation and support a role for CCRL2 in lung DC migration and function. Despite the reduced cell recruitment in BAL and of the reduced Th2 priming in lymph nodes, lung function, assessed as methacholine responsiveness, was only slightly decreased. These findings suggest that, in this model, lung function correlates better with the leukocyte content of lung parenchyma, rather than the airway lumen. Indeed, previous studies have shown that blocking CCL12 or CCL22 reduces leukocyte recruitment to the lung interstitium, but not to the airway lumen; this defect was associated with a decrease in airway hyper-responsiveness and mucus production.34,35 Of note, CCRL2−/− mice did not show any alteration when tested in a Th1 model of OVA/LPS-induced lung hypersensitivity36 (data not shown). This result highlights a peculiar role of CCRL2 in Th2-skewed responses.
In the past few years, it has been proposed that CCL2, CCL5, CCL7, and CCL8 could bind and activate CCRL2.37 However, we (data not shown) and others16 have not been able to confirm these data. Of note, we have previously reported that maturation of mouse DCs in vitro causes a marked decrease in the chemotactic response toward CCL2 and CCL5,21 whereas this study reports that CCRL2 is induced during maturation. CCRL2 presents a noncanonical DRYLAIV motif and the ability of this receptor to signal is still a matter of debate. It was recently shown that CCRL2 binds and presents chemerin in the absence of receptor internalization and signaling.16 Chemerin is a chemotactic protein that we and others have recently purified and characterized as the ChemR23 ligand.38,39 This study reports that chemerin induces the in vitro migration of WT and CCRL2−/− DCs, and does not modify the migration to CCR7 ligands (unpublished data). Chemerin expression in mediastinal lymph nodes was not modulated by OVA immunization and challenge in WT and CCRL2−/− mice (data not shown); therefore, the role of chemerin in the trafficking of CCRL2+ lung DCs is presently uncertain.38
The migration of DCs from peripheral tissues to draining lymph nodes relies on the functional expression of CCR7 and CCR8.5,6,26 However, CCR7 proper functioning requires accessory signals. In particular, it was reported that cysteinyl leukotrienes and their transporter protein, MRP1, were required for CCR7 activity.40 We have recently reported that also LTB4, through the activation of its high-affinity receptor, BLT1, promotes CCR7 expression and function in migrating DCs.9 Recent work has proposed that DC trafficking from peripheral tissues is controlled in a tissue-specific manner, with lung DC egression being independent of the expression of MRP1.11 In this article, we show that lung DC migration is impaired in CCRL2-deficient mice. However, we did not observe any defect in the migration of skin DCs in a model of FITC-skin painting and using CCRL2−/− bone marrow-derived DCs injected in the footpads of WT mice (data not shown). Therefore, we propose that MRP1-independent lung DC trafficking to lymph nodes may require a still-unknown accessory signal provided by CCRL2.
In summary, this study reports that CCRL2 plays a nonredundant role in lung DC migration to peripheral lymph nodes, and that this defective DC migration is responsible for the reduced Th2 response observed in CCRL2−/− mice. Therefore, CCRL2 may represent a new target for the control of lung inflammatory responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank P. Dalerba, S. Bernasconi, and M. De Bortoli for their initial contributions to this project and Silvia Lonardi, Cristina Rossigni, and Achille Anselmo for their technical support.
This work was supported by Istituto Superiore Sanità (ISS), Ministero Italiano Università e Ricerca (MIUR), Ministero Italiano della Sanità and Associazione Italiana per la Ricerca sul Cancro (AIRC), NOBEL Project Cariplo, by European Community (INNOCHEM and MOODINFLAME), and Piano Nazionale Richerche-Biotechnologie Avanzate, Tema 2.
Authorship
Contribution: K.O. performed research, analyzed data, and wrote the manuscript; A.V. and F.F. planned the experiments and analyzed the data; E.H., J.K, O.A., C.G., S.G.-F., A.D.P., W.V., and L.S. performed research and analyzed data; C.M.L. analyzed data; A.M. planned the experiments and discussed the data; and S.S. planned the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Silvano Sozzani, Section of General Pathology and Immunology, University of Brescia, viale Europa 11, 25123 Brescia, Italy; e-mail: sozzani@med.unibs.it.
REFERENCES
Author notes
This project was coordinated by A.M. and S.S.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal