Abstract
Leukocyte adhesion in the microvasculature influences blood rheology and plays a key role in vaso-occlusive manifestations of sickle cell disease. Notably, polymorphonuclear neutrophils (PMNs) can capture circulating sickle red blood cells (sRBCs) in inflamed venules, leading to critical reduction in blood flow and vaso-occlusion. Recent studies have suggested that E-selectin expression by endothelial cells plays a key role by sending activating signals that lead to the activation of Mac-1 at the leading edge of PMNs, thereby allowing RBC capture. Thus, the inhibition of E-selectin may represent a valuable target in this disease. Here, we have tested the biologic properties of a novel synthetic pan-selectin inhibitor, GMI-1070, with in vitro assays and in a humanized model of sickle cell vaso-occlusion analyzed by intravital microscopy. We have found that GMI-1070 predominantly inhibited E-selectin–mediated adhesion and dramatically inhibited sRBC-leukocyte interactions, leading to improved microcirculatory blood flow and improved survival. These results suggest that GMI-1070 may represent a valuable novel therapeutic intervention for acute sickle cell crises that should be further evaluated in a clinical trial.
Introduction
Sickle cell disease (SCD), one of the most common inherited blood disorders in the United States,1 results from a single amino acid substitution in the gene encoding the β-globin subunit.2 The β-globin subunit polymerizes in deoxygenation, producing less deformable sickle red blood cells (sRBCs) that can obstruct blood vessels.3 Recurrent vaso-occlusive episodes cause irreversible organ damage and contribute to morbidity and mortality in patients with sickle cell disease due to acute pain crises, chronic inflammation, and ischemic end-organ damage, such as pulmonary hypertension, renal failure, and cerebrovascular injury.4 Although the molecular basis of SCD has been well characterized, the complex cellular and molecular mechanisms underlying vaso-occlusion (VOC) have not been fully elucidated.
Recent studies have suggested that VOC is a complex cascade that involves multiple blood cells, adhesion, and signaling molecules.5 Intravital microscopy analyses in a SCD mouse model expressing exclusively human globin genes6 indicate that sRBCs interact primarily with adherent leukocytes (white blood cells [WBCs]) in postcapillary and collecting venules of cremasteric muscle and leading to vascular obstruction.7 The key role for leukocyte adhesion in sickle cell vascular occlusions has been suggested by the amelioration of flow abnormalities in sickle transgenic mice by anti-inflammatory therapies directed at nuclear factor-κB activation, reactive oxygen species, or endothelial adhesion molecules such as vascular cell adhesion molecule 1, intercellular adhesion molecule 1 (ICAM-1), or the selectins.7-9
The selectins comprise a family of 3 members that mediate adhesion events between blood cells and the endothelium. L-selectin is constitutively expressed on leukocytes and mediate lymphocyte recruitment in lymph nodes and secondary tethers between leukocytes in activated venules. Endothelial cells express 2 selectins, P-selectin that is stored in Weibel-Palade bodies and can be rapidly translocated to the cell surface on stimulation, and E-selectin whose expression is induced by inflammatory cytokines such as tumor necrosis factor α (TNF-α) or interleukin-1β (IL-1β).10 Selectins mediate leukocyte rolling along on the endothelium, allowing leukocytes to rapidly decelerate and to come into close contact with chemokines that will induce firm adhesion. Although mice lacking single selectin genes have relatively mild deficits in leukocyte recruitment, animals deficient in both P- and E-selectins exhibit severe defects in leukocyte adhesion11,12 and are protected from VOC.7 Most studies that evaluate the selectin functions in various animal models have confirmed their overlapping roles, suggesting that the greatest potential for therapy may involve the inhibition of more than 1 selectin and the need to balance anti-inflammatory activities with the risks of infections.13 However, recent studies of the individual function of single selectins in a mouse model of SCD have shown a key role for E-selectin, but not P-selectin, in sending activating signals leading to the up-regulation of the β2 integrin Mac-1, specifically at the leading edge of crawling neutrophils in inflamed venules14 All 3 selectins bind to sialylated and fucosylated moieties presented by glycoprotein or glycolipid ligands.
The involvement of the selectins in numerous disease states has spurred research into the development and rational design of drugs that target selectins and their ligands. By promoting the extravasation and migration of cells out of the bloodstream, the selectins have been identified as drug targets for inflammatory diseases15 and for metastasis of cancer cells.16,17 Past attempts to affect successfully these diseases with selectin antagonists have suffered from the construction of molecules with low affinity, inadequate specificity, and/or poor drug-like properties such as pharmacokinetics and stability.18-20
Here, we have evaluated the biologic effects of GMI-1070, novel small molecule glycomimetic pan-selectin antagonist, in leukocyte-endothelial interactions in vivo and VOC in a sickle cell mouse model.
Methods
Animals
Bone marrow nucleated cells from Berkeley sickle cell mice21 were transplanted into lethally irradiated C57BL/6 animals to generate age- and sex-matched genetically identical cohorts of SCD mice. Fully chimeric male sickle cell mice (expressing > 97% human globin, including βS) were subjected to intravital microscopy 3 to 5 months after bone marrow transplantation.7
ELISA binding protocol
The enzyme-linked immunoabsorbent assay (ELISA) to screen glycomimetic antagonists of the selectins are competitive binding assays, which allows the determination of values of the concentration that inhibits 50% (IC50). Briefly, chimera containing the extracellular domains of E-selectin fused to the immunoglobulin Fc portion (E-selectin/Ig) was immobilized by incubation at 37°C in 96-well microtiter plates for 2 hours. To reduce nonspecific binding, bovine serum albumin was added to each well and incubated at room temperature (RT) for 2 hours. P- or L-selectin/Ig chimera was immobilized by incubation at RT for 1 hour in 96-well microtiter plates precoated with human IgG antibody and blocked with bovine serum albumin. The plate was then washed, and serial dilutions of the test compounds were added to the wells in the presence of conjugates of biotinylated, sialyl Lewis a (sLea; for E-selectin) or sialyl Lewis x (sLex; for P- or L-selectin) polyacrylamide with streptavidin/horseradish peroxidase and incubated for 2 hours at RT. After washing, the peroxidase substrate, 3,3′,5,5′ tetramethyl benzidine was added to determine the amount of sLea bound to immobilized selectin. After 3 minutes, the enzyme reaction was stopped by the addition of H3PO4, and the absorbance of light at a wavelength of 450 nm was determined. The concentration of GMI-1070 required to inhibit binding by 50% was determined and reported as the IC50 value.
In vitro flow assay protocol
To model selectin-mediated cell adhesion in vitro under hydrodynamic shear force, a flow chamber was used, and adhesion events were recorded under low-light microscopy and analyzed by digital image analysis. Briefly, the parallel plate flow chamber with a silicon rubber gasket of a circular design to accommodate 35-mm tissue culture dishes was held in place under vacuum. Before the flow assay, the confluent human umbilical vein endothelial cell (HUVEC) monolayers were stimulated with TNF-α (30 U/mL) for 3 hours to induce the expression of E-selectin on the cell surface. For P-selectin expression, a combination of IL-4 (3 ng) and histamine (2.25μM) was used. The cell suspension of polymorphonuclear leukocytes (PMNs; 106 cells/mL) was perfused through the chamber containing the glycomimetic test compound at a shear rate corresponding to a wall shear stress of 0.9 dynes/cm2. The cell suspension was allowed to flow through the chamber for 3 minutes before digital images were collected to quantify each experiment.
The digital image acquisition and analysis for the flow assay uses Inovision's IC300 digital image system driven by a Silicon Graphics Indigo2 workstation. After 3 minutes of perfusing PMNs through the flow chamber, 10 digital images were acquired at 7 to 10 different locations on each of 3 dishes for every experimental condition. Each image was a result of a real-time minimization function of 3 frames to remove all moving cells in the bulk flow that were not in contact with the HUVEC monolayer. The 10 images at each location were combined into a single composite image with the use of a maximization function. Each rolling cell was then represented by a “streak” in the composite image. A summation of the area of all the streaks in each image is defined as the Rolling Index), which is a combined measure of the number and rolling velocity of PMN′s rolling on the HUVEC monolayer. Results were expressed as the percentage of inhibition of the Rolling Index.
Intravital experimental protocol
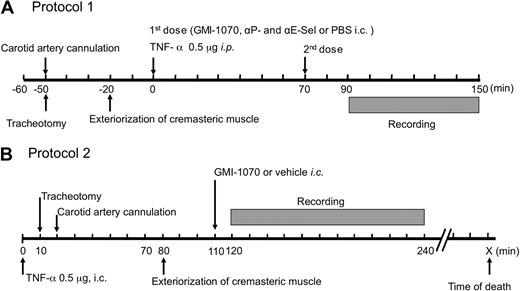
To characterize the effects of the selectin antagonist GMI-1070 on leukocyte rolling behavior, we used 2 protocols shown in Figure 1. In both protocols, SCD mice were anesthetized by intraperitoneal injection of a mixture of 2% chloralose and 10% α-urethane in phosphate-buffered saline (PBS; 6 mL/kg). A polyethylene tube was inserted into the trachea of anesthetized animals to facilitate spontaneous respiration, and then the right carotid artery was cannulated for administration of GMI-1070 (20 mg/kg, each dose), a mixture of antibodies against P-selectin (clone RB40.34; gift from Dietmar Vestweber, Max-Planck-Institute of Molecular Biomedicine) and E- selectin (clone 9A9; gift from Dr Barry Wolitzky, Immune Tolerance Network); in protocol 1, 1 mg/kg of each antibody or of vehicle PBS control. The cremaster muscle was gently exteriorized and then continuously superfused throughout the experiment with warmed (37°C) bicarbonate-buffered (pH 7.4) saline aerated with a mixture of 95% N2 and 5% CO2. In protocol 1, each experimental mouse received 2 doses of each antagonist, PBS saline containing antibodies, or equal volume of PBS control. The first injection was performed immediately after TNF-α injection (T0) and the second injection was made at 70 minutes (T70) after TNF-α administration. Twenty minutes after exposure to antagonists, PBS, or the antibody cocktail, 8 to 12 venules of each mouse were videotaped over a period of 60 minutes, with each venule recorded continuously for at least 2 minutes. To evaluate the therapeutic potential of GMI-1070 on SCD mice under sickle vasoocclusive crisis, we have also studied mice by a more clinically relevant protocol in which GMI-1070 was administered 110 minutes after TNF-α and the surgical trauma (protocol 2). This is the latest possible time point to treat the crisis because the control SCD mice begin to die shortly thereafter (∼ 120 minutes). Venules were visualized with a custom-designed intravital microscope (MM-40; Nikon), using a 60× water immersion objective (Nikon). Images were recorded with a charge-coupled device video camera (Hamamatsu) and video recorder (Sony SVHS, SVO-9500). Venular diameter was measured with a video caliper. Centerline red cell velocity (VRBC) was measured for each venule in real time with the use of an optical Doppler velocimeter (Texas A&M). Wall shear rate (γ) was calculated on the basis of Poiseuille law for a Newtonian fluid, γ = 2.12 (8Vmean) / Dv, where Dv is the venular diameter, Vmean is estimated as VRBC/1.6, and 2.12 is a median empirical correction factor obtained from actual velocity profiles measured in microvessels in vivo. Blood flow rate was calculated as Q = Vmean πd2/4, where Q is blood flow rate and where d is venule diameter and is expressed as 10−6 μL/s. Blood samples taken immediately after recording, through a cardiac puncture, were used to determine leukocyte counts with the use of a hemocytometer.
Schematic representation of intravital microscopy protocols. (A) In protocol 1, after anesthesia and surgical preparations, SCD mice were injected intraperitoneally (i.p.) with murine TNF-α (time 0) and then immediately with GMI-1070, or an antibody mixture containing anti–P- and –E-selectin monoclonal antibodies, or PBS through an intracarotid artery catheter (i.c.). To ensure activities of the selectin antagonist during the entire course of studies, SCD mice received a second dose of antagonists or vehicle controls 70 minutes after the first injection (T70). Images of the cremasteric venules under intravital microscopy were recorded between the time points of 90 and 150 minutes (T90 → T150). During filming, the hemodynamic parameters, including centerline velocity, venular diameter, and shear rate, were measured. (B) Protocol 2 was designed to assess the therapeutic effects of GMI-1070 on SCD mice with ongoing acute veno-occlusive crisis primed by TNF-α. SCD mice were infused with GMI-1070 at 110 minutes after animals were challenged with TNF-α (time 0). Images were recorded in the 2-hour interval between 120 and 240 minutes (T120 → T240) after the administration of GMI-1070.
Schematic representation of intravital microscopy protocols. (A) In protocol 1, after anesthesia and surgical preparations, SCD mice were injected intraperitoneally (i.p.) with murine TNF-α (time 0) and then immediately with GMI-1070, or an antibody mixture containing anti–P- and –E-selectin monoclonal antibodies, or PBS through an intracarotid artery catheter (i.c.). To ensure activities of the selectin antagonist during the entire course of studies, SCD mice received a second dose of antagonists or vehicle controls 70 minutes after the first injection (T70). Images of the cremasteric venules under intravital microscopy were recorded between the time points of 90 and 150 minutes (T90 → T150). During filming, the hemodynamic parameters, including centerline velocity, venular diameter, and shear rate, were measured. (B) Protocol 2 was designed to assess the therapeutic effects of GMI-1070 on SCD mice with ongoing acute veno-occlusive crisis primed by TNF-α. SCD mice were infused with GMI-1070 at 110 minutes after animals were challenged with TNF-α (time 0). Images were recorded in the 2-hour interval between 120 and 240 minutes (T120 → T240) after the administration of GMI-1070.
Image analyses
All analyses were made with playback assessment of videotapes as previously described.7,14,22 Briefly, rolling WBCs were defined as those moving at a velocity less than that of free-flowing erythrocytes in a given vessel and counted over 1-minute intervals per venule. Adherent WBCs were defined as those remaining stationary for at least 30 seconds over a 100-μm venular segment. RBCs were identified by their size and shape (discoid and sickle-shaped cells). An interaction between RBCs and adherent WBCs was defined as the arrest of an RBC on an adherent WBC for more than 2 video frames (> 0.07 second). Leukocyte rolling velocity was calculated by dividing the traveled distance by the tracking time or as the average translation over 2 seconds for 10 WBCs per venule, and expressed as μm/s. The flux fraction (F) of rolling leukocytes, corresponding to the percentage of leukocytes that are rolling per minute, was calculated by F = WBCr/(0.25πd2VRBC60 [WBC]), where WBCr is the number of rolling leukocytes past a fixed reference point in the venule per minute, d is venule diameter, VRBC is centerline velocity, and [WBC] is the systemic leukocyte count.
Statistical analyses
All data are shown as mean plus or minus SEM. Parametric data were analyzed with the use of analysis of variance. Statistical significance for nonparametric distributions (RBC-WBC interactions) was assessed with the use of the Mann-Whitney test. A value of P less than .05 was deemed significant.
Results
Generation and structure of GMI-1070
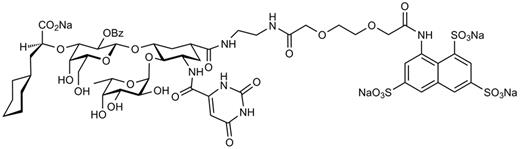
The selectin antagonist GMI-1070 was designed on the basis of the bioactive conformation of sLex in the carbohydrate-binding domain of E-selectin as determined by nuclear magnetic resonance techniques.23,24 Structural requirements for binding L- and P-selectins were also incorporated. The full synthetic scheme and molecular properties of GMI-1070 will be published elsewhere. The chemical structure of GMI-1070 is shown in Figure 2.
Chemical structure of GMI-1070. GMI-1070 is a glycomimetic pan-selectin inhibitor that was rationally designed on the basis of the bioactive conformation of sLex in the binding site of E-selectin as determined empirically by nuclear magnetic resonance methods. Incorporation of a sulfate-binding domain is important for interactions with P- and L-selectins.
Chemical structure of GMI-1070. GMI-1070 is a glycomimetic pan-selectin inhibitor that was rationally designed on the basis of the bioactive conformation of sLex in the binding site of E-selectin as determined empirically by nuclear magnetic resonance methods. Incorporation of a sulfate-binding domain is important for interactions with P- and L-selectins.
GMI-1070 antagonizes all 3 selectins
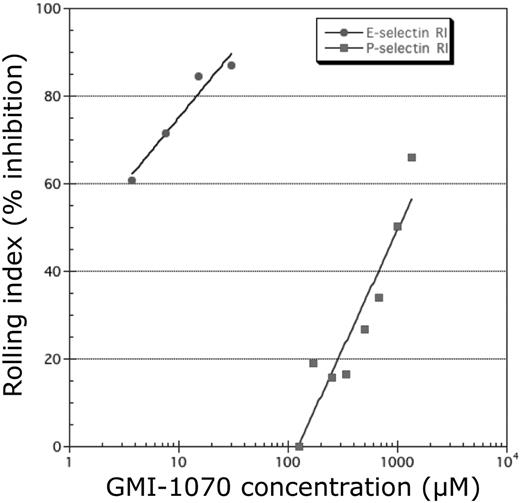
The ability of GMI-1070 to inhibit binding to E-, P-, and L-selectins was evaluated with the use of ELISA in which selectin chimeras were immobilized on microtiter plates and binding to sLea or sLex was determined. We found that the IC50 of GMI-1070 was 4.3μM for E-selectin, much lower than P-selectin (423μM) or L-selectin (337μM), suggesting that, although GMI-1070 inhibits binding to all 3 selectins, its activity is greatest toward E-selectin. To investigate further this issue, we assessed the effect of GMI-1070 on selectin-mediated cell adhesion in vitro with the use of a parallel plate flow chamber. Confluent HUVEC monolayers were stimulated with TNF-α to induce the expression of E-selectin or IL-4 and histamine to up-regulate P-selectin expression on the cell surface. PMNs were perfused through the chamber containing GMI-1070 under a shear stress of 0.9 dynes/cm2. Consistent with the ELISA findings, GMI-1070 inhibited the interactions of PMNs more effectively when E-selectin was induced on HUVECs than for P-selectin (Figure 3).
GMI-1070 inhibits selectin-mediated rolling. Studies in a parallel plate flow chamber showed a dose response of GMI-1070 to inhibit selectin-mediated rolling of isolated human neutrophils on monolayers of HUVECs activated to express E-selectin or P-selectin. Inhibition was greater on E-selectin–expressing HUVECs than on P-selectin–expressing HUVECs. RI indicates Rolling Index.
GMI-1070 inhibits selectin-mediated rolling. Studies in a parallel plate flow chamber showed a dose response of GMI-1070 to inhibit selectin-mediated rolling of isolated human neutrophils on monolayers of HUVECs activated to express E-selectin or P-selectin. Inhibition was greater on E-selectin–expressing HUVECs than on P-selectin–expressing HUVECs. RI indicates Rolling Index.
GMI-1070 alters in vivo leukocyte rolling and recruitment
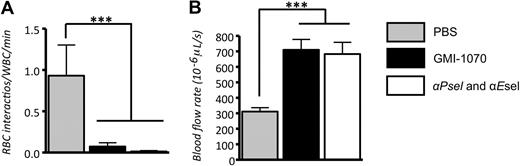
Preclinical studies on dosing and pharmacokinetics have established that GMI-1070 administered intravenously has a serum half-life of 1.25 hours in mice and 7.4 hours in humans (Helen Thackray and J.L.M., unpublished data, January 5, 2009). In the first protocol, to ensure adequate dosage during the entire course of studies, a second dose of GMI-1070 was administered 70 minutes after the TNF-α injection. Leukocyte behavior, including rolling, adhesion, and the capture rates of sRBCs, were analyzed in 51 venules of 5 mice treated with GMI-1070 and in 58 venules of 5 mice treated with P- and E-selectin antibodies. Administration of GMI-1070 did not significantly change systemic WBC counts in SCD mice under protocol 1, whereas significant increased WBC counts in mice treated with P- and E-selectin antibodies were observed (Table 1). Leukocyte rolling, adhesion, and RBC interaction with adherent leukocytes were monitored and analyzed in the 60-minute interval between 90 and 150 minutes after the inflammatory challenge. Leukocyte rolling was assessed as leukocyte rolling flux fraction, defined as the number of rolling leukocytes divided by the total number of leukocytes passing through the same vessel. Under inflammatory condition induced by combined surgical exteriorization of the cremaster muscle and exposure to TNF-α, rolling flux fraction was approximately 4.6% in PBS-treated SCD mice. Virtually all rolling during this time interval was mediated by P-and E-selectins because it was completely ablated by coinjection of anti–P-selectin and anti–E-selectin antibodies. Unexpectedly, the rolling fraction was increased to 11% in GMI-1070–treated mice. (Figure 4A; ***P < .001). The increased rolling fraction is reminiscent of experiments in which E-selectin is inactivated25 and confirms the in vitro studies described in the previous section, suggesting that GMI-1070 largely antagonizes E-selectin in vivo. The changes in rolling fractions were not due to alterations in leukocyte counts because similar conclusions can be drawn if the results are expressed as absolute numbers of rolling leukocytes per minute (10.9 ± 1.4 for PBS control; 41.6 ± 3.5 for GMI-1070, and 0.3 ± 0.1 for the group anti–P- and –E-selectin; all groups P < .01 compared with PBS). By contrast, the average number of WBCs adherent to endothelium was reduced by approximately 53% in SCD mice treated with GMI-1070 compared with SCD controls treated with PBS (Figure 4B-C; ***P < .001), suggesting significant blockade of P-selectin. Leukocyte adhesion was markedly reduced (by ∼ 97%) when endothelial selectins were blocked with the use of antibodies (Figure 4B-C).
GMI-1070 alters leukocyte recruitment. (A) The rolling flux fraction was increased by nearly 2-fold in SCD mice treated with GMI-1070, whereas it was completely inhibited when both endothelial selectins were blocked by antibody administration. (B) The average number of leukocytes adherent to endothelium was significantly reduced in SCD mice treated with GMI-1070 but completely inhibited by antiselectin antibodies. (C) Representative images of venules from sickle mice treated with PBS, GMI-1070, or anti–P- and –E-selectin antibodies. Each still frame was taken at the 30-minute time point after TNF-α injection. Both small molecule selectin antagonists and antiselectin antibodies significantly reduced the number of adherent leukocytes (circles) and RBCs interacting with adherent leukocytes (black arrows; see data in Figure 5). The small white arrow indicates an RBC interaction with the endothelium, and the large white arrow shows the direction of blood flow.
GMI-1070 alters leukocyte recruitment. (A) The rolling flux fraction was increased by nearly 2-fold in SCD mice treated with GMI-1070, whereas it was completely inhibited when both endothelial selectins were blocked by antibody administration. (B) The average number of leukocytes adherent to endothelium was significantly reduced in SCD mice treated with GMI-1070 but completely inhibited by antiselectin antibodies. (C) Representative images of venules from sickle mice treated with PBS, GMI-1070, or anti–P- and –E-selectin antibodies. Each still frame was taken at the 30-minute time point after TNF-α injection. Both small molecule selectin antagonists and antiselectin antibodies significantly reduced the number of adherent leukocytes (circles) and RBCs interacting with adherent leukocytes (black arrows; see data in Figure 5). The small white arrow indicates an RBC interaction with the endothelium, and the large white arrow shows the direction of blood flow.
GMI-1070 dramatically increases leukocyte rolling velocities
To understand further the mechanisms, we characterized leukocyte behavior in more detail by evaluating the velocity of leukocyte rolling in 193 leukocytes from 42 venules of 6 SCD mice treated with PBS and 481 leukocytes from 51 venules of 6 SCD mice treated with GMI-1070. Leukocytes in SCD mice treated with PBS rolled at an average velocity of 20.9 ± 1.1 μm/s, ranging from 0.3 to 86 μm/s (Figure 5A). In contrast, average leukocyte rolling velocity in SCD mice treated with GMI-1070 was significantly increased at 37.8 ± 1.2 μm/s, ranging from 2.5 to 207 μm/s (Figure 5B-C). Furthermore, cumulative frequency histograms showed that GMI-1070 shifted leukocyte rolling from slower to faster with approximately 1.6-fold higher median rolling velocities in PBS-treated control (Vmedian = 32 μm/s for GMI-1070 and Vmedian = 16 μm/s for PBS-treated control; Figure 5D). The dramatic effect of GMI-1070 on leukocyte rolling velocities is consistent with efficient in vivo blockade of E-selectin function.
Leukocyte rolling velocity histograms. Leukocyte rolling velocities in (A) PBS-treated control SCD mice and (B) GMI-1070–treated SCD mice. The arrowheads indicate the means. (C) Overlay of histograms of panels A and B shows the increased average leukocyte velocities in GMI-1070–treated mice. (D) Cumulative frequency histogram of leukocyte rolling velocities for these 2 groups. Median values are indicated by vertical lines; *** P < .001.
Leukocyte rolling velocity histograms. Leukocyte rolling velocities in (A) PBS-treated control SCD mice and (B) GMI-1070–treated SCD mice. The arrowheads indicate the means. (C) Overlay of histograms of panels A and B shows the increased average leukocyte velocities in GMI-1070–treated mice. (D) Cumulative frequency histogram of leukocyte rolling velocities for these 2 groups. Median values are indicated by vertical lines; *** P < .001.
GMI-1070 inhibits RBC interactions with WBCs
Our recent studies have shown that E-selectin–induced neutrophil activation was critical to enable their capture of circulating RBCs.14 To assess the effect of GMI-1070 on sickle cell adhesion, we quantified the interactions between circulating RBCs and adherent WBCs in venules recorded between 90 and 150 minutes. We found that the administration of GMI-1070 markedly reduced the number of RBCs interacting per adherent WBC (∼ 93% reduction; Figure 6A; P < .001). These data suggest that a selectin antagonist can affect the capture of RBCs even when adherent leukocytes are already recruited.
GMI-1070 inhibits RBC captures and improves blood flow in SCD mice. (A) Number of RBC interactions with adherent WBCs per minute. Both GMI-1070 and antiselectin antibodies reduced the capture rates of RBCs per adherent leukocytes. (B) The blood flow rates in SCD mice treated with GMI-1070 or anti–P- and –E-selectin antibodies were significantly higher than in mice treated with PBS control. This difference was not due to venular size because the average venular diameter was nearly identical (∼ 21 μm) among the 3 groups (Table 1); ***P < .001.
GMI-1070 inhibits RBC captures and improves blood flow in SCD mice. (A) Number of RBC interactions with adherent WBCs per minute. Both GMI-1070 and antiselectin antibodies reduced the capture rates of RBCs per adherent leukocytes. (B) The blood flow rates in SCD mice treated with GMI-1070 or anti–P- and –E-selectin antibodies were significantly higher than in mice treated with PBS control. This difference was not due to venular size because the average venular diameter was nearly identical (∼ 21 μm) among the 3 groups (Table 1); ***P < .001.
During intravital microscopy studies, hemodynamic parameters were measured with the use of an optical velocimeter, and leukocyte behavior was recorded and then analyzed. The mean centerline VRBC in mice treated with GMI-1070 or P- and E-selectin antibodies were almost equal and were significantly higher than that in PBS-treated control mice by near to 2-fold (P < .001; Table 1). This difference was not attributable to venular size because the average venular diameter (∼ 21 μm) was nearly identical among the 3 groups (Table 1). As a result, the wall shear rates (γ) in animals treated with GMI-1070 and anti–P- and –E-selectins were almost 2-fold greater than PBS-treated control. Because of reduced leukocyte recruitment and sRBC interactions in the microvasculature, the average blood flow rates were dramatically improved in mice treated with GMI-1070 or anti–P- and –E-selectins, compared with the PBS control group (Figure 6B; ***P < .001).
GMI-1070 administration inhibits leukocyte adhesion and sRBC interactions after the inflammatory trigger
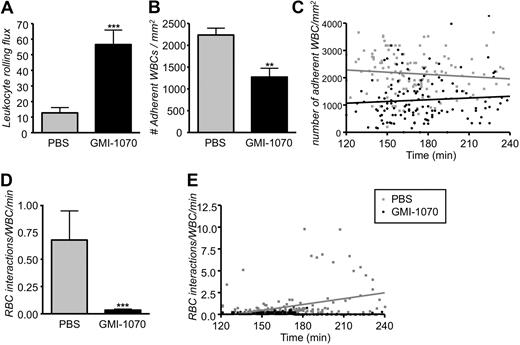
To evaluate the potential therapeutic effects of GMI-1070 in the painful sickle crisis, we have modified the protocol by delaying the infusion of GMI-1070 into SCD animals 110 minutes after TNF-α injection (Figure 1 protocol 2), a time at which leukocytes have already been recruited and a vaso-occlusive crisis is established.7 In protocol 2, venules were recorded immediately after the infusion and during the following 2 hours. Careful analyses of those recordings from 132 venules of 11 control mice treated with PBS and 130 venules of 8 SCD mice treated with GMI-1070 showed a 4.4-fold increase in leukocyte rolling flux in sickle mice treated with GMI-1070 (Figure 7A; ***P < .001). In addition, the average number of leukocytes adherent to the endothelium was reduced by approximately 44% in SCD mice treated with GMI-1070 compared with PBS-treated controls (Figure 7B; **P = .01). The effect of GMI-1070 on leukocyte adhesion was rapid because it was already detectable (***P < .001) in the first recorded venules approximately 20 minutes after the end of infusion (Figure 7C). The number of adherent leukocytes did not increase progressively over time in PBS-treated controls; however, SCD mice treated with GMI-1070 exhibited a slight increase over time in the number of leukocyte adherent endothelium, probably because of the relatively short half-life of the compound in mice. To evaluate the effect of GMI-1070 on the capabilities of adherent leukocytes to capture sickle cells, we quantified the interactions between circulating RBCs and adherent WBCs. Even after administration this late after the inflammatory stimuli, GMI-1070 treatment dramatically reduced the number of RBCs interacting per adherent WBC (20-fold reduction; Figure 7D; ***P < .001). Furthermore, we found the rate of RBC-WBC interactions (gray regression line) progressively increased over time in the control mice treated with PBS compared with SCD mice treated with GMI-1070, which shows a stable straight regression line near to abscissa (Figure 7E).
Administration of GMI-1070 after the inflammatory challenge produces the same biologic effects. Mice were treated with GMI-1070 or PBS as per protocol 2. (A) Leukocyte rolling flux (cells rolling per minute) was significantly elevated greater than 4-fold increase in SCD mice treated with GMI-1070; ***P < .001. (B) Recruitment of adherent leukocytes was severely inhibited in GMI-1070–treated SCD mice compared with PBS-treated SCD mice; **P < .01. (C) Scatter plots of the number of adherent leukocytes during the experimental period. Each dot represents data obtained from a single venule. GMI-1070 rapidly reduced the number of adherent leukocytes, compared with the PBS control group, but the number of adherent leukocytes remained almost unchanged over time after TNF-α exposure in both PBS-treated control (gray regression line) and GMI-1070–treated mice (black regression line). (D) Interactions between circulating RBCs and adherent leukocytes were abrogated (∼ 95% reduction) in SCD mice infused with GMI-1070. ***P < .001. (E) Scatter plots of the number of interactions between RBCs and adherent leukocytes during the experimental period. Each symbol represents data from a single venule. The increase in RBC-WBC interactions over time after TNF-α exposure was abrogated by the GMI-1070–treated compared with PBS-treated control mice. The gray and black regression lines represent PBS-treated and GMI-1070–treated mice, respectively.
Administration of GMI-1070 after the inflammatory challenge produces the same biologic effects. Mice were treated with GMI-1070 or PBS as per protocol 2. (A) Leukocyte rolling flux (cells rolling per minute) was significantly elevated greater than 4-fold increase in SCD mice treated with GMI-1070; ***P < .001. (B) Recruitment of adherent leukocytes was severely inhibited in GMI-1070–treated SCD mice compared with PBS-treated SCD mice; **P < .01. (C) Scatter plots of the number of adherent leukocytes during the experimental period. Each dot represents data obtained from a single venule. GMI-1070 rapidly reduced the number of adherent leukocytes, compared with the PBS control group, but the number of adherent leukocytes remained almost unchanged over time after TNF-α exposure in both PBS-treated control (gray regression line) and GMI-1070–treated mice (black regression line). (D) Interactions between circulating RBCs and adherent leukocytes were abrogated (∼ 95% reduction) in SCD mice infused with GMI-1070. ***P < .001. (E) Scatter plots of the number of interactions between RBCs and adherent leukocytes during the experimental period. Each symbol represents data from a single venule. The increase in RBC-WBC interactions over time after TNF-α exposure was abrogated by the GMI-1070–treated compared with PBS-treated control mice. The gray and black regression lines represent PBS-treated and GMI-1070–treated mice, respectively.
GMI-1070 reverses sickle cell crisis by improving blood flow and survival of SCD mice
Administration of GMI-1070 110 minutes after the inflammatory stimuli increased the mean VRBC by almost 2-fold compared with PBS-treated controls (P < .001; Table 2). This difference was also not attributable to venular size because the average venular diameter was nearly identical between the 2 groups (Table 2). The calculated mean blood flow rate, which can be regarded as a surrogate measure for VOC, was more than 2-fold higher in GMI-1070–treated mice than in control animals (Figure 8A; P < .001). After the end of the recording period, mice were monitored for up to 11 hours after the injection of TNF-α to assess the effect of the GMI-1070 therapy on mortality. Kaplan-Meier survival curves showed a dramatic improvement in survival of SCD mice treated with GMI-1070 compared with control SCD mice treated with PBS (Figure 8B; P = .007, log-rank test). The median survival in PBS-treated controls was at least 4 hours shorter than GMI-1070–treated SCD mice (5 hours vs 9.7 hours, respectively). Taken together, these results strongly suggest that GMI-1070 therapy may be beneficial in SCD during acute crisis.
GMI-1070 sustains blood flow rates and prolongs survival in TNF-α–exposed SCD mice challenged by surgical trauma. (A) Blood flow rates were 2-fold higher in GMI-1070–treated SCD mice compared with control SCD mice; ***P < .001. (B) The Kaplan-Meier survival curves for GMI-1070–treated or control SCD mice. Survival was significantly improved in the GMI-1070–treated group. Log-rank (Mantel-Cox) test, P = .007.
GMI-1070 sustains blood flow rates and prolongs survival in TNF-α–exposed SCD mice challenged by surgical trauma. (A) Blood flow rates were 2-fold higher in GMI-1070–treated SCD mice compared with control SCD mice; ***P < .001. (B) The Kaplan-Meier survival curves for GMI-1070–treated or control SCD mice. Survival was significantly improved in the GMI-1070–treated group. Log-rank (Mantel-Cox) test, P = .007.
Discussion
These studies provide the proof-of-principle of the treatment of sickle cell crises with selectin inhibitors. Our data support the notion that VOC in SCD is not a fixed irreversible event but a highly dynamic process that potentially could be reversed by targeted therapy.
Selectin ligands are composed of the tetrasaccharide sLex consisting of sialylated and fucosylated N-acetyllactosamine. Previous attempts to treat inflammatory diseases with selectin antagonists have suffered from the use of carbohydrate ligands (CY-1503) with inherently low-affinity and poor pharmacologic properties, such as a serum half-life of minutes.18 Other strategies to design glycomimetic antagonists (TBC-1269) have achieved more druglike properties, but have simplified the molecule at the expense of loss of activity and specificity.19 For example, both of these previous attempts produced molecules with relatively weak inhibitory activity for E-selectin (IC50 > 500μM). The structure of GMI-1070 was rationally designed on the basis of the bioactive conformation of sLex in the binding site of E-selectin as determined empirically by nuclear magnetic resonance methods rather than molecular modeling. This approach led to potent E-selectin inhibitory activity (IC50 = 4.3μM) along with good druglike properties such as low toxicity, metabolic stability, and adequate half-life profile.
Although all 3 selectins bind to sLex, the efficiency of interactions with P-selectin and L-selectin is greatly enhanced by additional interactions with negatively charged structures. For example, P-selectin glycoprotein ligand-1 harbors sulfated N-terminal tyrosine residues,26 whereas putative L-selectin ligands have sulfated glycoconjugates.27 Because soluble sLex displays mild selectin antagonistic activity in vivo,28 and because of disappointing results in clinical trials,29 GMI-1070 was rationally designed to contain both a more potent sLex mimetic with an extended sulfated domain to accommodate the binding requirements of P- and L-selectins and to confer druglike properties to the molecule.23,30,31
GMI-1070 is a pan-selectin inhibitor by design and appears to largely act as an E-selectin antagonist in vivo with partial inhibition of P-selectin at these doses, which is consistent with solid-phase in vitro assays and the results with the use of a parallel plate flow chamber in which the activity against E-selectin predominates. This conclusion from the intravital microscopy analyses is supported by the absence of any reduction in leukocyte rolling and mild effect on leukocyte adhesion. In fact, the leukocyte rolling fraction and rolling velocities were both significantly increased shortly after the administration of GMI-1070, a hallmark of E-selectin inhibition. Interestingly, the partial overall blockade of leukocyte adhesion by GMI-1070 appeared to be as efficient in protecting against VOC as the administration of 2 antibodies against either endothelial selectins. These data are consistent with our recent studies in which we have described a major role of E-selectin in sending activating signals into neutrophils that lead to the regional activation of Mac-1 at the leading edge of neutrophils, thereby allowing RBC capture, reduction in blood flow, and VOC.14 Indeed, GMI-1070 dramatically inhibited RBC-WBC interactions, improved blood flow, and survival of SCD mice, emphasizing a major role for E-selectin in these activities. In further support of these findings, the levels of circulating E-selectin in patients with SCD was shown to correlate positively with the rate of mortality over a 4-year period.32
In inflamed venules, leukocyte recruitment is an extremely dynamic process in which many adherent leukocytes continuously crawl to survey the venular endothelium while others detach to return in the circulation. Extravasated leukocytes account for a relatively minor subset of leukocytes that have adhered. Even after administration and long after the inflammatory challenge was initiated, GMI-1070 rapidly reduced the number of adherent leukocytes and also rapidly inhibited RBC-WBC interactions. These effects on leukocyte behavior are remarkably similar to those previously reported with intravenous γ-globulin administration,22 suggesting parallel mechanisms between the 2 drugs. The similar effects observed in the 2 protocols highlight the dynamic nature and potential reversibility of leukocyte adhesion and activation after targeted intervention, at least under the conditions studied herein.
It is important to mention the differences between this mouse model of SCD and the human disease. This mouse model of VOC is generally much more severe than the human disease in that it can lead to the death of most animals in several hours, in contrast with human sickle cell crises that are usually brought up over hours to days and are usually not lethal. However, we would argue that disease reversibility in the face of such a severe model bodes well for potential clinical relevance of a specific intervention. A phase 1 clinical trial has recently indicated that GMI-1070 has a good safety profile with a serum half-life of 7 to 8 hours. Metabolism is also minimal because greater than 90% of the drug is excreted intact and no serious adverse events because of toxicity were observed (J.L.M., unpublished data, January 14, 2009). The dramatic effects of GMI-1070, including the significant protection from death even when the drug is administered long after the inflammatory trigger, warrant further study in a clinical trial to treat acute VOC.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Dietmar Vestweber and Barry Wolitzky for providing reagents.
This work was supported in part by grants from GlycoMimetics Inc and by the National Institutes of Health (R01 HL69438; P.S.F.) and (T32 HL07824; J.C.). P.S.F. received an Established Investigator Award from the American Heart Association.
National Institutes of Health
Authorship
Contribution: J.C. designed the research, performed experiments, analyzed data, and wrote the manuscript; J.T.P. performed in vitro experiments and contributed a vital new reagent; A.S., and B.E. contributed a vital new reagent; J.L.M. contributed a vital new reagent, designed the research, analyzed data, and wrote the manuscript; and P.S.F. supervised the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: J.T.P., A.S., B.E., J.L.M., and P.S.F. have declared a financial interest in a company whose potential product was studied in the present work. P.S.F. received a research award and consulting fees including a stock option from GlycoMimetics that funded in part the present studies. J.C. declares no competing financial interests.
The current affiliation for J.C. is Graduate Institute of Medical Sciences Research Center for Biomedical Implants and Microsurgery Devices, Taipei Medical University, Taipei, Taiwan.
Correspondence: Paul S. Frenette, Ruth L. and David S. Gottesman Institute for Stem Cell and Regenerative Medicine, Albert Einstein College of Medicine, 1301 Morris Park Ave, Price Center, Rm 101B, Bronx, NY 10461; e-mail: paul.frenette@einstein.yu.edu; or John Magnani, GlycoMimetics Inc, 101 Orchard Ridge Dr, 1E, Gaithersburg, MD 20878; e-mail: jmagnani@glycomimetics.com.