Abstract
Cobalamin (Cbl, vitamin B12) deficiency in humans is a cause of hematologic and neurologic disorders. We show here that the cellular export of Cbl, in contrast to the carrier- and receptor-dependent cellular import of Cbl, occurs by transmembrane transport of “free” Cbl. Screening of candidate transporters by cellular gene silencing showed a role in cellular Cbl efflux of the ATP-binding cassette (ABC)–drug transporter, ABCC1, alias multidrug resistance protein 1 (MRP1), which is present in the basolateral membrane of intestinal epithelium and in other cells. The ability of MRP1 to mediate ATP-dependent Cbl transport was confirmed by vesicular transport experiments, and a physiologic role of MRP1 in mammalian Cbl homeostasis is indicated by the phenotype of knockout mice with targeted disruption of MRP1. These animals have a reduced concentration of Cbl in plasma and in the storage organs liver and kidney. In contrast, Cbl accumulates in the terminal part of the intestine of these mice, suggesting a functional malabsorption because of a lower epithelial basolateral Cbl efflux. The identification of this Cbl export mechanism now allows the delineation of a coherent pathway for Cbl trafficking from food to the body cells.
Introduction
Cobalamin (Cbl, vitamin B12) is a multifunctional organic tetrapyrrol structure synthesized by bacteria and archea. Mammals take up Cbl from their diet because they cannot synthesize Cbl and need this vitamin as an essential coenzyme for methylmalonyl coenzyme A mutase and methionine synthase. Deficiency of Cbl is the most common vitamin deficiency disease in industrialized countries and the cause of pernicious anemia, a serious disorder characterized by megaloblastic anemia and neurologic symptoms (for review see Reynolds1 and Depeint et al2 ). Deficiency of Cbl also causes a functional folate deficiency because of the role of methionine synthase in folate methylation (for review see Shane and Stokstad3 ).
Several proteins are involved in the uptake and transport of Cbl in mammals. In the gastrointestinal tract Cbl is bound to intrinsic factor (IF) secreted from the gastric epithelium, and the Cbl-IF complex is taken up by endocytosis after binding to the cubilin/amnionless receptor complex (cubam) present on the apical surface of the ileal epithelium.4,5 On internalization, IF is degraded in lysosomes and Cbl is released to the cytosol.6 Recent evidence from genetic analysis of a group of Cbl-deficiency patients with lysosomal accumulation of Cbl suggests that the lipocalin receptor-like protein LMBD1 is involved in Cbl transport across the lysosomal membrane.7
In plasma and interstitial fluids Cbl is bound to transcobalamin (TC) a protein homologous to IF. Cells internalize the TC-Cbl complex by an endocytic receptor, recently identified as the CD320 membrane protein.8 TC-Cbl filters through the kidney glomeruli and is efficiently taken up from the ultrafiltrate via endocytosis by binding to another receptor, megalin.9 Like CD320 and cubam, megalin causes the transfer of the TC-Cbl complex to the lysosomes where the protein moiety is degraded. Cbl is exchanged between cells and organs in the body, but it is not known how intracellular Cbl is exported from cells, including enterocytes. Because enterocytes have a high rate of synthesis of TC, it has been a common view10,11 that Cbl is secreted from the enterocytes in complex with TC. An alternative hypothesis is that free Cbl is transported from the cytosol across the basolateral cell surface into plasma, where it subsequently forms a complex with circulating TC. Whatever the pathway for cellular Cbl excretion, it has to involve transport across the plasma membrane or a vesicular membrane.
Prokaryotes are known to import nonprotein bound (“free”) Cbl in an ATP-dependent manner by a protein belonging to the ATP-binding cassette (ABC) family (BtuCD),12-14 but no eukaryotic transporters capable of transporting free Cbl across the plasma membrane have been identified so far. Here, we report that free Cbl is effluxed by cultured cells. With a small interfering RNA (siRNA)–based screening assay we identified ABCC1 (also known as multidrug resistance protein 1; MRP1) as a transport protein, capable of effluxing free Cbl. We demonstrate that MRP1 is able to transport Cbl in vesicular transport assays and that Cbl homeostasis is altered in mice that lack MRP1.
Methods
Materials and cell lines
DB M-270 amine magnetic beads were from Dynal Biotech. Colabeled cyano-Cbl ([57Co]-CN-Cbl) was from MP Biomedicals, and radiolabeled hydroxycobalamin ([57Co]-OH-Cbl) was prepared from [57Co]-CN-Cbl according to the method of Mahoney and Rosenberg.15 The cell lines used in the present study were differentiated and Epstein-Barr virus–immortalized Brown Norway rat yolk sac epithelial carcinoma cells (designated BN1616 ), human cervical cancer cell line (HeLa), Chinese hamster ovary (CHO) cells, and primary cultures of human dermal fibroblast cells and fibroblasts from mice. CHO cells were stably transfected with human TC cDNA17 (kind gift from Dr Sergey Fedosov, University of Aarhus) inserted in the pSecTag2 B vector (Invitrogen) containing the hygromycin resistance gene. HEK293 cells expressing MRP1 were generated by transfecting HEK293 cells with pCMVneoMRP1 by calcium phosphate precipitation.18 After 48 hours, cells were split, and G418 (800 μg/mL) was used to select for clones that express MRP1. G418-resistant clones were tested for MRP1 expression by immunoblot analysis with the use of the MRPr1 antibody.19 Plasma membrane localization of MRP1 was confirmed by immunofluorescence microscopy with the use of the same antibody (not shown). HEK293 parental and HEK293-MRP1 cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum and 100 U of penicillin/streptomycin/mL.
Cellular Cbl efflux assay
Subconfluent cells were grown in tissue culture flask until approximately 80% confluence. Cells were incubated with 40 pg/mL [57Co]-CN-Cbl in complex with recombinant IF or TC17 for 24 hours. The cells were subsequently washed 3 times with sterile phosphate-buffered saline, and the medium was changed to DMEM (Invitrogen) containing 1% glutamine, 1% penicillin, and 1% streptomycin, with or without 1μM CN-Cbl (Sigma-Aldrich). After 24 hours the medium was subjected to gel filtration (on Sephadex G25M column; GE Healthcare) or analyzed by size exclusion chromatography on a Superdex 200 HR 10/30 column (Amersham Pharmacia Biotech) with the use of a Dionex equipment (Dionex). Cells were collected after trypsination. TC and haptocorrin bound 57Co-Cbl was removed from the cell culture medium with the use of magnetic beads (Dynal Biotech) coated with monoclonal antibodies against TC and haptocorrin as previously described.20
RNA interference studies and RT-PCR analysis
All siRNA treatments were performed with Dharmacon ON-TARGETplus SMART pool siRNA probes (Dharmacon), using ON-TARGETplus siCONTROL Nontargeting Pool as a control (Dharmacon; D-001810-10-20). ON-TARGETplus SMART pool siRNA for ABCB6 (Dharmacon; L-007304-00), MRP1 (Dharmacon; L-007308-00), MRP3 (Dharmacon; L-007312-00), MRP5 (Dharmacon; L-007314-00), and ABCG2 (Dharmacon; L-009924-00) were transfected at a final concentration of 100nM into exponentially growing HeLa cells with DharmaFECT reagent 1 (Dharmacon; T-2001-03), according to the instructions of the manufacturer. Ninety-six hours after siRNA transfection, cells were subjected to the above-described pulse/chase experiments, and at the end of the experiment cells were harvested for the analyses of mRNA expressions by reverse transcription–polymerase chain reaction (RT-PCR) with the use of an avian-enhanced RT-PCR kit (Sigma-Aldrich). Oligonucleotides for ABC-A2 (GenBank accession no. AAK14335) RT-PCR analysis were 5′-TTCTCTGGGCTGTCTGCTGAGCT-3′ and 5′-AGGCCGGCCCAGAGCTGTACGAA-3′. For ABC-G2 (GenBank accession no. AAO14617) they were 5′-GGAGATGTTCTGATAAATGGAGC-3′ and 5′-CTGCCACTTTATCCAGACCTAAC-3′, for ABC-B9 (GenBank accession no. AAF89993) they were 5′-AAGCATGGATCAGTTCAGCACGG-3′ and 5′-TGTTCAGTCTGGCAAATATGAGG-3′, for ABC-B6 (GenBank accession no. AAH43423) they were 5′-TTCCTGTGCATGAGTCTTTACCT-3′ and 5′-TAGCGTTCTCCTGTGTGTTCATA-3′, for MRP3 (GenBank accession no. AAH50370) they were 5′-CGGACCTTTTTTACTTCCAT-3′ and 5′-TAAAAGGATCTTGGAGCGGAATG-3′, for MRP1(GenBank accession no. ABN79590) they were 5′-CTTCTTCTTCAAGGCCATCCACG-3′ and 5′-CTGGGGCCTTCGTGTCATTCAC-3′, for PLP2 (GenBank accession no. AAI09067) they were 5′-CCTTGCTGCTATTTTCTTTG-3′ and 5′-CTCTCTCAACAAGGACAACA-3′, for LAPTM4B (GenBank accession no. AAO64358) they were 5′-CTGCTTGTGCTGCCATGTCC-3′ and 5′-GCATCATCCATGAACTCAAA-3′, and for CD63 (GenBank accession no. CAG46893) they were 5′-ATGGCGGTGGAAGGAGGAAT-3′ and 5′-GCCCCCTGGATTATGGTCTG-3′. For β-actin (GenBank accession no. AAA51578) message analysis RT-PCR primer sequences were 5′-CGTGATGGTGGGCATGGGTCAG-3′ and 5′-TAATGTCACGCACGATTTCC-3′. All RT-PCR reactions were performed following the manufacturer's instructions. Cycles were performed at 50°C for 50 minutes, 94°C for 5 minutes, 94°C for 45 seconds, 55°C for 90 seconds, 72°C for 90 seconds (35 cycles), and 72°C for 10 minutes. PCR products were separated on a 2% agarose gel containing ethidium bromide. A 100-base pair DNA ladder standard was used as a size marker.
Western blotting
Proteins from cell lysates were separated by 4% to 16% SDS–polyacrylamide gel electrophoresis, followed by Western blotting with the use of Sequi-blot polyvinylidene diflouride membranes (Bio-Rad). MRP1 was detected with monoclonal antibody MRPr1 (5 μg/mL),19 and β-tubulin with an anti–β-tubulin antibody (1:50 000; Sigma-Aldrich), followed by a rabbit anti–rat horseradish peroxidase–conjugated antibody (1:1000; DakoCytomation) and visualization by enhanced chemiluminescence.
Preparation of membrane vesicles and vesicular transport assays
Vesicles were prepared from control and human MRP1-overproducing HEK293 cells. Cells were harvested by centrifugation at 500g for 5 minutes. The pellet was resuspended in ice-cold hypotonic buffer (0.5mM sodium phosphate, 0.1mM EDTA, pH 7.4) supplemented with a protease inhibitor cocktail (Roche) and incubated at 4°C for 90 minutes. The cell suspension was homogenized with the use of a tight-fitting Dounce homogenizer. After centrifugation at 500g at 4°C for 10 minutes, the supernatant was collected and centrifuged at 4°C at 100 000g for 40 minutes. The pellet was suspended in 10mM Tris-HCl pH 7.4 and passed through a 27-gauge needle 25 times. The vesicles were dispensed in aliquots, snap-frozen in liquid nitrogen, and stored at −80°C until use.
ATP-dependent transport of [57Co]-Cbl metabolites and [3H]–estradiol-17-β-glucuronide (E217βG) into inside-out membrane vesicles was measured with the rapid filtration technique as described previously.21 In short, vesicles were filtrated with the use of a MultiScreenHTS vacuum manifold in combination with MultiscreenHTS FB 96-well filter plates (Millipore). Membranes were washed 4 times with 200 μL of ice-cold phosphate-buffered saline, and the radioactivity retained on the membranes was counted by liquid scintillation counting ([3H]-E217βG) or γ-counting ([57Co]-Cbl metabolites). Glutathionyl-Cbl (GS-Cbl) was synthesized as previously described.22
Animals
Mrp1(−/−) mice have been described previously23 and were on a 99% Friend virus B–type background. Mice received food and water ad libitum and were housed in constant temperature rooms with a 12-hour light/12-hour dark cycle. Mouse handling and experimental procedures were conducted in accordance with institutional guidelines for animal care and use and approved by the Institutional Review Board for Animal Care and Use Committee, the Netherlands Cancer Institute.
Determination of the concentration of Cbl, methylmalonic acid, and homocysteine in mouse plasma, liver, kidney, and intestine
Concentrations of Cbl in plasma and homogenized tissues of 10 to 14 weeks old, age- and sex-matched mice were determined with the use of a commercially available competitive protein binding assay with IF as the binding protein (ACS; Centaur Automated Chemiluminescence System; Bayer A/S, Diamond Diagnostics). Concentrations of plasma folate were measured on the same platform. Levels of plasma methylmalonic acid and homocysteine were measured with the use of gas chromatography–mass spectrometry after derivatization with methylchloroformate as previously described.24
Results
Cellular efflux of Cbl
To measure cellular efflux of Cbl we loaded cells with 57Co-labeled Cbl and followed the efflux after washing. Rat yolk sac epithelial cells, which contain the IF-Cbl receptor (cubam), were loaded with the Cbl-IF complex; fibroblasts/HeLa cells, which contain the TC receptor, were loaded with the TC-Cbl complex. After wash and chase of the cells for 24 hours, most of the radioactivity was present in the medium. Gel filtration analysis (Figure 1A) of the medium showed that the vitamin migrated as a high-molecular- weight complex and subsequent high-resolution high-performance liquid chromatography gel filtration of this material showed that the elution profile of the [57Co]-label in the medium corresponded to that of Cbl bound to TC (Figure 1A). This was confirmed using established immunoprecipitation assays for TC and haptocorrin (another Cbl binder in humans).17 This showed that 85% to 90% of the 57Co-Cbl activity was bound to anti-TC antibody beads (Figure 1B). Including saturating amounts of unlabeled Cbl (1μM) in the culture medium in the chase phase resulted in the appearance of [57Co]-labeled Cbl in its free form (Figure 1A left, open circle). Similar results were obtained with the use of TC-transfected CHO cells (which have a high endogenous production of TC; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The free Cbl detected in the medium after a chase with excess Cbl was not derived from the dissociation of Cbl from the TC-Cbl complexes, because we found that less than 10% of the labeled Cbl in the TC-Cbl complex dissociated in cell medium within 24 hours at 37°C (data not shown) in agreement with previous observations.25 In summary, this set of experiments indicates that cells efflux Cbl in a free form, but after secretion, Cbl binds to TC released by the cells.
Analysis of 57Co-Cbl released from cells. (A left) Gel filtration chromatography of 57Co-Cbl in 24 hours of chase medium from cells loaded with 57Co-Cbl. The chase of the cells was performed in the absence (■) or presence of 1μM Cbl (○) in the chase medium. (A right) Analysis of the high-molecular-weight complex-form of 57Co-Cbl in a high-resolution SMART high-performance liquid chromatography gel filtration system (Superdex 200 column).  indicates known position of Cbl in complex with haptocorrin (HC), transcobalamin (TC), as well as free Cbl. (B) Precipitation of 57Co-Cbl in 24 hours of efflux medium of the human cells (HeLa cells and fibroblasts) with magnetic beads coated with monoclonal antibodies against human TC or human HC. Precipitation was carried out in chase medium with or without 1μM Cbl added at the initiation of the chase. The values are the means of triplicates ± 1 SD.
indicates known position of Cbl in complex with haptocorrin (HC), transcobalamin (TC), as well as free Cbl. (B) Precipitation of 57Co-Cbl in 24 hours of efflux medium of the human cells (HeLa cells and fibroblasts) with magnetic beads coated with monoclonal antibodies against human TC or human HC. Precipitation was carried out in chase medium with or without 1μM Cbl added at the initiation of the chase. The values are the means of triplicates ± 1 SD.
Analysis of 57Co-Cbl released from cells. (A left) Gel filtration chromatography of 57Co-Cbl in 24 hours of chase medium from cells loaded with 57Co-Cbl. The chase of the cells was performed in the absence (■) or presence of 1μM Cbl (○) in the chase medium. (A right) Analysis of the high-molecular-weight complex-form of 57Co-Cbl in a high-resolution SMART high-performance liquid chromatography gel filtration system (Superdex 200 column).  indicates known position of Cbl in complex with haptocorrin (HC), transcobalamin (TC), as well as free Cbl. (B) Precipitation of 57Co-Cbl in 24 hours of efflux medium of the human cells (HeLa cells and fibroblasts) with magnetic beads coated with monoclonal antibodies against human TC or human HC. Precipitation was carried out in chase medium with or without 1μM Cbl added at the initiation of the chase. The values are the means of triplicates ± 1 SD.
indicates known position of Cbl in complex with haptocorrin (HC), transcobalamin (TC), as well as free Cbl. (B) Precipitation of 57Co-Cbl in 24 hours of efflux medium of the human cells (HeLa cells and fibroblasts) with magnetic beads coated with monoclonal antibodies against human TC or human HC. Precipitation was carried out in chase medium with or without 1μM Cbl added at the initiation of the chase. The values are the means of triplicates ± 1 SD.
Screening for candidate proteins mediating Cbl efflux
To identify the transport protein involved in the efflux of free Cbl, we focused on ABC transporters because of their structural similarity to the known prokaryotic Cbl ABC transporter BtuCD.26 The ABC transporters selected for further investigation matched 2 of the following 3 criteria: (1) the ability to transport porphyrin structures (similarity to the corrin structure of Cbl), (2) a basolateral localization in polarized epithelial cells (such as enterocytes and yolk sac cells), and (3) a wide tissue distribution. This bioinformatic approach led to the selection of 5 ABC proteins: ABCB6, ABCG2, MRP1 (ABCC1), MRP3 (ABCC3), and MRP5 (ABCC5) of which ABCB6, ABCG2, and MRP3 have all been reported to transport porphyrins,27 and MRP1, MRP3, and MRP5 are expressed in basolateral surfaces of various polarized cells.28,29 Furthermore, they all have a relatively wide tissue distribution, although MRP5 is present at low levels (for review see Borst et al,29 Deeley et al,30 Borst and Elferink,31 and Kruh et al32 ).
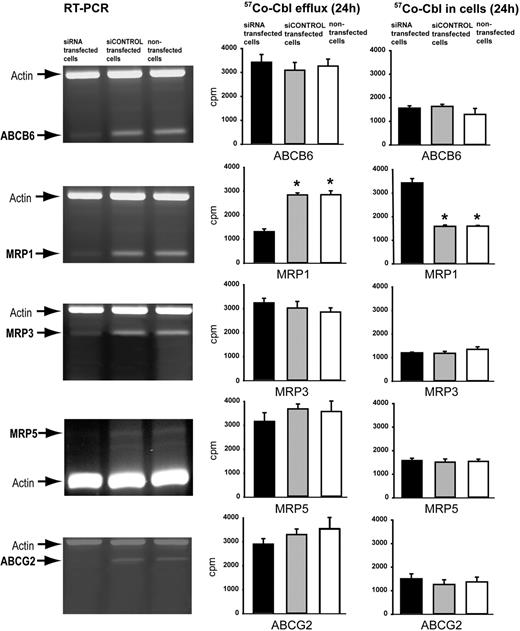
Human HeLa cells, which contain detectable mRNA levels (Figure 2A) for all of the candidate ABC proteins (but as expected a very low signal for MRP5), were used for siRNA-mediated ABC transporter mRNA knockdown experiments. RNA transcript levels were measured by RT-PCR band intensity. ABCB6, ABCG2, MRP1, and MRP3 RNAs could be reduced by greater than 80% by transcript-specific siRNAs transfections and for MRP5 to a level below detection (Figure 2). Knockdown of the MRP1 transcript resulted in greater than 50% reduction in the amount of 57Co-Cbl effluxed by the cells and a corresponding increase in the amount of Cbl present intracellularly (Figure 2 right). Western blotting confirmed that the knockdown had reduced the level of MRP1 protein greater than 80% (supplemental Figure 2). Similar results were obtained after knockdown of MRP1 in human fibroblasts and the monkey COS-1 epithelial cell line (data not shown). Knockdown of ABCB6, ABCG2, MRP3, and MRP5 did not significantly affect the Cbl efflux (Figure 2).
siRNA silencing of mRNA encoding 5 candidate proteins for efflux of Cbl. (A) The left lanes show RT-PCR analysis of ABCB6, MRP1, MRP3, MRP5, and ABCG2 RNA in HeLa cells incubated for 96 hours with specific siRNA probes targeting the mRNA encoding the indicated membrane transporters. The middle lanes show the same analysis of cells incubated with siCONTROL and the right lanes show the analysis of nontransfected cells. (B) 57Co-Cbl 24-hour efflux (left) and cellular accumulation (right) of HeLa cells transfected with the siRNA probes for ABCB6, MRP1, MRP3, PMRP5, and ABCG2 mRNA (■). ▩ and □ show the same data for cells transfected with siCONTROL and nontransfected cells, respectively. All data are shown as mean of triplicates ± 1 SD. Asterisks indicate the P values less than .001 (t test) for the difference between transfected and nontransfected cells.
siRNA silencing of mRNA encoding 5 candidate proteins for efflux of Cbl. (A) The left lanes show RT-PCR analysis of ABCB6, MRP1, MRP3, MRP5, and ABCG2 RNA in HeLa cells incubated for 96 hours with specific siRNA probes targeting the mRNA encoding the indicated membrane transporters. The middle lanes show the same analysis of cells incubated with siCONTROL and the right lanes show the analysis of nontransfected cells. (B) 57Co-Cbl 24-hour efflux (left) and cellular accumulation (right) of HeLa cells transfected with the siRNA probes for ABCB6, MRP1, MRP3, PMRP5, and ABCG2 mRNA (■). ▩ and □ show the same data for cells transfected with siCONTROL and nontransfected cells, respectively. All data are shown as mean of triplicates ± 1 SD. Asterisks indicate the P values less than .001 (t test) for the difference between transfected and nontransfected cells.
ATP-dependent transport of Cbl by MRP1 in vitro
Vesicular transport assays showed that MRP1 transports OH-Cbl in a time- and ATP-dependent manner (Figure 3A). MRP1-mediated transport of OH-Cbl was linear for at least 20 minutes (Figure 3A) and followed Michaelis-Menten kinetics. Nonlinear regression analysis yielded Km and Vmax values of 23μM and 76 pmol/mg per minute, respectively (Figure 3B). Cbl is found in several forms intracellularly, but we were unable to synthesize all these Cbl forms in a radiolabeled form. However, in vesicular transport experiments, competition with a known substrate is often used to screen for new substrates competing for the same binding/transport site on the transporter. We, therefore, tested whether OH-Cbl, CN-Cbl, and GS-Cbl competed with the established MRP1 substrate E217βG for transport (Figure 3C). Each of the known intracellular Cbl forms was found to inhibit E217βG transport. GS-Cbl was the strongest inhibitor, and the results presented in Figure 3D and E clearly show that the inhibition is competitive, with a Ki of approximately 6.2μM (± 1.6μM). This indicates that GS-Cbl has a relatively high affinity for MRP1.
Transport of Cbl metabolites by MRP1 in vesicular transport experiments. (A) Time course experiments of control and MRP1-containing vesicles incubated with 100nM [57Co]-OH-Cbl. (B) Concentration-dependent transport of [57Co]-OH-Cbl in MRP1-containing vesicles. (C) Inhibition of MRP1-mediated transport of 1μM [3H]-E217βG by various concentrations of unlabeled GS-Cbl, OH-Cbl, and CN-Cbl, as indicated. (D) Concentration-dependent transport of [3H]-E217βG in MRP1-containing membrane vesicles in the presence or absence of the indicated concentrations of GS-Cbl. (E) Lineweaver-Burk transformations of the data presented in panel D. Each data point represents the means ± 1 SD of an experiment performed in triplicate. MRP1-dependent transport was calculated by subtracting ATP-dependent transport in control vesicles from ATP-dependent transport in MRP1-containing vesicles.
Transport of Cbl metabolites by MRP1 in vesicular transport experiments. (A) Time course experiments of control and MRP1-containing vesicles incubated with 100nM [57Co]-OH-Cbl. (B) Concentration-dependent transport of [57Co]-OH-Cbl in MRP1-containing vesicles. (C) Inhibition of MRP1-mediated transport of 1μM [3H]-E217βG by various concentrations of unlabeled GS-Cbl, OH-Cbl, and CN-Cbl, as indicated. (D) Concentration-dependent transport of [3H]-E217βG in MRP1-containing membrane vesicles in the presence or absence of the indicated concentrations of GS-Cbl. (E) Lineweaver-Burk transformations of the data presented in panel D. Each data point represents the means ± 1 SD of an experiment performed in triplicate. MRP1-dependent transport was calculated by subtracting ATP-dependent transport in control vesicles from ATP-dependent transport in MRP1-containing vesicles.
Cbl homeostasis and cellular Cbl efflux in MRP1-deficient mice
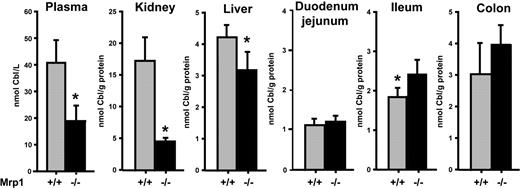
To test whether MRP1 has a physiologic role in the homeostasis/tissue disposition of Cbl, we determined Cbl levels in plasma, liver, kidney, and intestine of Mrp1(−/−) and wild-type (WT) mice. The absence of MRP1 in the Mrp1(−/−) mice resulted in a reduction of the Cbl levels in plasma (∼ 50%), liver (∼ 20%), and kidney (∼ 75%; Figure 4). In contrast, Cbl levels in the ileum and colon tissue were higher in Mrp1(−/−) than in WT mice (Figure 4). The reduced plasma levels of Cbl in Mrp1(−/−) mice did not lead to a significant increase in the plasma levels of methylmalonate and homocysteine (data not shown), the clinical indicators of human Cbl deficiency. The latter fits previous reports describing that Mrp1(−/−) mice are healthy and do not show an overt deficiency phenotype.23,33,34 We also determined Cbl levels in plasma, liver, and kidney in Mrp3(−/−) and Mrp1(−/−)/Mrp3(−/−) double knockout mice but did not find any difference in Cbl load compared with WT and Mrp1(−/−) mice, respectively (data not shown).
Cbl homeostasis in male and female MRP1-deficient mice and controls. The content of Cbl in blood plasma, kidney, liver, ileum, and colon was determined in control and Mrp1(−/−) mice. The content in plasma is shown as Cbl concentration (in nM) and in the other tissues as nanomolar Cbl per gram of tissue protein. Each measurement is the mean ± 1 SD of groups of 8 mice (4 males and 4 females). Asterisk indicates the P values less than .05 (t test) for the difference between means in the tissues.
Cbl homeostasis in male and female MRP1-deficient mice and controls. The content of Cbl in blood plasma, kidney, liver, ileum, and colon was determined in control and Mrp1(−/−) mice. The content in plasma is shown as Cbl concentration (in nM) and in the other tissues as nanomolar Cbl per gram of tissue protein. Each measurement is the mean ± 1 SD of groups of 8 mice (4 males and 4 females). Asterisk indicates the P values less than .05 (t test) for the difference between means in the tissues.
We also used the transgenic mice to verify at the cellular level that MRP1 contributes to Cbl homeostasis by comparing the efflux of Cbl from fibroblasts derived from a Mrp1(−/−) and a WT mouse. The results in Figure 5A show that Cbl efflux is diminished and Cbl accumulation is increased in the Mrp1(−/−) cells in agreement with the data in Figure 2. Transfection of a MRP1 cDNA construct into the Mrp1(−/−) cells rescued Cbl efflux to levels comparable to those of WT mouse fibroblasts (Figure 5B).
Time course of cellular 57Co-Cbl efflux from MRP1-deficient and control fibroblasts. (A) MRP1 levels in skin fibroblasts from Mrp1(−/−) mice, MRP1 cDNA-transfected Mrp1(−/−) mice and in WT skin fibroblast. The Western blots were probed with a MRP1-specific monoclonal antibody (top), before the blot was stripped and reprobed with an antibody against β-tubulin (bottom). The cellular protein loads are the same for each lane. (B) Time course of efflux of 57Co-Cbl in the 3 cell types. The result is normalized to cellular protein content. (C) Cellular content of 57Co-Cbl after efflux. The values are mean ± 1 SD of triplicate determinations.
Time course of cellular 57Co-Cbl efflux from MRP1-deficient and control fibroblasts. (A) MRP1 levels in skin fibroblasts from Mrp1(−/−) mice, MRP1 cDNA-transfected Mrp1(−/−) mice and in WT skin fibroblast. The Western blots were probed with a MRP1-specific monoclonal antibody (top), before the blot was stripped and reprobed with an antibody against β-tubulin (bottom). The cellular protein loads are the same for each lane. (B) Time course of efflux of 57Co-Cbl in the 3 cell types. The result is normalized to cellular protein content. (C) Cellular content of 57Co-Cbl after efflux. The values are mean ± 1 SD of triplicate determinations.
Discussion
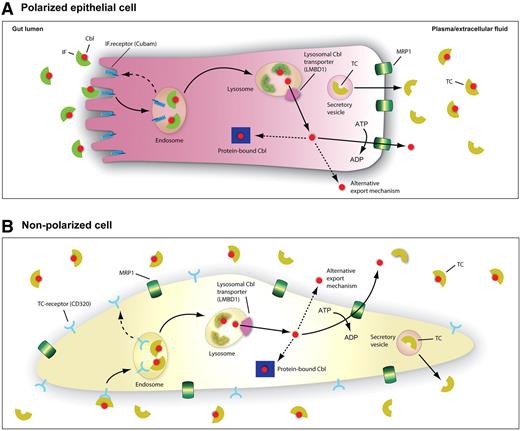
In the present study, we show that Cbl is released from cells in a nonprotein bound “free” form and that MRP1 is a physiologically relevant Cbl efflux transporter for ATP-dependent cellular Cbl efflux. Coherent transcellular Cbl transport pathways can now for the first time be depicted at the molecular level (Figure 6).
A schematic model for the tentative cellular pathways of Cbl. (A) Polarized ileal cell. After binding of Cbl to gastric IF in the gut lumen, the IF-Cbl complex binds to the IF-Cbl receptor, cubam, located in the ileal apical brush border. Once bound, IF-Cbl is internalized and processed via the classic endosome-lysosomal pathway. In the endosome IF-Cbl is released from cubam, which is recycled back to the cell surface in receptosomes (dashed line). In the lysosome, the lysosomal proteases degrade IF, releasing the vitamin for transport by the lysosomal transmembrane protein LMBD1. From the cytosol Cbl is exported at the basolateral side of the ileal cell by the ATP-driven exporter MRP1 or alternatively by a yet unknown mechanism. The vitamin remaining intracellularly is largely enzyme bound and serves as coenzyme for methionine synthase in the cytoplasm or for methylmalonyl CoA mutase in the mitochondria. Some Cbl may also be associated to the recently identified MMACHC protein involved in decyanation of CNCbl and the dealkylation of alkylcobalamins before conversion to coenzyme forms.35,36 In the plasma, free Cbl is immediately bound to transcobalamin (TC), synthesized and excreted in vesicles by the cells. (B) Nonpolarized cell expressing the TC receptor.
A schematic model for the tentative cellular pathways of Cbl. (A) Polarized ileal cell. After binding of Cbl to gastric IF in the gut lumen, the IF-Cbl complex binds to the IF-Cbl receptor, cubam, located in the ileal apical brush border. Once bound, IF-Cbl is internalized and processed via the classic endosome-lysosomal pathway. In the endosome IF-Cbl is released from cubam, which is recycled back to the cell surface in receptosomes (dashed line). In the lysosome, the lysosomal proteases degrade IF, releasing the vitamin for transport by the lysosomal transmembrane protein LMBD1. From the cytosol Cbl is exported at the basolateral side of the ileal cell by the ATP-driven exporter MRP1 or alternatively by a yet unknown mechanism. The vitamin remaining intracellularly is largely enzyme bound and serves as coenzyme for methionine synthase in the cytoplasm or for methylmalonyl CoA mutase in the mitochondria. Some Cbl may also be associated to the recently identified MMACHC protein involved in decyanation of CNCbl and the dealkylation of alkylcobalamins before conversion to coenzyme forms.35,36 In the plasma, free Cbl is immediately bound to transcobalamin (TC), synthesized and excreted in vesicles by the cells. (B) Nonpolarized cell expressing the TC receptor.
Mice deficient for MRP1 have substantially less Cbl in plasma, liver, and kidney but an increased content in the intestine. This may suggest that a reduced basolateral Cbl export from the intestinal epithelium causes a reduced loading of other tissues of these mice. A precedent for the deficiency of a nutrient caused by an impaired cellular export system is the recently identified iron deficiency caused by the inactivation of the iron exporter, ferroportin, present in the basolateral surface of the intestinal epithelium and in macrophages.37 The most profound effect on Cbl load by MRP1 deficiency was seen in the kidneys, which had a Cbl content of approximately 25% of that found in the WT mice. This in accordance with the finding that the kidney, which takes up Cbl from TC-Cbl complexes filtered in the renal glomeruli,9 is a short-time storage organ of nonenzyme-bound Cbl. A similar reduction in the kidney Cbl levels has been seen in rodents on a diet low in Cbl.38 The liver Cbl content, which is largely enzyme bound39 and less sensitive to the overall Cbl body load, was accordingly only reduced by approximately 25% in the Mrp1(−/−) mice.
The reduced Cbl content in plasma, liver, and kidney in Mrp1(−/−) mice was accompanied by higher levels in the ileum and colon. In ileum and colon, MRP1 is present at the basolateral surface of the epithelial cells,40 and we propose that the absence of MRP1 reduces the fraction of the Cbl taken up from the intestinal lumen that is subsequently released over the basolateral membrane toward the blood circulation. However, we found higher levels of Cbl in the colon than in the ileum, the primary site for the uptake of dietary Cbl complexed to IF in humans.5 How Cbl ends up in the mouse colon and whether it derives from Cbl produced in the bacterial flora of colon are unknown. The functional importance of MRP1 in the colon has been shown by the increased accumulation of etoposide in the colon of mice lacking MRP1.41
Although we attribute the lower levels of Cbl in plasma, liver, and kidney of Mrp1(−/−) mice to the absence of MRP1 in the gut, we have no direct evidence yet for this hypothesis, and caution should be taken when translating data of Cbl transport from mice to man. We tested the uptake of [57Co]-labeled HO-Cbl after oral administration, but the fraction of uptake in mice was below 10% in both WT and Mrp1(−/−) mice (data not shown), and the results of this experiment were therefore not conclusive. A low specific activity of the 57Co-HO-Cbl preparation resulting in high Cbl concentrations that saturate the Cbl receptor in the intestine may explain the low relative amount of [57Co]-labeled HO-Cbl taken up.
Our observation that cells are able to secrete free Cbl seems in contrast with the literature,10,11,42 because the predominant view has been that Cbl is released from TC-producing cells in complex with TC. It should be noted, however, that efflux of free Cbl has always been an implicit assumption in the classic Shilling test, used in the clinic for evaluation of intestinal Cbl uptake. In this test, a small oral dose of [57Co]-Cbl is administered followed by the injection of a large dose of unlabeled Cbl to prevent association of [57Co]-Cbl with Cbl-binding proteins in the plasma to allow the determination of the amount of labeled free Cbl excreted in the urine. The fact that orally administered [57Co]-Cbl ends up in the urine in persons with normal cubam-mediated intestinal uptake of [57Co]-Cbl-IF and receptor-mediated uptake of Cbl-TC in the renal proximal tubules9,43 is therefore a strong indication that free [57Co]-Cbl is released from intestinal cells and filtered in the glomeruli in a nonprotein bound form.
Intracellular Cbl is present in various forms, and it has been suggested that the high intracellular concentration of glutathione may cause a large part of cytosolic Cbl to be conjugated to glutathionyl groups (GS-Cbl).44,45 Direct experimental evidence for this plausible hypothesis remains to be provided. Although we were able to demonstrate transport of radiolabeled OH-Cbl and CN-Cbl by MRP1 in direct transport experiments, this was not possible for GS-Cbl because we were not able to make a stably radiolabeled GS-Cbl preparation (GS-Cbl transforms to OH-Cbl in water). However, unlabeled GS-Cbl most potently inhibited transport of the prototype MRP1 substrate E217βG, indicating that GS-Cbl is transported by MRP1 with high affinity. Because MRP1 is a high-affinity transporter of many GSH conjugates (reviewed in Deeley and Cole46 ), it is possible that GS-Cbl is a major form of Cbl transported by MRP1. It is clear from the efflux curve of Figure 5, however, that a fraction of radiolabeled Cbl loaded in the cells was not accessible for efflux and remained in the cells. The nature of this fraction is not known yet.
Mrp1(−/−) mice23,33 have no major morphologic or metabolic abnormalities. Besides the change in Cbl homeostasis reported here, an increased sensitivity to some cytostatic drugs and altered inflammatory responses are the only abnormalities observed.23,33,34,41,47,48 Redundant drug/substrate export by one or other transporters may compensate for the lack of MRP1. An alternative efflux pathway of Cbl is actually suggested by our results because MRP1 deficiency did not prevent Cbl efflux completely. The role of MRP1 may be further defined by studying other species, including species in which it is possible to establish a diet-induced Cbl deficiency condition, to investigate whether failure of MRP-1 has a worsening effect.
The role of MRP1 in Cbl efflux of specific tissues and cell types merits further investigation. For instance, human Cbl deficiency is characterized by neurologic and hematologic symptoms, and the high level of MRP1 in the blood-cerebrospinal fluid barrier31,49 and red blood cells (see de Wolf et al50 ) might make these tissues vulnerable to Cbl deficiency. MRP1 is also expressed at the basolateral surface of the syncytiotrophoblasts,51 but we did not find a role of MRP1 in fetal Cbl supply as the Cbl content of newborn offspring of the MRP1-deficient mice was not lowered (data not shown).
In conclusion, we have identified the ABC transporter MRP1 as the first known eukaryotic membrane efflux transporter capable of transporting free Cbl out of cells. Future studies may show other transporters assisting in the Cbl efflux and define whether polymorphisms or mutations in the genes encoding such transporters may affect the Cbl homeostasis in humans and contribute to Cbl deficiency disease. Moreover, the finding of MRP1-dependent Cbl transport may influence on future strategies on cancer treatment with cytotoxic drugs in which MRP-1 function induces resistance to these drugs.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Anna-Lisa Christensen, Jette Fisker Petersen, and Rikke Brok Jensen for technical assistance and Maciej Bogdan Maniecki for help with the illustrations.
This work was supported by the Novo Nordisk Foundation, the Lundbeck Foundation, and the Danish Medical Research Council (S.K.M.) and by ZonMw (TOP grant 40-00812-98-07-028; K.v.d.W. and P.B.).
Authorship
Contribution: R.B.-E. and K.v.d.W. performed research and contributed to writing the manuscript; T.H. performed specific analyses; E.N. performed specific analyses and contributed to data analysis; P.B. contributed to study design, data analysis, and writing the manuscript; and S.K.M. designed the study, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: S. K. Moestrup, Department of Medical Biochemistry, University of Aarhus, Ole Worms Alle, blgn 170, 8000 Århus C, Denmark; e-mail: skm@biokemi.au.dk.
References
Author notes
R.B.-E. and K.v.d.W. contributed equally to this study.


![Figure 3. Transport of Cbl metabolites by MRP1 in vesicular transport experiments. (A) Time course experiments of control and MRP1-containing vesicles incubated with 100nM [57Co]-OH-Cbl. (B) Concentration-dependent transport of [57Co]-OH-Cbl in MRP1-containing vesicles. (C) Inhibition of MRP1-mediated transport of 1μM [3H]-E217βG by various concentrations of unlabeled GS-Cbl, OH-Cbl, and CN-Cbl, as indicated. (D) Concentration-dependent transport of [3H]-E217βG in MRP1-containing membrane vesicles in the presence or absence of the indicated concentrations of GS-Cbl. (E) Lineweaver-Burk transformations of the data presented in panel D. Each data point represents the means ± 1 SD of an experiment performed in triplicate. MRP1-dependent transport was calculated by subtracting ATP-dependent transport in control vesicles from ATP-dependent transport in MRP1-containing vesicles.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/8/10.1182_blood-2009-07-232587/4/m_zh89990946830003.jpeg?Expires=1767835657&Signature=snptAZCpxD-dfE9jdAbts1sh7iuF54agsA2dODwwzWEewPa-mwAwS68nU6kkkSF89LRdps6jNLCvYGBI5od9jtuVSVWWoNXLIYg0rE7PHveZzu9PlsMTxt60coz4J3xUPFEA1Rr0Zv9tR3ZQonCZU8W8mTQqtbA1xGQnrDSBkOyyUU6oJ5oLc0vxmIac~OdahfolIMvtQlhvu~BFTo337kGVHRD60JIigw8kfPlPQXqTAEEKKfbxyFohKsakkau-17BWSj-vI4QPWq9qVZX~aVUiXVARHbxoajYX5gahnutViT5KRpNxfhWwi5dWabpYISeECuG5ueipJ8dpO38eBg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)