Quite a bit is now known about the complex mechanisms underlying how Cbl gets into cells. Just how the vitamin gets out for recycling remained a mystery until the findings by Beedholm-Ebsen and colleagues. In their article in this issue of Blood, cellular egress of Cbl is shown to occur by transport of “free” Cbl via the ABC drug transporter, ABCC1 (also known as the MRP1), present in the basolateral membrane of intestinal epithelium and in other cells.1
Physiological entry of cobalamin (Cbl) generally takes place through portals involving receptor-mediated endocytosis and specific protein janitors. For Cbl absorption, intrinsic factor (IF), a gastric glycoprotein, wends its way down the intestinal tract as it accompanies its charge to the luminal front portal of the ileal cell, and then facilitates its delivery via the highly specialized cubam receptor complex through a mechanism previously elucidated by Moestrup, Fyfe, and their groups.2,3 General cellular uptake of Cbl from circulation also occurs through receptor-mediated endocytosis. But here, the ostiary is the transcobalamin receptor, recently isolated and identified as the CD320 membrane protein by Quadros et al.4 Transit into the cell is tightly escorted by transcobalamin (TC), the more abundant and functionally better characterized of the 2 plasma Cbl binding proteins. The other, haptocorrin (HC), although less abundant, curiously binds a considerably greater fraction of Cbl in the plasma compartment. The function of HC, a glycoprotein, remains an enigma and little is known about how it picks up and drops off its cargo, save the fact that it has less specificity than either IF or TC for true Cbl. It appears to use the nonspecific asialoglycoprotein receptors for delivery to hepatocytes of Cbl and perhaps unwanted Cbl analogues for processing and storage of the vitamin in the liver.5
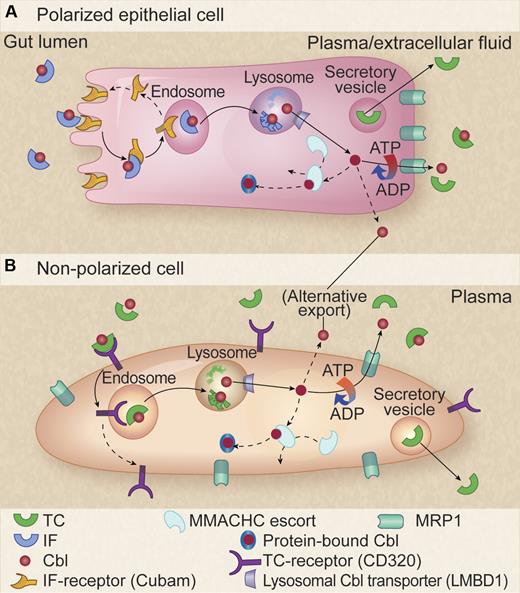
Model for cellular pathway of Cbl. (A) Polarized intestinal epithelial cell. The Cbl-intrinsic factor (Cbl-IF) complex is internalized through the cubam receptor for endosome-lysosome processing with subsequent cubam recycling to the cell surface and Cbl release into the cytosol via the LMBD1 lysosomal transporter. Cbl is then either escorted by MMACHC through reduction and activation processing to its destination enzyme partners in the mitochondria and cytosol or is exported through the basolateral membrane by the ATP-dependent MRP1. An as-yet-unknown alternative mechanism for export is also indicated. TC synthesized in the cells is separately secreted in vesicles and becomes available in the plasma for binding of Cbl released via MRP1. (B) Nonpolarized cells. A similar pathway is shown but with TC receptor serving as the entry portal. Professional illustration by Kenneth X. Probst.
Model for cellular pathway of Cbl. (A) Polarized intestinal epithelial cell. The Cbl-intrinsic factor (Cbl-IF) complex is internalized through the cubam receptor for endosome-lysosome processing with subsequent cubam recycling to the cell surface and Cbl release into the cytosol via the LMBD1 lysosomal transporter. Cbl is then either escorted by MMACHC through reduction and activation processing to its destination enzyme partners in the mitochondria and cytosol or is exported through the basolateral membrane by the ATP-dependent MRP1. An as-yet-unknown alternative mechanism for export is also indicated. TC synthesized in the cells is separately secreted in vesicles and becomes available in the plasma for binding of Cbl released via MRP1. (B) Nonpolarized cells. A similar pathway is shown but with TC receptor serving as the entry portal. Professional illustration by Kenneth X. Probst.
Study of the mechanisms used by primitive organisms to capture Cbl from the environment informs by ontogenetic example how membrane transit may be achieved by more highly evolved organisms. Thus, Escherichia coli and other prokaryotes import free Cbl in an adenosine triphosphate (ATP)–dependent manner via a protein belonging to the ATP-binding cassette (ABC) family (BtuCD).6 In more complex multicellular eukaryotes, a mechanism for cellular egress of cobalamin is vital for redistribution in body economy of this important nutrient cofactor. Using a combination of siRNA, vesicular transport, and knockout mice, Beedholm-Ebsen et al convincingly demonstrate that MRP1 (multidrug resistance protein 1) nicely subserves this role. With the caveat that Cbl metabolism in mice may not exactly mirror what occurs in humans, it appears that we have conserved the mechanism used by bacteria to take in Cbl to serve the purpose of expelling the vitamin from our cells.
The new information regarding the mechanism of cellular egress of Cbl through the MRP1 drug transporter conduit fills in an important missing piece in the picture of cellular handling of Cbl. However, in addition to events involving Cbl entry at the front and exit at the back doors of intestinal and other polarized cells, there is much yet to be learned about intracellular handling of Cbl. This is true also in the less-structured in-and-out trafficking that occurs in nonpolarized cells (see figure). The vitamin undergoes transformative reduction and activation to coenzymatically active forms (methyl- or adenosyl-Cbl) and traverses various cameral compartments, including endosomes, lysosomes, and mitochondria, aided by as yet anonymous or unidentified facilitating chaperones. This is not entirely a “black box.” Through the use of the painstaking approaches of fibroblast complementation experiments7 and their companion cell culture studies,8 much information has accrued from classic bedside-to-bench study of patients with puzzling disorders affecting Cbl-dependent pathways. For example, in a recent study, MMACHC, a versatile cytosolic Cbl trafficking agent that is impaired in the cblC group of inborn errors of Cbl metabolism, has been shown to catalyze the conversion of alkylcobalamins using the thiolate of glutathione to generate reduced cobalamin and the corresponding glutathione thioether.9 What is also intriguing about the present findings of Beedholm-Ebsen et al is that MRP1 is a high-affinity transporter of glutathionyl cobalamin (GS-Cbl), as is true of many GSH-conjugates. The possibility that GS-Cbl is a major form of Cbl transported by MRP1 raises anew the possible importance of this Cbl congener in Cbl coenzyme biosynthesis and trafficking.10
Is there any redundancy for Cbl efflux in the form of other transporters, and are there polymorphisms or mutations in the genes encoding such transporters that may disrupt Cbl homeostasis in humans with resulting disease? Future work on the MRP1-dependent Cbl transport pathway conceivably may also have implications with regard to manipulation of resistance to cytotoxic drugs processed through MRP1.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal