Abstract
Phagocytosis of nonopsonized bacteria is central to innate immunity, but its regulation is less defined. We show that overexpression of the P2X7 receptor greatly augments the phagocytosis of nonopsonized beads and heat-killed bacteria by transfected HEK-293 cells, whereas blocking P2X7 expression by siRNA significantly reduces the phagocytic ability of human monocytic cells. An intact P2X7-nonmuscle myosin complex is required for phagocytosis of nonopsonized beads because activation of P2X7 receptors by adenosine triphosphate (ATP), which dissociates myosin IIA from the P2X7 complex, inhibits this phagocytic pathway. Fresh human monocytes rapidly phagocytosed live and heat-killed Staphylococcus aureus and Escherichia coli in the absence of serum, but the uptake was reduced by prior incubation with ATP, or P2X7 monoclonal antibody, or recombinant P2X7 extracellular domain. Injection of beads or bacteria into the peritoneal cavity of mice resulted in their brisk phagocytosis by macrophages, but injection of ATP before particles markedly decreased this uptake. These data demonstrate a novel pathway of phagocytosis of nonopsonized particles and bacteria, which operate in vivo and require an intact P2X7-nonmuscle myosin IIA membrane complex. The inhibitory effect of ATP on particle uptake by the macrophage is regulated by the P2X7 receptor and defines this phagocytic pathway.
Introduction
Phagocytosis is a fundamental function of innate immune cells, and many receptors, including Fc receptors, complement receptors, various integrins, and scavenger receptors, have been shown to mediate phagocytosis of opsonized microbes.1 Several scavenger receptors have also been identified that directly interact with nonopsonized particles, and these are broadly classified as class A (macrophage receptor with collagenous structure) and class B (2 transmembrane receptors),2 whereas among others is the C-type lectin-like receptor, dectin-1.3-9 Assays for these scavenger receptors use nonselective inhibitors, polyinosinic acid, liposomes containing phosphatidylserine, oxidized low-density lipoprotein, or fucoidan, to inhibit this pathway. Phagocytosis of particles is accompanied by major rearrangements in the actin-myosin cytoskeleton, but there is little information on how scavenger receptors interact with proteins of the cytoskeleton. Nevertheless, cytochalasin D (CytD), a classic inhibitor of F-actin polymerization, is able to block particle uptake mediated by all these receptors.
The P2X7 receptor is a ligand-gated cation channel highly expressed on monocytes and macrophages and exists within the plasma membrane as a large multimolecular complex containing components of the cytoskeleton, including β-actin10 and nonmuscle myosin.11 Activation of P2X7 mediates actin reorganization and membrane blebbing in mouse macrophages,12-14 suggesting a close functional connection between the P2X7 receptor and the actin-myosin cytoskeleton. The P2X7 receptor has 2 transmembrane domains with intracellular amino and carboxyl termini and an extracellular domain containing some β-pleated sheets within a tertiary structure maintained by 5 disulphide bonds.15 Activation of the receptor by brief exposure to extracellular adenosine triphosphate (ATP) or the more potent 2′(3′)-O-(Benzoylbenzoyl)-ATP (BzATP) opens a cation-selective channel, whereas longer exposure to ATP for tens of seconds leads to secondary pore formation and massive K+ efflux. Cellular K+ depletion is thought to be a costimulus for formation of the inflammasome16,17 and secretion of proinflammatory cytokines interleukin-1β (IL-1β) and IL-18.18,19 Other downstream effects of P2X7 activation include blebbing of the plasma membrane,20 shedding of surface L-selectin and CD23,21 rapid secretion of matrix metalloproteinase-9,22 and activation of the caspase cascade leading to a form of apoptotic cell death.23,24 The role of P2X7 as a proinflammatory receptor is supported by results from the P2X7 gene-deleted mouse, which shows major reduction in inflammatory responses to various noxious stimuli.25 Extracellular ATP has also been shown to induce the killing of mycobacteria or chlamydiae after these pathogens have been phagocytosed by cells of monocytic-macrophage origin.26-28 Certain genetic variations in the P2X7 molecule reduce or abolish the ability of ATP to activate the receptor as well as the capacity of macrophages for ATP-induced killing of pathogens, and this evidence, together with the effect of P2X7 antagonists, point to the bactericidal effects of ATP working via the P2X7 receptor.26,29,30 It is noteworthy that these studies of P2X7-mediated killing of pathogens have generally examined the effect of ATP on viability of microbes engulfed within macrophages, and this study is the first to explore a function of the P2X7 molecule in the absence of extracellular ATP.
A major component of the cytoskeleton in nonmuscle cells, myosin II, is present in almost all nonmuscle cells,31 where it functions in the preservation of cell morphology32 and motility as well as in particle internalization during both complement receptor– and Fcγ receptor–mediated phagocytosis. Nonmuscle myosin is also required for actin cup assembly during complement receptor–mediated phagocytosis.33 There is also indirect evidence for the role of nonmuscle myosin heavy chain IIA (NMMHC-IIA) in phagocytosis from a study in which blebbistatin, a specific inhibitor for NMMHC-IIA ATPase,34 inhibited micropinocytosis and phagocytosis by HS1 cells transfected with NMMHC-IIA.35 In another study, lipoxin A4 caused dephosphorylation and redistribution of NMMHC-IIA as well as stimulated phagocytosis of apoptotic leukocytes by THP-1 cells.36 In a third study, the phosphorylation state of NMMHC-IIA was shown to be important for phagocytosis of red cells by differentiated THP-1 macrophages.37 Our recent studies have shown that NMMHC-IIA is tightly associated with P2X7 in human monocytic cells and that exposure of the cell to extracellular ATP leads to a slow dissociation of NMMHC-IIA from the P2X7 complex over a 10- to 15-minute time course.11 In this study, we examined the phagocytosis of beads and live or heat-killed bacteria by macrophages both in vitro and in vivo and studied the effect of ATP on the phagocytic process. Both monocytes and macrophages showed a brisk uptake of fluorescent beads and bacteria in the absence of ATP. Prior exposure of monocyte/macrophages to ATP strongly inhibited the uptake of nonopsonized beads or bacteria by this novel pathway of phagocytosis. Moreover, a monoclonal antibody (mAb) to the extracellular domain of P2X7 as well as recombinant P2X7 extracellular domain inhibited particle uptake. The data suggest that the P2X7 receptor in its unactivated conformation participates in the phagocytosis of nonopsonized particles, including live and heat-killed bacteria.
Methods
Details of methods are included in supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Sources of cells
Human peripheral blood mononuclear cells (PBMCs) were separated by density gradient centrifugation over Ficoll-Hypaque. Human monocytic THP-1 cells were stimulated to macrophage-like cells (iTHP-1) by interferon-γ plus lipopolysaccharide (LPS) according to a previously described method.38 The study was approved by the Human Research Ethics Committee of Sydney West Area Health Service (06/058). Informed consent was provided according to the Declaration of Helsinki. Murine macrophages were isolated from the peritoneal exudates from C57BL/6 mice elicited for 72 hours by 1% proteose peptone (Sigma-Aldrich). Animal experiments were conducted under ethical approval from the Sydney University Animal Ethics Committee.
Immunoprecipitation
P2X7 and its associated proteins were immunoprecipitated using anti-P2X7 mAb (clone L4) coated M-280 tosylactivated Dynabeads from cell lysis of THP-1 or HEK-293 cells as described previously.11
Transfection of HEK-293 cells
HEK-293 cells do not express P2X receptors in subconfluent cultures, so plasmid DNA was transfected into nearly confluent monolayers of HEK-293 and cultured for 40 to 44 hours. For live-cell imaging, HEK-293 cells were cultured and transfected in collagen-coated 27-mm MatTek culture dishes.
Phagocytosis of YG beads
The uptake of Fluoresbrite yellow green carboxylate latex microspheres (1-μm YG beads, Polysciences) by cells was analyzed by flow cytometry. Suspensions of PBMCs, THP-1, or HEK-293 in Na medium with 0.1mM CaCl2 were stirred and temperature controlled at 37°C using a Time Zero module, and YG beads were added 40 seconds later. The linear mean channel of fluorescence intensity for each gated subpopulation over successive 10-second intervals was analyzed by WinMDI software and plotted against time. The area under YG beads uptake curve in the first 6.5 minutes was calculated using a Microsoft Excel function.
siRNA blocking
The siRNA duplex for either P2RX7 or MYH9 gene was transfected into stimulated THP-1 cells. Cells were kept in culture for another 48 hours before collection for phagocytosis and Western blotting.
Phagocytosis in vivo by murine peritoneal macrophages
Wild-type or P2X7 deleted C57BL/6 mice were pretreated with 1% proteose peptone to elicit a peritoneal exudate and after 72 hours injected intraperitoneally with 1.0 mL of phosphate-buffered saline (PBS), ATP (5mM), or oxidized ATP (OxATP; 300μM). After 15 or 30 minutes, a peritoneal injection of YG beads or Alexa 488–conjugated Escherichia coli was made. The mice were killed after 30 minutes (beads) or 60 minutes (bacteria), and peritoneal exudates were collected, fixed, stained, and analyzed by a LSR II flow cytometer (BD Biosciences).
Phagocytosis of bacteria in vitro
PBMCs were incubated with or without ATP, OxATP, CytD, or KN62 followed by addition of Alexa 488–conjugated Staphylococcus aureus or E coli (Invitrogen; 2 mg/mL). Cells were incubated for 20 or 40 minutes, respectively, and fixed with 2% paraformaldehyde at 4°C. For transfected HEK-293 cells, this incubation time was 5 hours with S aureus and 10 hours with E coli. Trypan blue (1%, pH 4.5) was added to quench the fluorescence of bacteria attached on the cell surface. The samples were analyzed by BD FACSCalibur flow cytometry on the gated monocyte population.
Phagocytosis of live S aureus in vitro
Live S aureus (clinical standard) was labeled with carboxyfluorescein succinimidyl ester (CFSE) and incubated with human PBMCs for 60 minutes. After addition of trypsin (0.25% in PBS plus 1mM ethylenediaminetetraacetic acid), cells were vigorously stirred and fixed with equal volumes of 4% paraformaldehyde at 4°C.
Producing and purification of recombinant P2X7-ED
cDNA encoding P2X7 extracellular domain (His6-Met-Ala44 to Val335) was inserted into a pETMCSIII vector (kindly provided by Prof Nick Dixon, University of Wollongong, Australia). The construct was transformed into KRX one-step competent cells (Promega) and cultured in LB broth. L-Rhamnose (0.1%) was added as the stimulator. The KRX cells were collected and sonicated, the pellet was dissolved in 6M guanidine HCl, and the recombinant protein was purified using Ni-NTA superflow resin (QIAGEN).
Results
P2X7 expression on HEK-293 cells confers phagocytic ability
Our previous study has shown that P2X7 has a tight molecular association with NMMHC-IIA in monocytic cells.11 Because NMMHC-IIA has been suggested to play a role in phagocytosis,33 we studied whether transfection with P2X7 would confer phagocytic ability on HEK-293 cells, a kidney cell line with poor phagocytic ability. Figure 1A shows the uptake of nonfluorescent 3-μm beads by HEK-293 cells cotransfected with NMMHC-IIA-AcGFP and P2X7-DsRed. Bright field illumination showed many refractile floating beads that are not attached to cells. The nonrefractile beads on bright field are also identified by the 4,6-diamidino-2-phenylindole fluorescent counterstain showing that these same beads have an intracellular location. Fluorescent microscopy shows that both P2X7 and NMMHC-IIA were present on the surface of the engulfed beads, whereas the overlay image shows colocalization of these 2 molecules on the beads (Figure 1A). Phase-contrast microscopy was also performed on nonfluorescent latex beads incubated with monocyte-derived macrophages (supplemental Figure 1), which showed that most beads had an intracellular location.
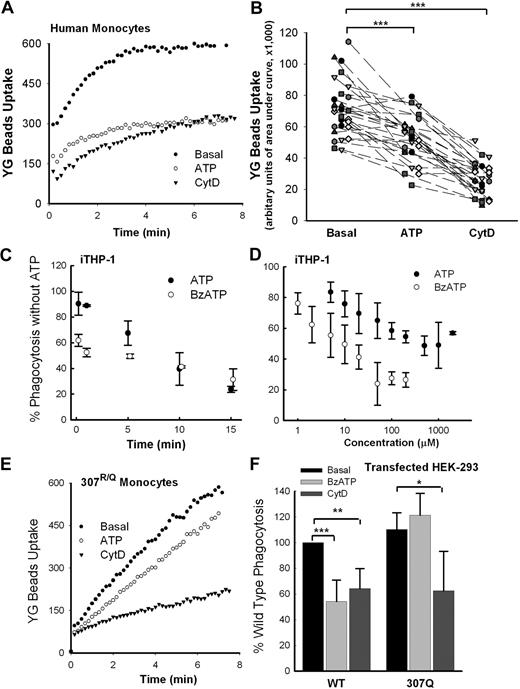
Transfection with P2X7 confers phagocytic ability. (A) Bright field and fluorescent images showing engulfed nonfluorescent 3-μm beads within a monolayer of HEK-293 cells cotransfected with both NMMHC-IIA-AcGFP and P2X7-DsRed. Cells were counterstained with 5μM 4,6-diamidino-2-phenylindole for 30 minutes and washed 3 times during a 1-hour period before incubation with 10 μL of beads for 10 minutes at 23°C. The images were captured by an Olympus IX81 fluorescent microscope with a 60× oil-immersion lens. (B) Phagocytosis of YG beads by HEK-293 cells transfected with P2X7-DsRed or NMMHC-IIA-DsRed. Time-resolved flow cytometry dot plots allowed cells to be gated and analyzed as 2 populations according to their DsRed intensity (high or low). Control cells were transfected with DsRed alone. (C) Quantitation of YG bead phagocytosis by HEK-293 cells transfected with DsRed (mock) or wild-type P2X7-DsRed. Cells were pretreated with IgG2b isotype control mAb (clone WMD7, 100 μg/mL) and anti-P2X7 mAb (clone L4, 100 μg/mL). Additional control was CytD added 20 minutes before the YG beads. Phagocytosis index of untreated cells (Basal) in each separate experiment (n = 3-5) was used to calculate the basal level (100%).
Transfection with P2X7 confers phagocytic ability. (A) Bright field and fluorescent images showing engulfed nonfluorescent 3-μm beads within a monolayer of HEK-293 cells cotransfected with both NMMHC-IIA-AcGFP and P2X7-DsRed. Cells were counterstained with 5μM 4,6-diamidino-2-phenylindole for 30 minutes and washed 3 times during a 1-hour period before incubation with 10 μL of beads for 10 minutes at 23°C. The images were captured by an Olympus IX81 fluorescent microscope with a 60× oil-immersion lens. (B) Phagocytosis of YG beads by HEK-293 cells transfected with P2X7-DsRed or NMMHC-IIA-DsRed. Time-resolved flow cytometry dot plots allowed cells to be gated and analyzed as 2 populations according to their DsRed intensity (high or low). Control cells were transfected with DsRed alone. (C) Quantitation of YG bead phagocytosis by HEK-293 cells transfected with DsRed (mock) or wild-type P2X7-DsRed. Cells were pretreated with IgG2b isotype control mAb (clone WMD7, 100 μg/mL) and anti-P2X7 mAb (clone L4, 100 μg/mL). Additional control was CytD added 20 minutes before the YG beads. Phagocytosis index of untreated cells (Basal) in each separate experiment (n = 3-5) was used to calculate the basal level (100%).
The kinetics of bead phagocytosis was studied by time-resolved flow cytometry in which cells were incubated with 1-μm carboxylated YG beads at 37°C with constant stirring using a time-zero module attachment. This allowed the mean fluorescence intensity of cell-associated beads to be recorded over 10-second intervals for 8 to 10 minutes by flow cytometry. Brisk uptake of YG beads was observed in fresh human monocytes but not lymphocytes (supplemental Figure 2), suggesting little nonspecific binding of beads to cell surface using this flow method. We then transfected HEK-293 cells with either P2X7-DsRed or NMMHC-IIA-DsRed and measured their ability to phagocytose 1-μm YG beads. Cells were gated separately with lower or higher DsRed expression, and the phagocytic ability was analyzed for each gated population. Cells with high expression of P2X7 or NMMHC-IIA showed a brisk uptake of the beads over 5 minutes (Figure 1B). In contrast, in the same test tube, cells with low expression of either molecule showed little or no uptake of beads (Figure 1B). This result shows that overexpression of P2X7 promotes phagocytosis in HEK-293 cells. Our data showing increased phagocytosis by HEK-293 cells overexpressing NMMHC-IIA (Figure 1B) agree with reports showing that NMMHC-IIA may facilitate phagocytosis in many different situations.35-37 The effect of an mAb (clone L4) to P2X7 on the phagocytosis of beads by HEK-293 cells transfected with P2X7-DsRed was studied. Preincubation of cells with this mAb inhibited the phagocytosis of YG beads by P2X7-transfected HEK-293 cells down to the level observed with CytD, a classic inhibitor of phagocytosis (Figure 1C).
Activation of P2X7 by ATP attenuates phagocytosis of beads
Our previous data show that exposure of human monocytes to ATP rapidly dissociates NMMHC-IIA from the P2X7 complex.11 Figure 2A shows that preincubation of monocytic cells with ATP inhibited the phagocytosis of YG beads by fresh human monocytes to an extent approaching that induced by CytD. Both in fresh human monocytes and in cultured THP-1 monocytes, the maximum uptake of YG beads was attained by 6 to 8 minutes of incubation. Fresh monocytes were isolated from 28 healthy control subjects, and these cells were pretreated with 1mM ATP for 10 to 15 minutes. This pretreatment significantly reduced the phagocytosis of beads to approximately 60% of basal (P < .001), whereas the addition of 20μM CytD reduced the phagocytosis further to approximately 40% of basal (P < .001; Figure 2B). This inhibitory effect of a P2X7 agonist was studied in interferon-γ and LPS-stimulated human monocytic THP-1 cells (iTHP-1), which are known to up-regulate large amounts of P2X7. Inhibition of bead uptake by agonist was time and dose dependent, with maximal inhibition observed after 10 to 15 minutes of preincubation of cells with either ATP or BzATP, a more potent P2X7 agonist (Figure 2C). The inhibitory effect followed a sigmoid dose-response relation with IC50 values for BzATP and ATP of 8μM and 80μM, respectively (Figure 2D), similar to the EC50 values for these agonists to activate the P2X7 receptor.15,38-41 However, the inhibitory effect of ATP on phagocytosis was attenuated when the human monocytes were isolated from a subject heterozygous for the R307Q single nucleotide polymorphism, which abolishes ATP binding to the receptor42 (Figure 2E). A similar observation was made in HEK-293 cells, in which ATP inhibited phagocytosis by cells transfected with wild-type P2X7 but not by those transfected with 307Q mutant P2X7 (Figure 2F), suggesting that ATP inhibits phagocytosis via activation of the P2X7 receptor.
Extracellular ATP attenuates phagocytosis of nonopsonized beads via the P2X7 receptor. (A) Typical time course of uptake of 1.0-μm YG beads by fresh human monocytes with wild-type P2X7. (B) The composite values for YG bead uptake by monocytes from 28 randomly selected human subjects. Cells were labeled with allophycocyanin (APC)–conjugated anti-CD14 mAb and pretreated with 1mM ATP for 15 minutes or 20μM CytD for 30 minutes before the addition of YG beads. Values for uptake are in arbitrary units of area under uptake curve over 6.5 minutes. ***P < .001. (C) Preincubation of iTHP-1 cells with ATP (1mM) or BzATP (100μM) causes time-dependent inhibition of phagocytosis of YG beads. (D) Preincubation of iTHP-1 cells with ATP or BzATP for 10 minutes causes dose-dependent inhibition of phagocytosis of YG beads. Different levels of bead uptake were standardized so that values with CytD were 40% of basal. (E) Typical uptake curves of YG beads by human monocytes from a subject with genetic loss of P2X7 pore function (R307Q). (F) Phagocytosis of YG beads by HEK-293 cells transfected with P2X7-DsRed and P2X7-R307Q-DsRed. Phagocytosis index of basal cells transfected with wild-type P2X7 in each separate experiments (n = 5) was normalized to 100%. *P < .05; **P < .02; ***P < .01. n = 4 or 5.
Extracellular ATP attenuates phagocytosis of nonopsonized beads via the P2X7 receptor. (A) Typical time course of uptake of 1.0-μm YG beads by fresh human monocytes with wild-type P2X7. (B) The composite values for YG bead uptake by monocytes from 28 randomly selected human subjects. Cells were labeled with allophycocyanin (APC)–conjugated anti-CD14 mAb and pretreated with 1mM ATP for 15 minutes or 20μM CytD for 30 minutes before the addition of YG beads. Values for uptake are in arbitrary units of area under uptake curve over 6.5 minutes. ***P < .001. (C) Preincubation of iTHP-1 cells with ATP (1mM) or BzATP (100μM) causes time-dependent inhibition of phagocytosis of YG beads. (D) Preincubation of iTHP-1 cells with ATP or BzATP for 10 minutes causes dose-dependent inhibition of phagocytosis of YG beads. Different levels of bead uptake were standardized so that values with CytD were 40% of basal. (E) Typical uptake curves of YG beads by human monocytes from a subject with genetic loss of P2X7 pore function (R307Q). (F) Phagocytosis of YG beads by HEK-293 cells transfected with P2X7-DsRed and P2X7-R307Q-DsRed. Phagocytosis index of basal cells transfected with wild-type P2X7 in each separate experiments (n = 5) was normalized to 100%. *P < .05; **P < .02; ***P < .01. n = 4 or 5.
Inhibitors of P2X7 or NMMHC-IIA block phagocytosis
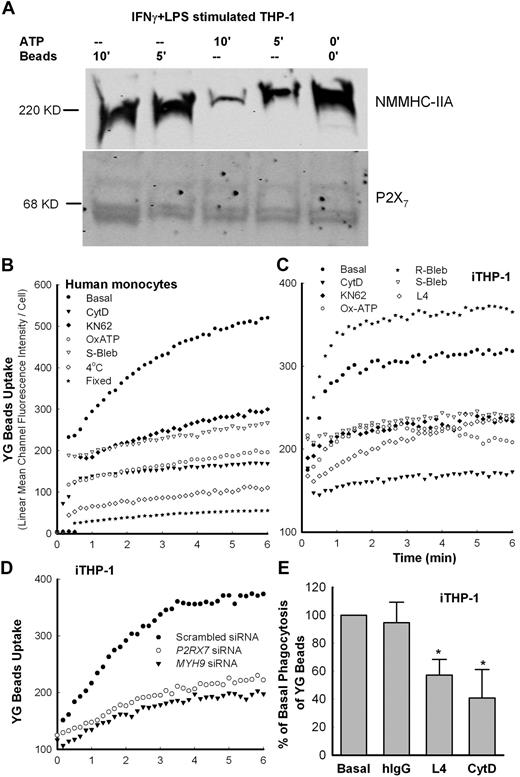
We confirmed our previous study,11 showing that NMMHC-IIA was present in the P2X7 membrane complex obtained by immunoprecipitation with anti-P2X7 mAb-coated Dynabeads from iTHP-1 cells (Figure 3A). Pretreatment of cells with ATP-dissociated NMMHC-IIA from this complex over a 10-minute time course, whereas addition of beads did not alter the amount of nonmuscle myosin in the complex (Figure 3A). Thus, ATP may inhibit phagocytosis of beads by monocytes via dissociating the P2X7-NMMHC-IIA complex. To further study the role of P2X7 and NMMHC-IIA in phagocytosis of beads, human PBMCs were pretreated with antagonists of either P2X7 (1μM KN-62 or 0.3mM OxATP)39,40 or NMMHC-IIA (100μM S-(−)-blebbistatin [S-Bleb]). These antagonists partially inhibited phagocytosis (Figure 3B-C), whereas R-(+)-blebbistatin (R-Bleb), an inactive analog of S-Bleb, showed no inhibitory effect (Figure 3C). Addition of CytD reduced both the rate and the amount of uptake by more than 60%, although the inhibition of bead uptake was never as complete as observed with uptake at 4°C or by fixed cells (Figure 3A). Neither fixation nor low temperature nor CytD affected initial nonspecific binding (adherence) of bacteria to fresh monocyte surface (supplemental Figure 3), suggesting that the cell surface binding characteristics are not affected in these conditions. Moreover, pretreatment of cells with ATP, CytD, OxATP, or KN-62 did not significantly alter the surface expression of P2X7 in human monocytes or in transfected HEK293 cells (supplemental Figure 4). When the cells were transfected with siRNA to P2RX7 or MYH9 (myosin heavy chain 9, the gene encoding NMMHC-IIA), the protein levels of P2X7 and NMMHC-IIA were significantly reduced to approximately half of the control (supplemental Figure 5), causing significant impairment of phagocytosis (Figure 3D). As shown in Figure 1C for transfected HEK-293 cells, P2X7-dependent phagocytosis in iTHP-1 cells was blocked by pretreatment with anti-P2X7 antibody (clone L4, final concentration 10 μg/mL; Figure 3C,E), which binds to the extracellular domain of P2X7 and inhibits its pore formation,43,44 suggesting that P2X7 may directly or indirectly contact the beads.
Inhibitors of P2X7 and NMMHC-IIA block phagocytosis. (A). Western blot images of NMMHC-IIA from iTHP-1 cell lysate immunoprecipitated by Dynabeads coated with P2X7 mAb. Cells pretreated with 1mM ATP show loss of coprecipitated NMMHC-IIA. Control cells were exposed to 1-μm YG beads (5 μL/mL) for the indicated time before wash and lysis. (B-C) Typical time-resolved flow cytometry dot plots showing phagocytosis of YG beads by human CD14+ monocytes and iTHP-1 cells. Cells were preincubated with inhibitors (CytD, 20μM for 20 minutes; KN-62, 1μM for 15 minutes; OxATP, 0.3mM for 30 minutes; S- and R-bleb, 100μM for 60 minutes; L4 mAb: 100 μg/mL) before the addition of beads. (D) Uptake of YG beads by iTHP-1 cells transfected with siRNA to P2RX7 or MYH9. All figures are representative of at least 3 experiments. (E) Inhibition of YG bead uptake by cells pretreated with L4 mAb or CytD before the addition of YG beads. Control values with human IgG (250 μg/mL) are shown. The arbitrary unit of area under YG bead uptake curve over 6.5 minutes is used to quantitate the phagocytosis level. *P < .05. n = 3 or 4.
Inhibitors of P2X7 and NMMHC-IIA block phagocytosis. (A). Western blot images of NMMHC-IIA from iTHP-1 cell lysate immunoprecipitated by Dynabeads coated with P2X7 mAb. Cells pretreated with 1mM ATP show loss of coprecipitated NMMHC-IIA. Control cells were exposed to 1-μm YG beads (5 μL/mL) for the indicated time before wash and lysis. (B-C) Typical time-resolved flow cytometry dot plots showing phagocytosis of YG beads by human CD14+ monocytes and iTHP-1 cells. Cells were preincubated with inhibitors (CytD, 20μM for 20 minutes; KN-62, 1μM for 15 minutes; OxATP, 0.3mM for 30 minutes; S- and R-bleb, 100μM for 60 minutes; L4 mAb: 100 μg/mL) before the addition of beads. (D) Uptake of YG beads by iTHP-1 cells transfected with siRNA to P2RX7 or MYH9. All figures are representative of at least 3 experiments. (E) Inhibition of YG bead uptake by cells pretreated with L4 mAb or CytD before the addition of YG beads. Control values with human IgG (250 μg/mL) are shown. The arbitrary unit of area under YG bead uptake curve over 6.5 minutes is used to quantitate the phagocytosis level. *P < .05. n = 3 or 4.
Both P2X7 mAb and P2X7 extracellular domain inhibit phagocytosis
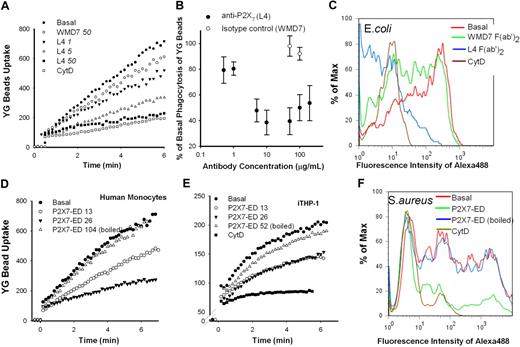
The anti-P2X7 mAb (clone L4) is known to inhibit P2X7 by binding to the extracellular domain of the receptor.43,44 Phagocytosis of both YG beads (Figure 4A-B) and E coli (Figure 4C) by fresh human monocytes was inhibited by F(ab′)2 fragment of P2X7 mAb, with IC50 of 5 and 10 μg/mL, respectively. In contrast, the isotype control mAb showed no inhibitory action on the uptake of particles (Figure 4). To further confirm the specificity of this inhibition, we synthesized recombinant P2X7 extracellular domain (P2X7-ED). The purity (> 99%) of this P2X7-ED was assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blotting, whereas solubility was confirmed by enzyme-linked immunosorbent assay (supplemental Figure 6). P2X7-ED effectively inhibited phagocytosis of beads (Figure 4D-E) and heat-killed S aureus (Figure 4F) at final concentrations approximately 15 to 30 μg/mL. This result together with the effect of the P2X7 mAb strongly suggests that the ectodomain of P2X7 is required for phagocytosis of beads or bacteria.
Both anti-P2X7 mAb (L4) and recombinant P2X7-ED inhibit phagocytosis of YG beads and bacteria. (A) A representative flow cytometry dot plot showing the inhibitory effect of L4 mAb on bead uptake. Human PBMCs prelabeled with APC-conjugated anti-CD14 mAb (2 × 106 in 100 μL) were incubated with L4 or the isotype control mAb WMD7 at indicated concentrations for 30 minutes at 20°C before dilution to 1.0 mL and the addition of 5-μL YG beads. The fluorescence from bead uptake was measured in gated CD14+ monocytes. The dose-response curve is shown in panel B (n = 3 or 4). (C) A representative flow cytometry histogram showing the inhibitory effect of L4 F(ab′)2 fragments on phagocytosis of E coli. Human PBMCs (3 × 106 in 150 μL) were incubated with L4 or WMD7 F(ab′)2 fragments at indicated concentrations for 30 minutes at 20°C before the addition of 20 μg of Alexa 488–conjugated E coli (10 μL). After a 60-minute incubation at 37°C with gentle shaking, cells were fixed and the fluorescence was measured immediately after the addition of trypan blue. (D-E) A total of 5 μL of YG beads was incubated in Na medium with or without P2X7-ED at indicated concentrations (μg/mL) in a total of 30 μL volume for 10 minutes. Heat-treated P2X7-ED (95°C for 10 minutes) was used as a control. The mixture was added into 1 mL of fresh human mononuclear cells (D) or THP-1 cells pretreated with or without 20μM CytD (E). (F) A total of 10 μL of Alexa 488–conjugated S aureus was incubated in Na medium with or without 52 μg/mL P2X7-ED in a total of 30 μL volume for 10 minutes. Heat-treated P2X7-ED was used as a control. A total of 130 μL of cells (∼ 3 × 106) pretreated with or without 20μM CytD was added. The mixture was incubated at 37°C with gentle shaking for 20 minutes, and stopped by 150 μL of 4% paraformaldehyde. Equal volume of 1% trypan blue was added immediately before analysis by flow cytometry.
Both anti-P2X7 mAb (L4) and recombinant P2X7-ED inhibit phagocytosis of YG beads and bacteria. (A) A representative flow cytometry dot plot showing the inhibitory effect of L4 mAb on bead uptake. Human PBMCs prelabeled with APC-conjugated anti-CD14 mAb (2 × 106 in 100 μL) were incubated with L4 or the isotype control mAb WMD7 at indicated concentrations for 30 minutes at 20°C before dilution to 1.0 mL and the addition of 5-μL YG beads. The fluorescence from bead uptake was measured in gated CD14+ monocytes. The dose-response curve is shown in panel B (n = 3 or 4). (C) A representative flow cytometry histogram showing the inhibitory effect of L4 F(ab′)2 fragments on phagocytosis of E coli. Human PBMCs (3 × 106 in 150 μL) were incubated with L4 or WMD7 F(ab′)2 fragments at indicated concentrations for 30 minutes at 20°C before the addition of 20 μg of Alexa 488–conjugated E coli (10 μL). After a 60-minute incubation at 37°C with gentle shaking, cells were fixed and the fluorescence was measured immediately after the addition of trypan blue. (D-E) A total of 5 μL of YG beads was incubated in Na medium with or without P2X7-ED at indicated concentrations (μg/mL) in a total of 30 μL volume for 10 minutes. Heat-treated P2X7-ED (95°C for 10 minutes) was used as a control. The mixture was added into 1 mL of fresh human mononuclear cells (D) or THP-1 cells pretreated with or without 20μM CytD (E). (F) A total of 10 μL of Alexa 488–conjugated S aureus was incubated in Na medium with or without 52 μg/mL P2X7-ED in a total of 30 μL volume for 10 minutes. Heat-treated P2X7-ED was used as a control. A total of 130 μL of cells (∼ 3 × 106) pretreated with or without 20μM CytD was added. The mixture was incubated at 37°C with gentle shaking for 20 minutes, and stopped by 150 μL of 4% paraformaldehyde. Equal volume of 1% trypan blue was added immediately before analysis by flow cytometry.
P2X7 regulates phagocytosis of live and dead bacteria
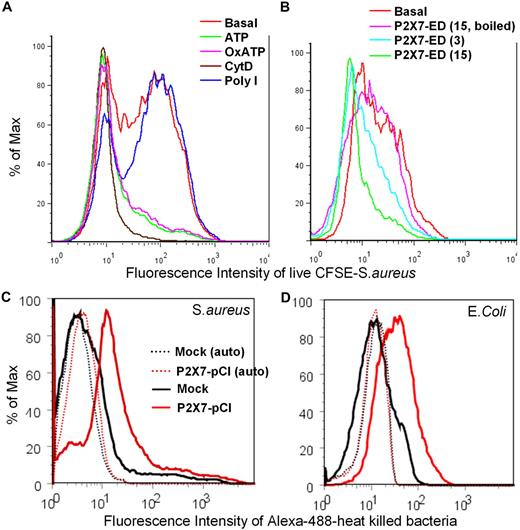
We explored the physiologic significance of this phagocytic pathway regulated by P2X7 by measuring the uptake of live S aureus by human monocytes. When low concentrations of CFSE-labeled S aureus (final concentration < 2 μg/mL) were used, trypsin digestion effectively removed most of membrane-associated bacteria and CytD showed a clear inhibitory effect (Figure 5A). The phagocytosis of live S aureus by human CD14+ monocytes was significantly inhibited by pretreatment of cells with ATP and OxATP (Figure 5A), whereas in contrast, poly I, the classic inhibitor of scavenger receptors, failed to alter the phagocytosis of live S aureus (Figure 5A). P2X7-ED also inhibited phagocytosis of live S aureus by human monocytes at concentrations from 10 to 20 μg/mL (Figure 5B). We also studied the phagocytosis of both heat-killed E coli and S aureus conjugated with Alexa 488. HEK 293 cells transfected with P2X7 were incubated with bacteria followed by fixation and the addition of 0.5% trypan blue, which efficiently quenched membrane-associated fluorescence (supplemental Figure 7). Transfection of HEK-293 cells with P2X7 constructs conferred the ability to engulf both S aureus and E coli, although the time course of bacterial uptake was far slower than for beads requiring 3 to 10 hours (Figure 5C-D). The ability of a nonphagocytic cell, HEK-293, to take up beads or bacteria when transfected with P2X7 constructs provides strong evidence for a role for P2X7 in the uptake of nonopsonized particles, whereas the uptake results with live bacteria give this P2X7 pathway physiologic significance.
P2X7 regulates phagocytosis of live or dead bacteria in vitro. Human PBMCs (2 × 107/mL in 150 μL of Na medium) were labeled with APC-conjugated anti-CD14 mAb and incubated with or without (A) 1mM ATP for 15 minutes, 0.3mM OxATP for 30 minutes, 20μM CytD for 30 minutes, or 50 μg/mL poly I for 30 minutes; or (B) 3 or 15 μg/mL P2X7-ED for 20 minutes at room temperature before the addition of 10 μL of CFSE-labeled live S aureus. After 60 minutes, cells were incubated with 100 μL of 0.25% trypsin for 2 minutes, followed by vigorous stirring and fixation. (C-D) Typical flow cytometry histograms showing phagocytosis of S aureus (C) and E coli (D) by HEK-293 cells transfected with P2X7-pCI constructs. Cells (3 × 106) were incubated with 20 μg of Alexa 488–conjugated bacteria in 150 μL of Na medium with 0.5mM Ca2+ for 5 hours (S aureus) or 10 hours (E coli). Cells were fixed, and fluorescence from surface-attached bacteria was quenched by trypan blue. Dotted lines show autofluorescence of transfected HEK-293 cells.
P2X7 regulates phagocytosis of live or dead bacteria in vitro. Human PBMCs (2 × 107/mL in 150 μL of Na medium) were labeled with APC-conjugated anti-CD14 mAb and incubated with or without (A) 1mM ATP for 15 minutes, 0.3mM OxATP for 30 minutes, 20μM CytD for 30 minutes, or 50 μg/mL poly I for 30 minutes; or (B) 3 or 15 μg/mL P2X7-ED for 20 minutes at room temperature before the addition of 10 μL of CFSE-labeled live S aureus. After 60 minutes, cells were incubated with 100 μL of 0.25% trypsin for 2 minutes, followed by vigorous stirring and fixation. (C-D) Typical flow cytometry histograms showing phagocytosis of S aureus (C) and E coli (D) by HEK-293 cells transfected with P2X7-pCI constructs. Cells (3 × 106) were incubated with 20 μg of Alexa 488–conjugated bacteria in 150 μL of Na medium with 0.5mM Ca2+ for 5 hours (S aureus) or 10 hours (E coli). Cells were fixed, and fluorescence from surface-attached bacteria was quenched by trypan blue. Dotted lines show autofluorescence of transfected HEK-293 cells.
ATP attenuates phagocytosis of beads by murine macrophages in vitro and in vivo
We measured phagocytosis of beads by peritoneal macrophages from C57BL/6 mice with and without deletion of the P2RX7 gene.25 Wild-type macrophages phagocytosed beads with a time course of uptake similar to human monocytes. Pretreatment of these murine cells with ATP reduced the uptake of beads to the level observed with CytD (Figure 6A-B). However, peritoneal macrophages from the P2RX7−/− mice showed an identical uptake of beads with or without preincubation of cells with ATP (Figure 6B). The modulating effect of extracellular ATP on phagocytosis by macrophages was also studied in vivo in a mouse model of inflammation induced by intraperitoneal proteose peptone.45 Mice were injected intraperitoneally with PBS or ATP followed 15 minutes later by the injection of YG beads. After 30 minutes, the peritoneal exudate was collected and analyzed by flow cytometry. The macrophage population was identified by forward and side scatter and strong positivity for CD11b or CD68. Particular care was taken to exclude cell doublets. The uptake of beads by macrophages was not uniform with 2 populations evident; a highly phagocytic population (> 10 beads/cell) and a moderately phagocytic population containing one to 4 beads per cell (Figure 6C-D). When ATP was infused into the peritoneal cavity 15 minutes before the beads, the highly phagocytic macrophage population disappeared with little effect on the moderately phagocytic population (Figure 6C). This dramatic effect contrasted with the results in P2RX7−/− mice. In these gene-deleted mice, the highly phagocytic population composed most of the macrophages present, and ATP had little effect in removing this population (Figure 6D). This result shows that ATP can inhibit phagocytosis of beads both in vivo and in vitro, and this inhibitory effect is mediated via the P2X7 receptor.
In vitro and in vivo phagocytosis of YG beads by murine peritoneal macrophages. In vitro (A,B top panel) and in vivo (C-D bottom panels) phagocytosis of YG beads by stimulated peritoneal macrophages from wild-type and P2X7-knockout C57BL/6 mice. For in vitro assay, cells were treated with or without 1mM ATP for 15 minutes before the addition of beads. For in vivo assay, mice were injected intraperitoneally with either 1 mL of PBS or 5mM ATP followed 15 minutes later by injection of 1-μm YG beads. The mice were killed after 30 minutes, and peritoneal exudates were collected. Monocyte-macrophages were gated by forward scatter-height and side scatter-height (A-B) plus CD11b+ (C-D). Doublets were excluded by side scatter-height and side scatter-area gating (supplemental Figure 9). *P < .05; **P < .02 (n = 5).
In vitro and in vivo phagocytosis of YG beads by murine peritoneal macrophages. In vitro (A,B top panel) and in vivo (C-D bottom panels) phagocytosis of YG beads by stimulated peritoneal macrophages from wild-type and P2X7-knockout C57BL/6 mice. For in vitro assay, cells were treated with or without 1mM ATP for 15 minutes before the addition of beads. For in vivo assay, mice were injected intraperitoneally with either 1 mL of PBS or 5mM ATP followed 15 minutes later by injection of 1-μm YG beads. The mice were killed after 30 minutes, and peritoneal exudates were collected. Monocyte-macrophages were gated by forward scatter-height and side scatter-height (A-B) plus CD11b+ (C-D). Doublets were excluded by side scatter-height and side scatter-area gating (supplemental Figure 9). *P < .05; **P < .02 (n = 5).
ATP attenuates phagocytosis of bacteria in vitro and in vivo
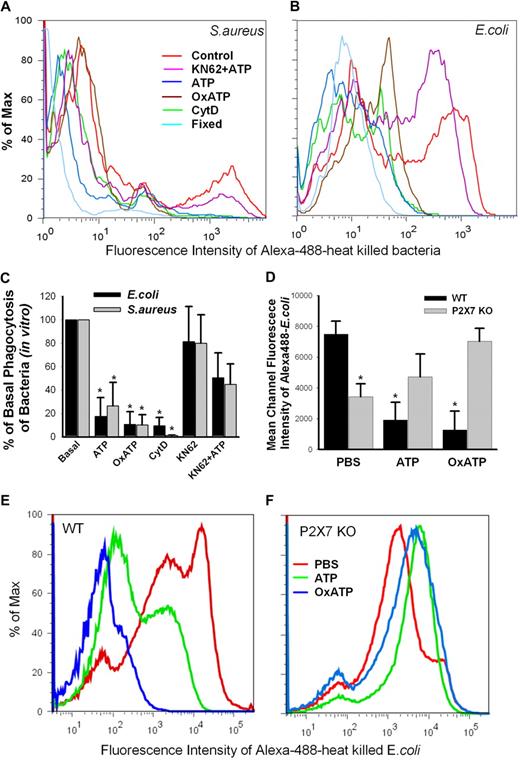
The physiologic significance of this P2X7-dependent uptake pathway was explored by measuring the phagocytosis of heat-killed bacteria by monocytes and macrophages. Brisk uptake of heat-killed Gram-positive S aureus or Gram-negative E coli (both conjugated with Alexa 488) was observed at 20 and 40 minutes of incubation, respectively, with fresh human monocytes (Figure 7A-B). Pretreatment of fresh human monocytes with 1mM ATP for 15 minutes, or with 0.3mM OxATP (an irreversible inhibitor of P2X7 receptor)39 for 30 minutes almost completely abolished their ability to uptake the bacteria (Figure 7A-C, P < .01, n = 6). Another specific P2X7 inhibitor, KN-62, showed only partial inhibition of phagocytosis; however, it partially blocked the inhibitory effect of ATP (Figure 7C, P < .05, n = 6). Moreover, to observe the in vivo effect of ATP and OxATP, wild-type or P2X7-deficient C57BL/6 mice were injected intraperitoneally with PBS, ATP, or OxATP followed 30 minutes later by the injection of heat-killed Alexa 488–conjugated E coli. After 60 minutes, the peritoneal exudate was collected, fixed, and analyzed by flow cytometry. The uptake of E coli by macrophages showed 2 cell populations (Figure 7E-F), similar to that observed for phagocytosis of beads (Figure 6). When ATP was infused into the peritoneal cavity 30 minutes before E coli, the highly phagocytic macrophage population disappeared with little change of the moderately phagocytic population (Figure 7E, P < .01, n = 5,). Furthermore, preinjection with OxATP almost completely inhibited the phagocytosis of E coli (Figure 7E, P < .001, n = 5). These dramatic effects contrasted with the results in P2X7−/− mice whose macrophages had significant impairment of phagocytosis compared with wild-type macrophages (Figure 7D, P < .01, n = 3). In contrast to wild-type mice, these P2X7−/− mice preinjected with ATP or OxATP showed a slight increase of E coli phagocytosis by their peritoneal macrophages (Figure 7D,F). This result confirms that ATP can inhibit phagocytosis of bacteria both in vivo and in vitro, and this inhibitory effect is mediated via the P2X7 receptor
In vitro and in vivo phagocytosis of bacteria. (A-C) In vitro phagocytosis of Alexa 488–conjugated S aureus and E coli by human monocytes. (*P < .01, vs Basal, n = 6). PBMCs (2.0 × 107/mL) in 150 μL were incubated in Na medium containing 0.5mM Ca2+ with 1mM ATP for 15 minutes, 0.3mM OxATP for 30 minutes, 20μM CytD for 30 minutes, 1μM KN-62 for 15 minutes followed by addition of 1mM ATP, or fixed with 2% paraformaldehyde. (D-F) In vivo phagocytosis of Alexa 488–conjugated E coli by peritoneal macrophages from wild-type and P2X7-knockout C57BL/6 mice. Mice were injected intraperitoneally with 1 mL of PBS, 5mM ATP, or 0.6mM OxATP followed 30 minutes later by injection of E coli. The mice were killed after 60 minutes, and peritoneal exudates were collected. The fluorescence was measured immediately after the addition of trypan blue. *P < .01, vs wild-type PBS control group; n = 5 for wild-type and n = 3 for knockouts.
In vitro and in vivo phagocytosis of bacteria. (A-C) In vitro phagocytosis of Alexa 488–conjugated S aureus and E coli by human monocytes. (*P < .01, vs Basal, n = 6). PBMCs (2.0 × 107/mL) in 150 μL were incubated in Na medium containing 0.5mM Ca2+ with 1mM ATP for 15 minutes, 0.3mM OxATP for 30 minutes, 20μM CytD for 30 minutes, 1μM KN-62 for 15 minutes followed by addition of 1mM ATP, or fixed with 2% paraformaldehyde. (D-F) In vivo phagocytosis of Alexa 488–conjugated E coli by peritoneal macrophages from wild-type and P2X7-knockout C57BL/6 mice. Mice were injected intraperitoneally with 1 mL of PBS, 5mM ATP, or 0.6mM OxATP followed 30 minutes later by injection of E coli. The mice were killed after 60 minutes, and peritoneal exudates were collected. The fluorescence was measured immediately after the addition of trypan blue. *P < .01, vs wild-type PBS control group; n = 5 for wild-type and n = 3 for knockouts.
P2X7 regulated phagocytosis is independent of Fcγ receptors or scavenger receptors
Fcγ receptors are a major class of phagocytic receptors that participate in recognition and internalization of IgG-opsonized particles. Although phagocytosis by human monocytes varied between different persons, pretreatment of these cells with 1mM ATP for 15 minutes nearly always inhibited phagocytosis of nonopsonized beads. In contrast, ATP failed to inhibit phagocytosis of hIgG-opsonized beads in most of subjects studied (supplemental Figure 8A). A similar lack of response to ATP pretreatment was also shown for phagocytosis of nonopsonized beads and hIgG-opsonized beads by iTHP-1 (supplemental Figure 8B). Scavenger receptors on human monocytes/macrophages have also been shown to mediate phagocytosis of nonopsonized particles.2 Polyinosinic acid (poly I), a specific polyanionic inhibitor of scavenger receptors, partially inhibited bead uptake to a similar extent as ATP pretreatment of human monocytes (supplemental Figure 8C). In contrast, poly I had no significant inhibitory effect on phagocytosis of beads by P2X7-transfected HEK-293 cells (supplemental Figure 8D), suggesting scavenger receptors are not involved in P2X7-regulated phagocytosis. Although poly I inhibited the phagocytosis of heat-killed S aureus (supplemental Figure 8E) or E coli (supplemental Figure 8F), the inhibitory effect of ATP is still significant even at the presence of poly I (supplemental Figure 8E-F). Moreover, poly I failed to inhibit the phagocytosis/binding of live S aureus by human monocytes (Figure 7A; supplemental Figure 8G), indicating divergent roles of scavenger receptors and P2X7 receptors in phagocytosis. Poly C, the inactive control for poly I, had no effect in phagocytosis of nonopsonized particles (supplemental Figure 8).
Discussion
In this study, we first measured the uptake of YG latex beads and bacteria by macrophages in the absence of added ATP and without serum present. A brisk uptake was observed, and multiple lines of evidence showed that P2X7 receptors regulate phagocytosis of these nonopsonized particles. First, transfection of the nonphagocytic cell line HEK-293 with P2X7 constructs conferred an ability of these cells to rapidly take up the YG beads, provided the density of expressed receptors was sufficiently high. Moreover, P2X7 could also be visualized on the phagosome membrane surrounding the engulfed beads. A second line of evidence is the observation that an inhibitory P2X7 mAb (clone L4) blocks the phagocytosis of beads (Figure 4A-B) and E coli (Figure 4C), providing strong evidence for the involvement of P2X7 in the uptake of nonopsonized particles. Whether the ectodomain of P2X7 or some coassociated adhesion molecule binds to or recognizes the particle is uncertain, although the observation that recombinant P2X7 extracellular domain (Figure 4D-F) inhibits the phagocytosis of beads and bacteria is consistent with an effect of P2X7 in recognition or at least in regulation of the uptake of nonopsonized particles. Third, blocking P2X7 at a transcriptional level using siRNA also diminished the phagocytosis of beads in interferon-γ stimulated THP-1 cells. Fourth, there is evidence that ATP acts via the P2X7 receptor both in vitro and in vivo to activate the receptor and inhibit further phagocytosis. Finally, the irreversible P2X7 antagonist, OxATP, inhibited the uptake of YG beads and bacteria to almost the same extent as CytD. Collectively, these results reveal a unique property of the P2X7 receptor in its unactivated conformation, namely, to directly or indirectly participate in the uptake of nonopsonized particles. The inhibitory effect of ATP on uptake of bead and bacteria in wild-type mice (but not in P2X7−/− mice) shows that this pathway operates effectively in vivo. For comparison, we studied the Fc-mediated phagocytosis of beads opsonized by human IgG, the uptake of which showed no involvement of P2X7 receptors (supplemental Figure 8A-B). In human monocytes, the uptake of nonopsonized beads was partially inhibited by poly I, suggesting that scavenger receptors accounted for part of the bead uptake. However, bead uptake by P2X7-transfected HEK-293 cells as well as live S aureus uptake by fresh monocytes were not affected by poly I, which makes an interaction between scavenger and P2X7 receptors unlikely. However, it cannot be excluded that P2X7 may also couple with other opsonin-independent pathways, such as mediated by β1-integrin, which has been detected on the membrane surrounding engulfed nonopsonized beads taken up by microglia.46 Whether P2X7 has a recognition function for foreign particles remains uncertain, but it is of interest that P2X7 has a similar 2-transmembrane topology as CD163 and CD36, 2 members of the scavenger receptor group B family that have been shown to directly interact with bacteria or apoptotic cells, respectively.5,6
Exposure of human monocytes to extracellular ATP for 10 to 15 minutes greatly reduced the phagocytosis of YG beads by human monocytes or monocytic THP-1 cells stimulated by interferon-γ and LPS (iTHP-1). ATP is a physiologic ligand for several purinergic receptors, and it is known that monocyte-macrophages express P2X4, P2Y1, P2Y2, P2Y4, and P2Y6, as well as the P2X7 receptor.47 We considered the possibility that ATP reduced phagocytosis via one of these purinergic receptors other than P2X7. Our study of murine peritoneal macrophages showed that the reduction of phagocytosis of beads and bacteria in vitro and in vivo after pretreatment of ATP was observed only in wild-type mice but not in P2X7 gene-deleted animals. Moreover, ATP also failed to inhibit phagocytosis in HEK-293 cells transfected with the Arg-307 to Gln mutant P2X7, which abolishes ATP binding to this receptor.42 Finally, the inhibitory effect of ATP and BzATP (a potent P2X7 agonist) on phagocytosis showed a sigmoid dose effect, with IC50 values of 80μM and 8μM, similar to values needed to activate the P2X7 receptor in native or transfected cells. Thus, P2X7 is probably the major target for ATP in regulating phagocytosis. It is noteworthy that activation of P2X7 has an opposing effect to P2Y6 on phagocytosis of nonopsonized beads because uridine diphosphate has been shown to stimulate phagocytosis of 1.0-μm beads by microglia.46 Extracellular ATP had the unexpected effect of dissociating myosin-IIA from the P2X7-myosin IIA membrane complex in monocytic cells, although the effect of ATP was slow and full dissociation did not reach completion until 15 minutes at 37°C.11 This time frame fits well with the time (10-15 minutes) required for ATP to inhibit phagocytosis, suggesting that the dissociation of myosin IIA from P2X7 and the inhibition of phagocytosis by ATP are linked events.
Activation of the P2X7 receptor by ATP is a major pathway leading to the processing and secretion of IL-1β as well as IL-18 from cells of monocyte-macrophage origin.18,19 This pro-inflammatory role for P2X7 contrasts with the initial P2X7-mediated uptake of bacteria, which requires an absence of ambient ATP. Phagocytosis of bacteria would internalize interacting P2X7 molecules, and our fluorescent images show P2X7 in the phagosome membrane surrounding the engulfed YG beads (Figure 1A). Recent reports suggest that P2X7 within the phagosome membrane together with a coassociated pannexin-1 molecule may facilitate the delivery of bacterial products from phagocytic vesicles into the cytosol, where they can activate inflammasome assembly.48,49 Monocytes migrate with neutrophils to enter an infective focus, and P2X7-regulated uptake of bacteria may be one of the earliest innate immune responses. However, accumulation of ATP within the focus leads to opening of the P2X7 channel/pore, which initiates a second and distinct biologic effect to inhibit further uptake of bacteria by monocytes resulting from dissociation of nonmuscle myosin from the P2X7 complex. As a sequel to this second phase, the activation of cell surface P2X7 receptors by ATP drives a process of phagosome-lysosome fusion, elevation of cytosolic calcium, and killing of engulfed bacteria.26,27,29
P2X7 regulated phagocytosis represents a new biologic function for this membrane receptor, which has clearly separate features from the well-described P2X7 cation-selective channel.50 Thus, CytD, a potent phagocytosis inhibitor, has no effect on P2X7 pore formation measured by ATP-induced ethidium+ uptake.11 In this study, the R307Q mutation, which completely abolishes P2X7 pore formation,42 did not inhibit P2X7-regulated phagocytosis of beads. Thus, ATP, which is an agonist for opening the P2X7 channel, functions as an antagonist for P2X7-regulated phagocytosis. This new biologic effect of P2X7 requires an intact P2X7-NMMHC-IIA complex because extracellular ATP causes slow dissociation of NMMHC-IIA from the complex and inhibits further phagocytosis of nonopsonized particles. We speculate that the role of P2X7 in this pathway is to regulate the uptake of foreign particles or bacteria and after some minutes, as ambient ATP increases, first to limit excessive phagocytosis by the macrophage and second to promote release of proinflammatory cytokines.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Leanne Stokes for helpful comments, Mr Chun Sun for technical assistance, and Ms Ellie Kable in the Electron Microscopy Unit, University of Sydney, for assistance with confocal microscopy.
This work was supported by the Cure Cancer Australia Foundation, the Leukemia Foundation of Australia, National Health and Medical Research Council of Australia, and a Sesqui Fellowship from the University of Sydney (B.J.G.).
Authorship
Contribution: B.J.G. designed the study, did most of the experimental work, analyzed the data, and wrote the manuscript; B.M.S. performed the in vivo study and was involved in discussions; C.J. performed some flow cytometry; and J.S.W. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James S. Wiley, Florey Neuroscience Institutes, Level 3 Alan Gilbert Bldg, University of Melbourne, Parkville 3052, Australia; e-mail: james.wiley@florey.edu.au.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal