Abstract
During the course of homing to lymph nodes (LNs), T cells undergo a multistep adhesion cascade that culminates in a lymphocyte function-associated antigen 1 (LFA-1)–dependent firm adhesion to the luminal surface of high endothelial venules (HEVs). The importance of LFA-1 affinity regulation in supporting T-cell arrest on HEVs has been well established, however, its importance in the postadhesion phase, which involves intraluminal crawling and diapedesis to the extravascular space, remains elusive. Here we have shown that LFA-1 affinity needs to be appropriately regulated to support these essential steps in the homing cascade. Genetically engineered T cells that were unable to properly down-regulate LFA-1 affinity underwent enhanced, chemokine-independent arrest in HEVsbut showed perturbed intravascular crawling to transmigration sites and compromised diapedesis across HEVs. By contrast, the extravascular migration of T cells was insensitive to the affinity-enhancing LFA-1 mutation. These results highlight the requirement for balanced LFA-1 affinity regulation in intravascular and transvascular, but not extravascular, T-cell migration in LNs.
Introduction
The constant recirculation of naive T cells through secondary lymphoid organs is critical for immune surveillance.1 A central event in this process is homing of T cells to lymph nodes (LNs) via high endothelial venules (HEVs). A current model of the homing cascade includes a sequence of at least 4 distinct steps2-5 : (1) recruitment of circulating T cells to the luminal HEV surface, involving a rolling interaction and its subsequent conversion to firm adhesion upon chemokine activation; (2) intravascular migration of luminally adherent T cells that allows the translocation from the initial attachment site to a suitable exit site; (3) transendothelial diapedesis across HEVs; and (4) random migration of T cells within the extravascular compartment in LN parenchyma. Considerable information is available on the molecular and cellular mechanisms involved in the first and last step in this homing cascade; however, little is known about the rules that control the access of luminally adherent cells to the LN parenchyma.
Integrin lymphocyte function-associated antigen 1 (LFA-1; αLβ2) is the predominant cell adhesion molecule present on T cells.6-8 LFA-1 is an α/β heterodimeric transmembrane membrane protein that contains the ligand binding inserted (I) domain at the most distal part of the extracellular portion.9 LFA-1 undergoes dynamic and regulated conformational changes in response to internal cues (eg, the intracellular signaling elicited by chemokine and T-cell receptors) as well as in response to external cues (eg, ligand densities and shear stress).10-12 A series of in vitro investigations propose a model that explains how these sequential engagements of internal and external cues regulate LFA-1 conformations in T-cell interactions with intercellular adhesion molecule-1 (ICAM-1), the major LFA-1 ligand on endothelial cells.13 In naive unstimulated T cells, LFA-1 is predominantly in a default bent form containing a low-affinity (LA) I domain. Upon encountering endothelial cell–bound chemokines that trigger G-protein–coupled receptor (GPCR) signaling, this latent form of LFA-1 is converted into a “primed” extended form possessing an intermediate-affinity (IA) I domain. In physiologically perfused microvessels, the IA LFA-1 is rapidly stabilized into a fully active extended form with a high-affinity (HA) I domain via the interaction with ICAM-1, supporting T-cell arrest on ICAM-1.14 In T cells laterally migrating on ICAM-1 substrates in vitro, LFA-1 affinity needs to be spatiotemporally regulated: whereas HA LFA-1 mediates adhesion at the anterior of polarized cells, the heterodimer reverts to the LA form and, thus promotes de-adhesion at the posterior end, supporting balanced cycles of adhesion and de-adhesion.15,16
Previous studies using LFA-1 blocking antibody17 and LFA-1–deficient mice18 have shown that lymphocyte homing to LNs is critically dependent on LFA-1. Intravital microscopy (IVM) investigations of lymphocyte behavior in LNs have revealed that inhibitors of LFA-119,20 block intravascular lymphocyte arrest on HEVs. In addition, similar loss-of-function strategies have been used to suggest that LFA-1 might be dispensable for leukocyte migration in the LN interstitial space. For example, Woolf et al reported that β2 integrin–deficient T cells lacking LFA-1 exhibited only moderately impaired interstitial motilities.21 In addition, Lämmermann et al reported entirely integrin-independent interstitial migration of dendritic cells (DCs).22 However, it is still unclear how the conformational regulation of LFA-1 activation and, in particular, regulated LFA-1 de-adhesion affect T-cell homing. Moreover, loss-of-function approaches that abrogate LFA-1 function are not suitable to explore the role of LFA-1 in the postadhesion phase of the homing cascade prior to entry into the extravascular space. Thus, it is currently unknown whether LFA-1 contributes to intravascular T-cell crawling or diapedesis in vivo. To fill this gap in knowledge, we have generated a mutant mouse, in which LFA-1 de-adhesion is perturbed by a knock-in mutation αL-I306A that constitutively up-regulates ligand binding affinity of LFA-1. By studying the adhesive interactions of αL-I306A T cells with HEVs using multiphoton intravital microscopy (MP-IVM), here we show the importance of properly down-regulating LFA-1 affinity in promoting the intravascular crawling and diapedesis of T cells during physiologic homing to peripheral LNs.
Methods
Additional information is in supplemental Methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
MP-IVM to study T-cell interactions with HEVs
To visualize the endothelial layer of LN vessels, β-actin green fluorescent protein (GFP) mice23 were irradiated with 13 Gy (divided by 2 doses of radiation 6 hours apart) and reconstituted by intravenous injection of bone marrow cells from wild-type (WT) C57BL/6J mice. Resulting GFP-chimeric mice were used as recipients, 5 to 6 weeks after hematopoietic reconstitution. GFP-chimeric mice were prepared for MP-IVM imaging as described.24,25 WT and knock-in (KI) T cells (107), fluorescently labeled with 7-amino-4-chloromethylcoumarin (CMAC) and 5-(and-6)-(((4-chloromethyl) benzoyl) amino) tetramethylrhodamine (CMTMR), were adoptively transferred to the GFP-chimeric mice preparation. In some experiments, fluorescent dyes CMAC and CMTMR were switched. In other experiments, donor T cells were pretreated with 100 ng/mL pertussis toxin (PTX) for 1 hour at 37°C prior to injection. The kinetics of intravascular behaviors of donor T cells were evaluated by MP-IVM imaging on the HEVs from time 0 to 180 minutes after injection. Volocity software (Improvision) was used for semiautomated tracking of cell motility in 3 dimensions inside vessels. These regions were defined by the delimitation of a region of interest containing GFP+ vessels and both T-cell types. Parameters of cell motility were determined as previously described.24,25 Analysis of migratory paths of donor T cells arresting to HEVs and the quantification of cell numbers within HEVs were performed manually and offline by playback of digital video files. Donor cells crawling on the apical surface of HEVs and those transmigrating HEVs at transendothelial migration (TEM) sites were visually distinguished. The measurements of time of TEM (TTEM) were performed with ImageJ software (W. S. Rasband, ImageJ; National Institutes of Health, http://rsb.info.nih.gov/ij) using the Manual Tracking plug-in.
Statistical analysis
Unless otherwise stated, data are expressed as the mean values plus or minus SEM. Two-tailed Student t test was used for statistical analyses unless otherwise indicated. Statistical significance was defined as P less than .05, less than .01, or less than .001.
Results
Constitutive affinity up-regulation of LFA-1 by a genetic perturbation of the I domain α-helical conformational constraint residue
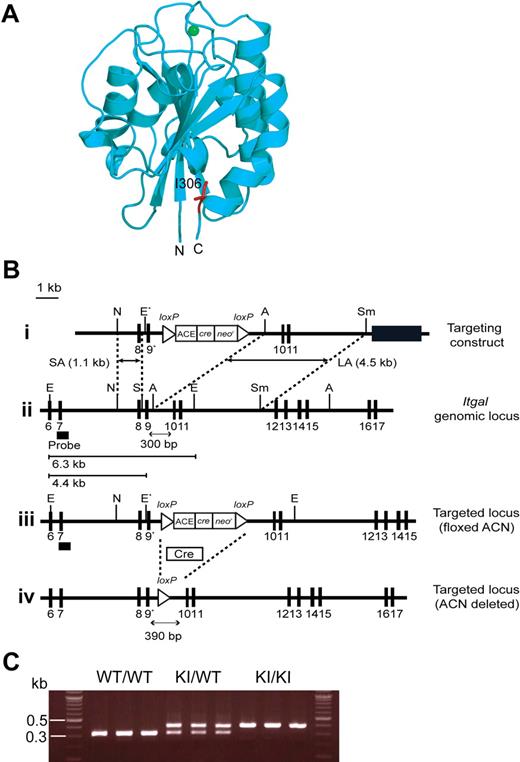
To perturb LFA-1 de-adhesion in vivo, we sought to use a knock-in mutation that constitutively up-regulates LFA-1 affinity. To this end, we investigated the structural information of human LFA-1 I domain.10 The I domain adopts a Rossmann fold with a metal ion–dependent adhesion site at the “top” of the domain, whereas its N- and C-terminal connections occur on the distal “bottom” face (Figure 1A).10,26,27 Upon cellular activation, the C-terminal α7 helix is induced to slide down along the side of the I domain, thereby triggering the conformational conversion of the default LA I domain to the IA, and then to the HA form.10 In the LA I domain, an invariant isoleucine (I306 in the αL I domain), located at the second half of the α7 helix, intercalates deeply into a hydrophobic pocket, thus providing the stabilization needed for this conformation (Figure 1A).28-30 In this way, I306 constrains the movement of the α7 helix, thereby maintaining LFA-1 in a default LA nonadhesive state. Indeed, the alanine substitution of I306 has been shown to constitutively activate human LFA-1 in transfectants.29
Generation of αL-I306A mice. (A) Structure of the mouse αL I domain built by homology modeling using a crystal structure of the human αL I domain (1ZON) as a template. The Mg2+ ion at the ligand binding site (ie, metal ion–dependent adhesion site) is shown with a green sphere and the side chain of I306 is in red. The amino (N) and the carboxyl (C) termini are labeled. Note that I306 is located in the hydrophobic pocket underneath the C-terminal helix. (B) Targeted insertion to the Itgal locus of the floxed ACN cassette and the mutated exon 9 (9*) that contains αL-I306A. The (1) targeting vector, (2) wild-type Itgal locus, (3) targeted Itgal allele containing floxed ACN cassette, and (4) mutated Itgal (I306A) allele are shown. Exons are shown as filled boxes. Long arm (LA) and short arm (SA) of homology, as well as the diphtheria toxin (DT) are shown. The floxed ACN cassette is deleted in chimeric male mice during spermatogenesis, leaving one loxP site (4). An engineered EcoRI site (E*) was designed to identify the targeted allele by Southern blot analysis. N indicates NcoI; E, EcoRI; A, AvrII; Sm, SmaI; and S, SpeI. The thick black line indicates the probe used to screen for homologous recombinations. (C) Genotyping and confirmation of deleted ACN cassette by polymerase chain reaction (PCR). Genomic DNA isolated from tails was used for PCR analyses. PCR bands are shown for wild-type (WT/WT, 300 bp), heterozygote (KI/WT, 300 and 390 bp), and homozygote (KI/KI, 390 bp) samples.
Generation of αL-I306A mice. (A) Structure of the mouse αL I domain built by homology modeling using a crystal structure of the human αL I domain (1ZON) as a template. The Mg2+ ion at the ligand binding site (ie, metal ion–dependent adhesion site) is shown with a green sphere and the side chain of I306 is in red. The amino (N) and the carboxyl (C) termini are labeled. Note that I306 is located in the hydrophobic pocket underneath the C-terminal helix. (B) Targeted insertion to the Itgal locus of the floxed ACN cassette and the mutated exon 9 (9*) that contains αL-I306A. The (1) targeting vector, (2) wild-type Itgal locus, (3) targeted Itgal allele containing floxed ACN cassette, and (4) mutated Itgal (I306A) allele are shown. Exons are shown as filled boxes. Long arm (LA) and short arm (SA) of homology, as well as the diphtheria toxin (DT) are shown. The floxed ACN cassette is deleted in chimeric male mice during spermatogenesis, leaving one loxP site (4). An engineered EcoRI site (E*) was designed to identify the targeted allele by Southern blot analysis. N indicates NcoI; E, EcoRI; A, AvrII; Sm, SmaI; and S, SpeI. The thick black line indicates the probe used to screen for homologous recombinations. (C) Genotyping and confirmation of deleted ACN cassette by polymerase chain reaction (PCR). Genomic DNA isolated from tails was used for PCR analyses. PCR bands are shown for wild-type (WT/WT, 300 bp), heterozygote (KI/WT, 300 and 390 bp), and homozygote (KI/KI, 390 bp) samples.
To determine the structural basis of the I306 constraint in the mouse LFA-1 I domain, we first built a structural model, because there is no crystal or solution structure currently available. We carried out homology modeling using the closed LA conformation of a human LFA-1 I domain crystal structure (pdb code: 1ZON31 ) as a template. This model showed that the conserved I306 is located in a hydrophobic cavity of the closed conformation of the mouse I domain (Figure 1A), thus supporting the hypothesis that this isoleucine likely helps maintain the mouse LFA-1 I domain in the LA state.
To disable the I306 I domain conformational constraint in vivo, we used standard gene targeting approaches; specifically, we mutated the conserved I306 into alanine in the murine αL I domain, thereby generating a mutant Itgal-I306A (Figure 1B-C). We have obtained KI mice homozygous for the mutant αL allele ItgalI306A/I306A, which were designated αL-I306A. αL-I306A mice did not exhibit any gross abnormalities or developmental defects except for a smaller sized peripheral LNs (WT, 1.62 ± 0.11 mm; αL-I306A, 1.06 ± 0.08 mm [diameter] P < .001), which contained fewer lymphocytes compared with WT (supplemental Table 1).
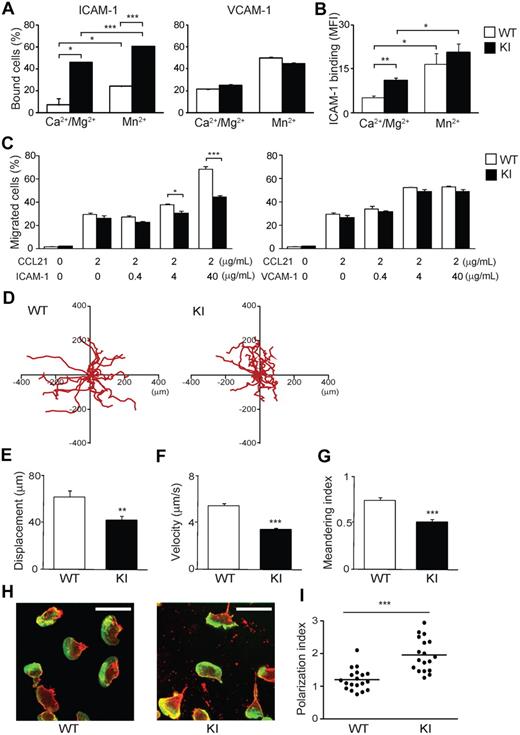
Increased binding to and perturbed migration on ICAM-1 of αL-I306A LFA-1 in vitro
To characterize the effect of αL-I306A mutation to LFA-1 on the cell surface, we initially analyzed mutant and WT lymphocyte adhesion in vitro. αL-I306A lymphocytes showed reduced cell surface expression of LFA-1 but not other integrins (supplemental Figure 1). Despite reduced LFA-1 expression, αL-I306A lymphocytes exhibited enhanced basal activity to bind to ICAM-1, but not to vascular cell adhesion molecule-1 (VCAM-1), substrates in the presence of physiological concentrations of Mg2+ and Ca2+, which favor the default LA state of integrins9 (Figure 2A and supplemental Figure 2A-B). The increased basal adhesion of αL-I306A cells to ICAM-1 was further enhanced by extracellular Mn2+ stimulation, which mimics cellular stimulation, thus inducing the HA conformation9 (Figure 2A left). As measuring monomeric affinity of LFA-1 on the cell surface is technically difficult,32 we sought to assess LFA-1 affinity status using dimeric ICAM-1–Fc. Consistent with the cell adhesion assays, the assessment of LFA-1 affinity status using soluble ICAM-1–Fc showed that αL-I306A LFA-1 adopts a constitutively active IA conformation, which can be reversibly activated to the HA conformation upon exposure to Mn2+ (Figure 2B).
Adhesive interactions of WT and αL-I306A (KI) cells with ICAM-1 in vitro. (A) Cell adhesion of splenocytes to ICAM-1 and VCAM-1 substrates studied using a V-bottom-well plate. Cell suspensions were added to the plates and immediately centrifuged at 200g for 15 minutes to remove unbound cells. (B) Binding of soluble ICAM-1–Fc to WT and KI splenocytes. Bound ICAM-1–Fc was detected with indirect immunofluorescent cytometry using fluorescein isothiocyanate (FITC) anti–human immunoglobulin G (Fc-specific) antibody. (C) Transmigration of WT and KI splenocytes toward a CCL21 gradient through mock-, ICAM-1–coated, and VCAM-1–coated permeable inserts was examined using a modified Boyden chamber assay with a Transwell system (Corning Inc). (A-C) Data are expressed as the mean ± SEM of triplicates from 4 independent experiments. (D-I) 2D migration of T lymphocytes on ICAM-1. (D) 2D tracking of WT and αL-I306A (KI) TCM migrating ICAM-1/CXCL12 substrates. Each track (red) represents migratory paths of individual WT (n = 20) and KI (n = 20) over a 25-minute period. Mean displacement (E), mean velocity (F), and meandering index (G) of laterally migrating TCMs on ICAM-1/CXCL12 substrates were obtained from analysis of live-cell imaging. (E-G) Data are expressed as mean values ± SEM. (H) Representative confocal images of T lymphocytes migrating on ICAM-1/CXCL12 stained for actin (Alexa 488) and αL integrin (cyanin 3) are shown. White bars represent 10 μm. Confocal imaging was performed with a Radiance 2000 Laser-scanning confocal system (Bio-Rad Laboratories) using an Olympus BX50BWI microscope outfitted with a 100×/1.0 numeric aperture water-immersion objective coupled to a photomultiplier tube (PMT) detection system. Imaging medium was PBS. Images were acquired with BioRad2000 Version 2 software and subsequently processed with OpenLab Version 3.0 software. (I) The polarization index16 of T cells from randomly selected fields. Thick horizontal bars indicate mean values. (A-C,E-F,I) Data are expressed as the mean values ± SEM. Two-tailed Student t test was used for statistical analyses. Statistical significance was defined as *P < .05, **P < .01, or ***P < .001.
Adhesive interactions of WT and αL-I306A (KI) cells with ICAM-1 in vitro. (A) Cell adhesion of splenocytes to ICAM-1 and VCAM-1 substrates studied using a V-bottom-well plate. Cell suspensions were added to the plates and immediately centrifuged at 200g for 15 minutes to remove unbound cells. (B) Binding of soluble ICAM-1–Fc to WT and KI splenocytes. Bound ICAM-1–Fc was detected with indirect immunofluorescent cytometry using fluorescein isothiocyanate (FITC) anti–human immunoglobulin G (Fc-specific) antibody. (C) Transmigration of WT and KI splenocytes toward a CCL21 gradient through mock-, ICAM-1–coated, and VCAM-1–coated permeable inserts was examined using a modified Boyden chamber assay with a Transwell system (Corning Inc). (A-C) Data are expressed as the mean ± SEM of triplicates from 4 independent experiments. (D-I) 2D migration of T lymphocytes on ICAM-1. (D) 2D tracking of WT and αL-I306A (KI) TCM migrating ICAM-1/CXCL12 substrates. Each track (red) represents migratory paths of individual WT (n = 20) and KI (n = 20) over a 25-minute period. Mean displacement (E), mean velocity (F), and meandering index (G) of laterally migrating TCMs on ICAM-1/CXCL12 substrates were obtained from analysis of live-cell imaging. (E-G) Data are expressed as mean values ± SEM. (H) Representative confocal images of T lymphocytes migrating on ICAM-1/CXCL12 stained for actin (Alexa 488) and αL integrin (cyanin 3) are shown. White bars represent 10 μm. Confocal imaging was performed with a Radiance 2000 Laser-scanning confocal system (Bio-Rad Laboratories) using an Olympus BX50BWI microscope outfitted with a 100×/1.0 numeric aperture water-immersion objective coupled to a photomultiplier tube (PMT) detection system. Imaging medium was PBS. Images were acquired with BioRad2000 Version 2 software and subsequently processed with OpenLab Version 3.0 software. (I) The polarization index16 of T cells from randomly selected fields. Thick horizontal bars indicate mean values. (A-C,E-F,I) Data are expressed as the mean values ± SEM. Two-tailed Student t test was used for statistical analyses. Statistical significance was defined as *P < .05, **P < .01, or ***P < .001.
Chemokine-driven transmigration of WT and αL-I306A lymphocytes across uncoated and VCAM-1–coated Transwell inserts proved comparable, suggesting that chemokine responses and α4 integrin–mediated transmigration in αL-I306A cells are intact (Figure 2C right). By contrast, transmigration of αL-I306A cells across ICAM-1–coated inserts was suppressed compared with that of WT cells (Figure 2C left). The more ICAM-1 density on inserts increased, the more severe was the perturbation of αL-I306A T cells (Figure 2C left).
We next attempted to study 2-dimensional (2D) migrations of naive WT lymphocytes, in which basal LFA-1 adhesiveness was maintained at low levels. However, these cells failed to exhibit efficient 2D migration on either ICAM-1/CXCL12- or ICAM-1/CCL21-coated substrates (data not shown). Similar observations have been made in a previous report.21 As an alternative, we therefore used interleukin-15–induced central memory–like T cells (TCMs), because in these cells LFA-1 is known to display little basal adhesiveness to ICAM-1, while still readily up-regulating adhesion upon chemokine stimulation.33 WT TCMs robustly migrated on ICAM-1/CXCL12 (Figure 2D and supplemental Video 1A). In contrast, αL-I306A TCMs showed efficient adhesion, but reduced 2D migration, on the same substrate (Figure 2D-G and supplemental Video 1B). During the migration of αL-I306A cells, the leading and trailing edges were not well coordinated. Whereas WT cells polarized in a characteristic fashion, displaying a typically hand mirror–like shape with a flattened leading edge followed by a short narrow tail, αL-I306A cells exhibited extremely extended uropods that were highly enriched in αL-integrin (Figure 2H-I). Continuous forward movement of both the leading edge and the body of the αL-I306A cell appeared to be frustrated by the inefficient detachment of the uropod. In addition, migrating αL-I306A, but not WT, T cells left behind LFA-1–rich membrane debris on ICAM-1 substrates, suggesting that the mutant LFA-1 present in the tail was unable to detach from the ICAM-1 substrates (Figure 2H). The discoordination between the body of αL-I306A cells and their trailing edge reduced not only the migratory velocity, but also their propensity to make a constant forward movement in the same direction, resulting in approximately 34% reduced meandering index (Figure 2G). Thus, the decreased migration efficiency of αL-I306A cells on and across ICAM-1 substrates appears to be related to the perturbed detachment of the uropod.
αL-I306A LFA-1 enhances the interactions of T cells with lymph node venules
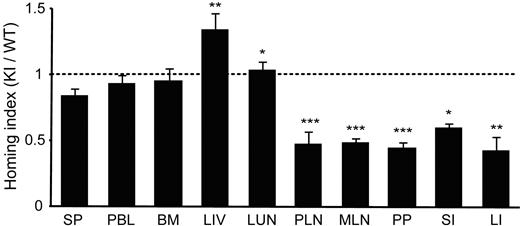
Next, we sought to study how LFA-1 de-adhesion would affect T-cell homing to LNs. We performed a competitive homing assay, in which WT and αL-I306A cells labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and CMTMR, respectively, were mixed and intravenously administered into C57BL/6J-Ly5.1 WT recipient mice. Organs were harvested 2 hours after injection and the homing indices were determined. We found that αL-I306A T cells homed approximately 50% less well to the peripheral and mesenteric LNs, Peyer patches, and small and large intestines, whereas mutant and WT T cells were equally represented in peripheral blood, spleen, bone marrow, and lung (Figure 3). In contrast, more αL-I306A T cells accumulated in the liver than WT cells (Figure 3).
In vivo homing of T lymphocytes. Equal numbers of differentially labeled cells were mixed and injected into C57BL/6J-CD45.1+ congenic recipient mice. The number of homed donor cells and homing indices were determined 2 hours after injection. Data are expressed as the mean values ± SEM. Two-tailed Student t test was used for statistical analyses. Statistical significance was defined as *P < .05, **P < .01, or ***P < .001 vs SP. SP indicates spleen; PBL, peripheral blood lymphocytes; PLN, peripheral lymph node; MLN, mesenteric lymph node; PP, Peyer patch; SI, small intestine; LI, large intestine; BM, bone marrow; LIV, liver; and LUN, lung.
In vivo homing of T lymphocytes. Equal numbers of differentially labeled cells were mixed and injected into C57BL/6J-CD45.1+ congenic recipient mice. The number of homed donor cells and homing indices were determined 2 hours after injection. Data are expressed as the mean values ± SEM. Two-tailed Student t test was used for statistical analyses. Statistical significance was defined as *P < .05, **P < .01, or ***P < .001 vs SP. SP indicates spleen; PBL, peripheral blood lymphocytes; PLN, peripheral lymph node; MLN, mesenteric lymph node; PP, Peyer patch; SI, small intestine; LI, large intestine; BM, bone marrow; LIV, liver; and LUN, lung.
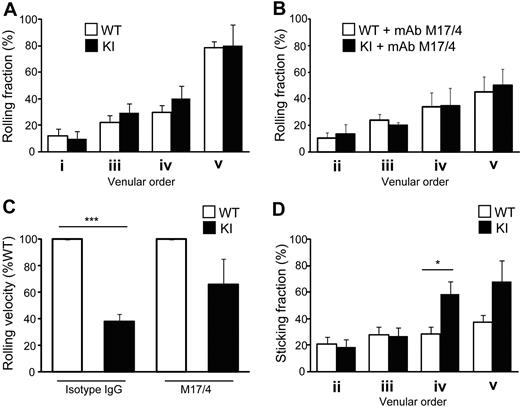
Reduced homing of αL-I306A T cells to LNs raised the possibility that although αL-I306A cells showed enhanced adhesion to ICAM-1 under static condition in vitro, constitutively IA LFA-1 might confer to T cells a diminished ability to arrest on HEVs. To directly address this possibility, we used epifluorescence-IVM imaging of peripheral (inguinal) LNs, investigating the adhesive interactions of WT and αL-I306A T cells with physiologically perfused LN venules. The venular tree in inguinal LNs is composed of 5 different venular branching orders: orders III to V are cortical HEVs, whereas orders I and II are medullary collecting venules with flat endothelia.34 Only high-order venules simultaneously express ligands for L-selectin, ligands for LFA-1, and CCR7-activating chemokines that trigger LFA-1 activation and support the bulk of naive lymphocyte traffic into peripheral LNs.19 Thus, we focused our analysis on the ability of fluorescently tagged WT and mutant T cells to roll and arrest in order II-V HEVs of WT recipient mice.
Although both cell types displayed comparable rolling fractions (Figure 4A), αL-I306A T cells exhibited a slower rolling velocity than did WT cells on high-order venules (Figure 4C). Both cell types express comparable levels of L-selectin, and their rolling was mediated primarily by L-selectin, because a monoclonal antibody (mAb) to L-selectin abrogated all rolling interactions (data not shown). The reduction in rolling velocity of KI T cells was mediated by αL-I306A LFA-1, because LFA-1 mAb treatment reverted it to levels comparable with those of WT cells (Figure 4C) without affecting rolling fractions (Figure 4B). Compared with WT, αL-I306A T cells also exhibited markedly increased sticking fractions in order IV and V venules (Figure 4D). LFA-1 mAb treatment abrogated the firm adhesion of both cell types in HEVs (data not shown). Thus, reduced homing of αL-I306A T cells to LNs is unlikely due to their diminished ability to arrest on HEVs.
Adhesive interactions of T cells with lymph node vessels studied by epifluorescent IVM on inguinal LNs. (A-B) Rolling fractions. WT and αL-I306A T cells showed comparable rolling fractions in the absence (A) or presence (B) of LFA1 blocking antibody M17/4. (C) Rolling velocity on high-order venules. αL-I306A T cells rolled slower on high-order LN venules than WT. After LFA-1 blockade by M17/4 antibody, both cell types showed comparable rolling velocity. (D) Sticking fractions of WT and KI cells. The fraction of sticking cells was increased for αL-I306A T exclusively on IV and V order venules. Data are expressed as the mean ± SEM of 3 independent experiments. Two-tailed Student t test was used for statistical analyses. Statistical significance was defined as *P < .05 or ***P < .001.
Adhesive interactions of T cells with lymph node vessels studied by epifluorescent IVM on inguinal LNs. (A-B) Rolling fractions. WT and αL-I306A T cells showed comparable rolling fractions in the absence (A) or presence (B) of LFA1 blocking antibody M17/4. (C) Rolling velocity on high-order venules. αL-I306A T cells rolled slower on high-order LN venules than WT. After LFA-1 blockade by M17/4 antibody, both cell types showed comparable rolling velocity. (D) Sticking fractions of WT and KI cells. The fraction of sticking cells was increased for αL-I306A T exclusively on IV and V order venules. Data are expressed as the mean ± SEM of 3 independent experiments. Two-tailed Student t test was used for statistical analyses. Statistical significance was defined as *P < .05 or ***P < .001.
αL-I306A T cells exhibit perturbed intravascular crawling and diapedesis
To investigate the mechanisms underlying the reduced homing of αL-I306 T cells despite enhanced sticking to HEVs, we conducted a series of MP-IVM experiments in intact popliteal LNs by injecting WT and mutant T cells into bone marrow (BM) chimeric mice. These chimeric mice were made by irradiating and reconstituting actin promoter-driven GFP-transgenic mice23 with WT BM cells. In these mice, only radioresistant stromal cells, particularly LN endothelial cells, are GFP positive. Indeed, we found that LN endothelial cells exhibited much higher GFP levels than any other cell type in BM chimeras, thus allowing us to discriminate individual vessels from the surrounding stromal cells (Figure 5A and supplemental Video 2). Unlike IVM methods involving the use of fluorescent plasma markers that highlight only the luminal compartment of LN vessels, the use of GFP chimeras enabled us to directly visualize and delineate the vascular wall of HEVs and to undertake a detailed examination of interactions between donor T cells and endothelial cells during the entire homing process.
MP-IVM investigations on T-cell interactions with HEVs. (A) A representative image of adoptively transferred WT T cells (blue) and αL-I306A T cells (red) interacting with popliteal lymph node vessels (green) of GFP-chimeric recipient mice. (B) Gαi-independent arresting of KI cells. Firm adhesion of αL-I306A T cells to HEV endothelial cells was independent of Gαi signals, whereas WT T-cell adhesion was reduced by pertussis toxin (PTX) treatment. (C-D) Motility parameters of T cells in the LN vessel compartment. Meandering index (C) and 3D-IV (D) are shown. (E) Overall transendothelial migration efficacy of arrested cells. Each migratory path of arrested WT and KI T cell on HEVs was traced and analyzed. The line in the box-plot indicates the median, the box-part represents the interquartile range, the whiskers depict the 5th and 95th percentiles, and the crosses represent the mean of 3 independent experiments. (F) A graphic representation of the crawling and TEM steps during adhesive interactions of T cells with HEVs. (G-H) Velocity (G) and traveling distance (H) during the crawling step. αL-I306A T cells migrated slower and a shorter distance on HEVs than did WT T cells. (I) Time required for completing the TEM step. (J-K) Representative migration velocity profiles of WT (J) and KI (K) T cells during the crawling and TEM steps. (B-D,G) Data are expressed as the mean values ± SEM of 3 independent experiments. Two-tailed Student t test was used for statistical analyses. Statistical significance was defined as *P < .05 or ***P < .001. (C-D,H-I) A representative result from 3 independent experiments is shown. (C-D,I) Thick horizontal bars indicate mean values.
MP-IVM investigations on T-cell interactions with HEVs. (A) A representative image of adoptively transferred WT T cells (blue) and αL-I306A T cells (red) interacting with popliteal lymph node vessels (green) of GFP-chimeric recipient mice. (B) Gαi-independent arresting of KI cells. Firm adhesion of αL-I306A T cells to HEV endothelial cells was independent of Gαi signals, whereas WT T-cell adhesion was reduced by pertussis toxin (PTX) treatment. (C-D) Motility parameters of T cells in the LN vessel compartment. Meandering index (C) and 3D-IV (D) are shown. (E) Overall transendothelial migration efficacy of arrested cells. Each migratory path of arrested WT and KI T cell on HEVs was traced and analyzed. The line in the box-plot indicates the median, the box-part represents the interquartile range, the whiskers depict the 5th and 95th percentiles, and the crosses represent the mean of 3 independent experiments. (F) A graphic representation of the crawling and TEM steps during adhesive interactions of T cells with HEVs. (G-H) Velocity (G) and traveling distance (H) during the crawling step. αL-I306A T cells migrated slower and a shorter distance on HEVs than did WT T cells. (I) Time required for completing the TEM step. (J-K) Representative migration velocity profiles of WT (J) and KI (K) T cells during the crawling and TEM steps. (B-D,G) Data are expressed as the mean values ± SEM of 3 independent experiments. Two-tailed Student t test was used for statistical analyses. Statistical significance was defined as *P < .05 or ***P < .001. (C-D,H-I) A representative result from 3 independent experiments is shown. (C-D,I) Thick horizontal bars indicate mean values.
For MP-IVM imaging, an equal number of WT and KI T cells were fluorescently labeled with CMAC (blue) and CMTMR (red), respectively, and adoptively transferred by intravenous injection (in some experiments, the fluorescent dyes were swapped with identical results). Adhesive interactions of donor T cells with GFP+ LN venules were recorded. HEVs were morphologically and functionally identified as those GFP+ LN venules in which WT T cells could be detected (Figure 5A and supplemental Video 2). During the course of our observations (0 to ∼180 minutes after adoptive transfer), we continually noted that approximately 2 times more αL-I306A cells firmly adhered to the apical sides of HEVs than WT cells (Figure 5B). This finding is consistent with the increased sticking fraction of αL-I306A cells to high-order venules observed in epifluorescence-IVM. To determine whether αL-I306A LFA-1 can support firm adhesion to HEVs independently of GPCR activation, WT and αL-I306A T cells were pretreated with pertussis toxin (PTX) and adoptively transferred into GFP-chimeric mice. Although PTX treatment greatly reduced the number of arresting WT T cells as expected,19 it barely affected the number of arresting KI cells (Figure 5B). However, neither PTX-treated WT nor KI T cells that arrested in HEVs underwent subsequent diapedesis, indicating that this step requires guidance by GPCR-dependent chemoattractants (supplemental Video 3).
To study the motility of T cells on the apical surface of HEVs, we analyzed those T cells present on the luminal side of GFP+ vessels by delimiting a region of interest to the vessel compartment. This analysis approach confirmed that more KI cells were present in the vessel compartment than were WT cells (Figure 5C-D). Compared with WT T cells, αL-I306A T cells displayed aberrant, “frustrated” movements characterized by a decreased meandering index and an increased mean 3-dimensional instantaneous velocity (3D-IV; Figure 5C-D). This type of movement of KI T cells suggests an inefficient migration as seen in our in vitro 2D migration data (supplemental Video 1B), in which they fail to move forward because of an impaired release of the trailing edge from the substrate, while rapidly displacing the leading edge. This result led us to study in detail how efficiently WT and KI T cells migrate on and across HEVs (Figure 5E-K).
Whereas approximately 81% of arrested WT T cells completed both steps of HEV transmigration in a 60-minute recording, only approximately 52% of KI T cells did so (Figure 5E). While tracing the migratory paths of individual T cells that had successfully transmigrated, we noted that arrested T cells underwent distinct sequential steps while emigrating from HEVs: an initial crawling step identified by a migratory path on the apical surface of HEVs from the initial arresting point to an exit site; and the TEM step, which encompassed the movement of T cells that had reached an exit site across the HEVs (Figure 5F and supplemental Video 2). To better characterize these steps of T-cell transmigration, we focused on the WT and KI T cells that had successfully transmigrated across HEVs, and excluded those that had not transmigrated. We found that αL-I306A T cells crawled more slowly and shorter distances to exit sites than did WT cells (Figure 5G-H,J-K). During the TEM step, αL-I306A T cells took approximately 6 times longer to transverse the endothelial layer and reach the LN parenchyma than did WT cells (Figure 5I). Representative migration profiles show that compared with a WT T cell (Figure 5J), a KI T cell crawled for a shorter time period with a slower velocity, potentially scanning less surface areas of HEVs, and then took longer to complete transmigration (Figure 5K). These findings suggest that the perturbed cell migration during both crawling on and transmigrating across HEVs was responsible for the lower overall homing efficacy of KI T cells.
Impact of perturbed crawling and diapedesis on T-cell homing to LNs
In the conventional homing assays that we had performed initially (Figure 3), labeled cells were considered to have “homed” when they could be recovered from recipient tissues irrespective of whether they reside in the vascular or in the interstitial compartment. To selectively assess the capacity of T cells to complete the entire homing cascade including TEM, we administered LFA-1 blocking mAb M17/4 after allowing donor cells to home to LNs for 1 hour. Lymphoid organs were harvested 1 hour after mAb injection and the homing indices were determined. This delayed LFA-1 mAb treatment not only blocked further accumulation of new circulating T cells to HEVs, but also detached those T cells that were already adherent on the luminal surface of HEVs but had not undergone diapedesis. Although the delayed LFA-1 mAb treatment did not affect the homing indices to the spleen, bone marrow, and peripheral blood (Figure 6), it decreased the homing indices to the peripheral and mesenteric LNs by approximately 50% (Figure 6). These results strongly suggest, but may not conclude definitively, that the perturbed intravascular crawling and diapedesis steps observed in KI T cells at the HEV microenvironment have an impact on T-cell homing and extravasation to LNs at a systemic level.
Impact of the delayed LFA-1 inhibition on in vivo T-cell homing. Equal numbers of fluorescently labeled WT and KI T cells were mixed and intravenously injected into C57BL/6J-CD45.1+ congenic mice. One hour after cell injection, the mice were injected intravenously with vehicle or 100 μg of anti–LFA-1 mAb M17/4. One hour later, selected organs were harvested and the homing index (KI/WT) was determined. Homing index in LNs was compared with and without delayed M17/4 treatment. Data are expressed as the mean values ± SEM of at least 4 independent experiments. Two-tailed Student t test was used for statistical analyses. Statistical significance was defined as *P < .05, **P < .01, or ***P < .001 vs SP. (#P < .05 or ###P < .001 in groups with and without mAb [M17/4] treatment.) SP indicates spleen; PBL, peripheral blood lymphocytes; BM, bone marrow; PLN, peripheral lymph node; and MLN, mesenteric lymph node.
Impact of the delayed LFA-1 inhibition on in vivo T-cell homing. Equal numbers of fluorescently labeled WT and KI T cells were mixed and intravenously injected into C57BL/6J-CD45.1+ congenic mice. One hour after cell injection, the mice were injected intravenously with vehicle or 100 μg of anti–LFA-1 mAb M17/4. One hour later, selected organs were harvested and the homing index (KI/WT) was determined. Homing index in LNs was compared with and without delayed M17/4 treatment. Data are expressed as the mean values ± SEM of at least 4 independent experiments. Two-tailed Student t test was used for statistical analyses. Statistical significance was defined as *P < .05, **P < .01, or ***P < .001 vs SP. (#P < .05 or ###P < .001 in groups with and without mAb [M17/4] treatment.) SP indicates spleen; PBL, peripheral blood lymphocytes; BM, bone marrow; PLN, peripheral lymph node; and MLN, mesenteric lymph node.
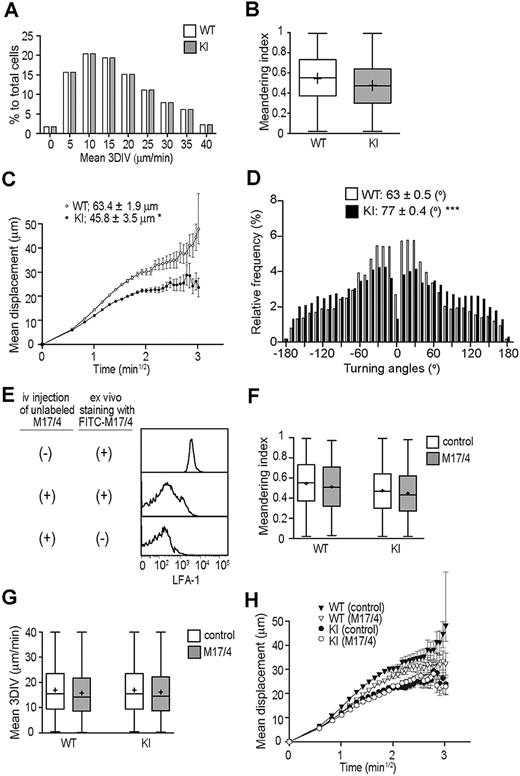
αL-I306A T cells do not display aberrant interstitial motility
Next we studied WT and KI T-cell migration in the LN interstitial microenvironment, which contains fibroblastic reticular cells (FRCs) and DCs expressing detectable levels of ICAM-1, albeit low levels compared with HEVs (supplemental Figure 3). To examine T-cell interstitial motility with MP-IVM, we used nonirradiated WT mice as recipients of differentially labeled WT and KI T cells. Both WT and KI T cells crawled rapidly, displaying random walklike behaviors (supplemental Video 4) with comparable motility parameters, including mean 3D-IV and meandering indices (Figure 7A-B). We observed small, but statistically significant, decrease in motility coefficients of KI T cells (Figure 7C), which was associated with increased turning angles (Figure 7D). These results suggest that LFA-1 is minimally engaged in ligand binding during the rise in interstitial T-cell motility, even when the integrin was in a constitutive IA state. To confirm this hypothesis, we acutely blocked LFA-1 by administering mAb M17/4 after allowing adoptively transferred T cells to enter the LN interstitium (ie, 24 hours after adoptive T-cell transfer). Administered M17/4 (unlabeled) bound to T cells in the LN interstitium, as it competed for LFA-1 with a fluorescently labeled M17/4 when the labeled mAb was applied ex vivo (Figure 7E) However, this anti–LFA-1 treatment had no effect on mean 3D-IV, meandering indices, or the motility coefficients of WT and KI T cells in the LN interstitium (Figure 7F-H), thereby indicating that neither an enhanced (intermediate) ligand binding activity nor complete functional inhibition of LFA-1 has a major impact on interstitial T-cell motility in the steady state. We observed similar results in additional experiments in which WT and αL-I306A cells were alternately labeled (data not shown).
MP-IVM investigations on T-cell interstitial motilities. Motility parameters were measured 18 to 24 hours after adoptive transfer of WT and αL-I306A T cells in C57BL/6 mice. (A) Mean 3D instantaneous velocity (3D-IV). (B) Meandering index. (C) Motility coefficient. (D) Tuning angles. (E) FACS histograms showing a staining of PLN T cells with FITC-labeled anti–LFA-1 mAb M17/4. T cells were isolated from either mice that had been administered with unlabeled M17/4 (middle panel) or those that had been mock treated (top panel). Please note that prior in vivo administration of unlabeled M17/4 competed for LFA-1 with FITC-M17/4. (F-G) Motility parameters after LFA-1 inhibition with M17/4. M17/4 treatment affected neither meandering index (F), mean 3DIV (G), nor motility coefficient (H) of WT and KI cells. (A-H) A representative result from 3 independent experiments is shown. (C,H) Data are expressed as the mean values ± SEM. Data are expressed as mean values ± SEM (B,F-G). The line in the box-plot indicates the mean values, and the top and the bottom of the box represent the maximum and minimum values, respectively. The data are from 3 independent experiments. Two-tailed Student t test was used for statistical analyses. Statistical significance was defined as *P < .05 or ***P < .001.
MP-IVM investigations on T-cell interstitial motilities. Motility parameters were measured 18 to 24 hours after adoptive transfer of WT and αL-I306A T cells in C57BL/6 mice. (A) Mean 3D instantaneous velocity (3D-IV). (B) Meandering index. (C) Motility coefficient. (D) Tuning angles. (E) FACS histograms showing a staining of PLN T cells with FITC-labeled anti–LFA-1 mAb M17/4. T cells were isolated from either mice that had been administered with unlabeled M17/4 (middle panel) or those that had been mock treated (top panel). Please note that prior in vivo administration of unlabeled M17/4 competed for LFA-1 with FITC-M17/4. (F-G) Motility parameters after LFA-1 inhibition with M17/4. M17/4 treatment affected neither meandering index (F), mean 3DIV (G), nor motility coefficient (H) of WT and KI cells. (A-H) A representative result from 3 independent experiments is shown. (C,H) Data are expressed as the mean values ± SEM. Data are expressed as mean values ± SEM (B,F-G). The line in the box-plot indicates the mean values, and the top and the bottom of the box represent the maximum and minimum values, respectively. The data are from 3 independent experiments. Two-tailed Student t test was used for statistical analyses. Statistical significance was defined as *P < .05 or ***P < .001.
These results support the possibility that the conversion of the primed LFA-1 to the HA conformation that occurs during intravascular arrest is apparently absent from the LN interstitial space. A potential absence of shear stress in the LN interstitium might explain the mechanism that prevents such conversion to the HA conformation. Alternatively, as the LFA-1 ligand expression levels potentially affect LFA-1 activation, we studied the spatial and differential distribution of ICAM-1 and ICAM-2 in the vessel and interstitial compartments. Immunofluorescence histology revealed that although ICAM-2 was highly colocalized with PNAd (HEVs), it barely colocalized with ERTR-7+ (FRCs) or CD11c+ (DCs) (supplemental Figure 3E), confirming a previous report that ICAM-2 is expressed predominantly in endothelial cells and only at low levels on platelets and some leukocyte subsets.35 By contrast, ICAM-1 was broadly expressed in the T-cell zone, where it partly colocalized with PNAd, ERTR-7, and CD11c (supplemental Figure 3A,C). To assess the density of ICAM-1, we compared the mean fluorescence intensity of ICAM-1 in HEVs, FRCs, and DCs. Using quantitative image analysis with a Photoshop CS2 software application (Adobe Systems),36 we found that the ICAM-1 mean fluorescence intensity values in HEVs were significantly higher than those in FRCs and DCs (supplemental Figure 3B,D). Therefore, relatively low ICAM-1 and ICAM-2 densities in the interstitial space might prevent ligand engagement of LFA-1.
Discussion
LFA-1 conformation is thought to be spatially and temporally regulated, playing an important role in the promotion of T-cell migration.15,16 Whereas T cells migrated on ICAM-1 substrates, LA LFA-1 was found to selectively localize at the trailing edge, where it was associated with myosin heavy chain IIA, which would provide the necessary force to retract the uropod.16 To investigate the regulated conformational activation of LFA-1, and in particular LFA-1 de-adhesion in T-cell homing to LNs, we used αL-I306A knock-in mice. The αL-I306A LFA-1 adopts the constitutively active IA conformation that supports high-avidity cell adhesion to ICAM-1 substrates in an in vitro adhesion assay. As previously proposed,37 IA LFA-1 establishes the initial contact, which is subsequently transformed into a stable high-avidity cell adhesion. One should take into consideration that V-bottom adhesion assays use shear stress via centrifugal force to remove unbound cells.38 The shear stress generated by this technique might help the conversion of the IA αL-I306A LFA-1 to the HA conformation, thereby supporting high-avidity cell adhesion in this in vitro setting. Although αL-I306A KI cells exhibited constitutively enhanced adhesion to ICAM-1 substrates, they showed perturbed transmigration across ICAM-1–coated chambers and 2D migration on ICAM-1 substrates. Our in vitro observations are consistent with a previous study involving another strain of knock-in mice (Lfa-1d/d) expressing constitutively active LFA-1 via a deletion of the membrane-proximal αL cytoplasmic region.39 Perturbation of either the I domain constraint in αL-I306A mice or the cytoplasmic α/β association in Lfa-1d/d mice resulted in aberrantly activated ligand binding by LFA-1 and suppressed cell migration on ICAM-1. Importantly, by leaving the cytoplasmic domains intact, the I domain mutation in αL-I306A mice rules out the possibility that the altered interactions with cytoskeletal and cytoplasmic signaling molecules are induced by the partial deletion of cytoplasmic tails, a potential caveat associated with the use of Lfa-1d/d cells.39
Our epifluorescence-IVM investigations showed that whereas αL-I306A LFA-1 did not affect the rolling fraction, it decreased rolling velocity, suggesting that IA αL-I306A LFA-1 can stabilize rolling interactions once they are initiated by L-selectin. LFA-1 has been shown to help stabilize selectin-mediated rolling of neutrophils along inflamed mesenteric venules in vivo,40 but LFA-1 does not appear to contribute to physiological lymphocyte rolling in HEVs,19 presumably because WT LFA-1 on naive T cells adopts a predominantly LA conformation.13 The present data demonstrate that LFA-1 in the IA conformation can stabilize L-selectin–mediated T-cell rolling along HEVs, suggesting that the conformational regulation of LFA-1 differs in lymphocyte and myeloid leukocytes. Consistent with this interpretation, Green et al suggested that IA LFA-1 cooperates with selectins in reducing the rolling velocity of neutrophils.41 Stabilization of αL-I306A T-cell rolling, which occurred in high-order LN venules, apparently also promoted the subsequent firm adhesion of rolling T cells. This can account for the increased sticking fraction observed preferentially in high-ordered venules, although LFA-1 ligands are broadly expressed throughout the venule tree.34
Our MP-IVM investigations in GFP-chimeric mice enabled, for the first time, a detailed examination of T-cell interactions with HEVs during the postadhesion phase of homing to LNs. We identified a sequence of 3 distinct steps that require the fine-tuned regulation of LFA-1 to enable T-cell entry into LNs: (1) firm arrest; (2) intravascular crawling to a TEM site; and (3) TEM across HEVs into the extravascular space. Monocytes42 and neutrophils43,44 have been previously shown to undergo β2 integrin–mediated intravascular crawling on inflamed vessels to the extravasation sites. However, to our knowledge, this is the first demonstration that T cells crawl along the luminal surface of microvessels to undergo transmigration at TEM sites. Although αL-I306A T cells showed increased firm arrest to HEVs, their capacity for intravascular crawling and TEM was severely compromised. This disturbed migration likely stems from the inability of adherent αL-I306A T cells to efficiently detach the uropod, thereby locking them in place in the intravascular compartment. Thus, T cells must appropriately down-regulate LFA-1 affinity to efficiently crawl within and across HEVs. Our in vivo observations are in good agreement with recent in vitro findings by Shulman et al5 who showed that human T cells must be able to regulate LFA-1 to migrate on and across a monolayer.5
It has been suggested that lymphocytes preferentially leave HEVs through discrete exit sites (termed “hot spots”).45 The inability to crawl within HEVs likely limited the ability of αL-I306A T cells to reach these hot spots. Indeed, modifying a traditional homing assay by using a delayed LFA-1 mAb treatment, we were able to distinguish between donor cells that had successfully accessed the LN parenchyma from those that remained arrested in the intravascular compartment. These experiments indicate that the perturbed intravascular crawling and diapedesis of αL-I306A T cells reduced their capacity to home into the LN parenchyma by as much as 60%.
The aberrant motility profiles of αL-I306A cells in the vessel compartment have also been characterized. αL-I306A cells showed lower meandering indices, but higher 3D-IV, than did WT cells. In addition, αL-I306A cells tended to change direction more frequently (ie, higher turning angles). Consistent with these data, we observed that many αL-I306A cells in the vessel compartment displayed unproductive jerky oscillations, as if frustrated by their inability to detach from endothelial cells. These oscillatory movements appear to reflect an active (albeit largely thwarted) migratory apparatus (ie, chemotactic sensing and lamellipodia formation), as is reflected by the increased mean 3D-IV. Similarly, during 2D migration on ICAM-1/CXCL12 substrates in vitro, αL-I306A cells displayed disturbed migratory movements that appeared to result from the inability of aberrantly activated LFA-1 to support efficient tail detachment. Jerky migratory behaviors have also been seen in the disturbed TEM of human lymphocytes aberrantly activated by a small-molecule agonist for LFA-1.46 Alternatively, aberrantly regulated signaling through constitutively, or irreversibly, active αL-I306A LFA-1 might perturb appropriate LFA-1 redistribution on the membrane, thereby suppressing efficient cell migration, as regulated redistribution of IA LFA-1 at the leading edge and HA in the midbody have been proposed to be important for facilitating T-cell migration.47
Since its first implementation, MP-IVM has revealed the unexpectedly motile nature of naive T cells in the LN interstitial compartment.24,48,49 However, the impact of LFA-1 activation status in this physiologic mode of migration has remained elusive. Woolf et al observed minimal engagement of LFA-1 on naive T cells migrating in LNs, suggesting that the LFA-1 activation cascade precipitating the conversion to enhanced affinity might be inactive.21 However it had been unclear whether a shift in LFA-1 to the IA affinity form could impact on interstitial T-cell motility. We show here that IA αL-I306A LFA-1 exhibited enhanced firm adhesion to HEVs under shear stress in the absence of GPCR signaling. However, the expression of IA LFA-1 was apparently insufficient to engage its ligand in the extravascular space, although one could assume that such engagement would result in detectable changes in interstitial T-cell motility. We observed mild changes in the motility coefficient and turning angle of KI T cells compared with WT T cells. These parameters were unaffected after LFA-1 inhibition in vivo, thereby suggesting that these changes were independent of LFA-1 engagement. Although we did not observe an enrichment of activated T cells in LNs of KI mice, compared with those of WT mice (not shown), it is difficult to experimentally exclude the possibility that WT and KI T-cell subsets that gained access to the LN interstitium might not be identical. These results suggest that the LN interstitial space lacks essential external cues that must convert the IA LFA-1 to the HA form. It has been based on earlier studies of intravascular lymphocyte-endothelial interactions11,14 that chemokine-induced IA LFA-1 is converted to the HA form by interactions with its ligand combined with fluid shear stress. Because the interstitial milieu is proposed to lack shear stress,21 αL-I306A LFA-1 might remain in the IA conformation, which is apparently insufficient to engage in a strong adhesive interaction with ICAM-1. However, it is possible that in the LN parenchyma, cells continuously slide against each other under a high cell density microenvironment, potentially producing a physical friction that could influence LFA-1 conformations. Alternatively, as shown in our quantitative fluorescent histology data of the LN HEVs and interstitium (supplemental Figure 3), lower levels of ICAM-1 and ICAM-2 expression in the LN parenchyma, compared with HEVs, might prevent the conversion of IA LFA-1 on KI T cells to an HA form.
In summary, our data demonstrate that the appropriate balance of LFA-1 affinity is critical for T-cell homing to LNs. Whereas LFA-1 affinity up-regulation is required for T-cell arrest on the luminal surface of HEVs, LFA-1 affinity needs to be properly down-regulated to facilitate intraluminal crawling and diapedesis. Perturbed de-adhesion limits the ability of T cells to crawl within and diapedese across HEVs, thereby interfering with T-cell homing to LNs. In contrast to the interactions with HEVs, LFA-1 engagement is dispensable for T-cell migration in the interstitial space presumably due to the absence of shear and/or reduced LFA-1 ligand expression, thereby failing to convert the IA LFA-1 to the ligand competent HA form.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge Jianghai Zhu and Can Xie for modeling; Timothy A. Springer for his valuable advice; Mario R. Capecchi for the self-excision cassette used in pACN-TV vector; Klaus Rajewsky for Bruce-4 embryonic stem cells; and Myriam Wlodarczyk for helpful discussions. We thank Mary Mohrin, Ronnie Yoo, Whitney Silkworth, Yoshiteru Sasaki, Thomas Schurpf, and Harry Leung for technical assistance.
This work was supported by grants from the Arthritis Foundation (C.V.C.) and the National Institutes of Health grants AI061663, HL56949, AR42689, AI069259, AI072252, and AI078897 (U.H.v.A.), AI063421, and HL048675 (M.S.). E.J.P. is partly supported by the GlaxoSmithKline-Immune Disease Institute alliance fellowship.
National Institutes of Health
Authorship
Contribution: E.J.P. and A.P. designed, performed, and analyzed all experiments unless stated otherwise; Y.I. established positive embryonic stem cell clones and discussed the data; A.G. and G.C. performed epifluorescence IVM experiments; C.V.C. designed, performed, and analyzed in vitro imaging; C.V.C., U.H.v.A., and M.S. designed experiments and secured funding; and E.J.P., A.P., C.V.C., U.H.v.A., and M.S. discussed the data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ulrich H. von Andrian, 77 Ave Louis Pasteur, Boston, MA 02115; e-mail: uva@hms.harvard.edu; or Motomu Shimaoka, 3 Blackfan Cir, Rm 3041, Boston, MA 02115; e-mail: shimaoka@idi.harvard.edu.
References
Author notes
E.J.P. and A.P. contributed equally to this work.


![Figure 6. Impact of the delayed LFA-1 inhibition on in vivo T-cell homing. Equal numbers of fluorescently labeled WT and KI T cells were mixed and intravenously injected into C57BL/6J-CD45.1+ congenic mice. One hour after cell injection, the mice were injected intravenously with vehicle or 100 μg of anti–LFA-1 mAb M17/4. One hour later, selected organs were harvested and the homing index (KI/WT) was determined. Homing index in LNs was compared with and without delayed M17/4 treatment. Data are expressed as the mean values ± SEM of at least 4 independent experiments. Two-tailed Student t test was used for statistical analyses. Statistical significance was defined as *P < .05, **P < .01, or ***P < .001 vs SP. (#P < .05 or ###P < .001 in groups with and without mAb [M17/4] treatment.) SP indicates spleen; PBL, peripheral blood lymphocytes; BM, bone marrow; PLN, peripheral lymph node; and MLN, mesenteric lymph node.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/8/10.1182_blood-2009-08-237917/4/m_zh89990949120006.jpeg?Expires=1763515697&Signature=iDcHkpbD51Y1IWRS-YuDDG4uxdpsS8zw835mlUd0TEfd6lpVhXlUZJRTvElHmWvAleN7s6e6x9teYbe1sWZPwHEOwguxIzRE75wspCV1FT4x9ymz6gKwSadFF7Cx4VRQLnV~Uc1F7OXRB~-C~gjT4UIP94IjFyre-Q7Cdo4SZWINRhVxEy4RZ5xYKLKrrJtdniehUUUn8SBbmRx2Nx9oxD1OmLWQwaxG8oAvwSRhtYpIW4pMm9SXunpBOBRSDN6HEKI6tqVI-2iYogRLTIWuPC2hGOnvPstAHrKpD3k7KEwWxpQVceSzZKSmwn-GTgd~Smm~ORy-dq4yEQ1v6YdjJw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)