Abstract
Cytotoxic T lymphocytes and natural killer cells destroy target cells via the polarized exocytosis of lytic effector proteins, perforin and granzymes, into the immunologic synapse. How these molecules enter target cells is not fully understood. It is debated whether granzymes enter via perforin pores formed at the plasma membrane or whether perforin and granzymes are first endocytosed and granzymes are then released from endosomes into the cytoplasm. We previously showed that perforin disruption of the plasma membrane induces a transient Ca2+ flux into the target cell that triggers a wounded membrane repair response in which lysosomes and endosomes donate their membranes to reseal the damaged membrane. Here we show that perforin activates clathrin- and dynamin-dependent endocytosis, which removes perforin and granzymes from the plasma membrane to early endosomes, preserving outer membrane integrity. Inhibiting clathrin- or dynamin-dependent endocytosis shifts death by perforin and granzyme B from apoptosis to necrosis. Thus by activating endocytosis to preserve membrane integrity, perforin facilitates granzyme uptake and avoids the proinflammatory necrotic death of a membrane-damaged cell.
Introduction
Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells eliminate virus-infected cells and tumors by releasing the contents of cytotoxic granules into the immunologic synapse formed with the target cell.1,2 The granule mediators of cell death, serine proteases known as granzymes (Gzms), are delivered into the target cell cytosol by the granule pore-forming protein perforin (PFN).3,4 The ability of Gzms to induce target cell apoptotic death is entirely PFN-dependent.2 PFN-deficient mice are highly susceptible to viruses and carcinogen-induced neoplasia and spontaneously develop B-cell lymphomas. Human congenital defects that result in impaired PFN synthesis, function, or dysregulated release lead to familial hemophagocytic lymphohistiocytosis, a disease associated with profound immunodeficiency.5,6
How PFN delivers Gzms into target cells is not fully understood.7,8 Because PFN multimerizes in a Ca2+-dependent manner in membranes to form pores, the original model was that Gzms enter target cells via plasma membrane pores formed by multimerized PFN.9,10 An influx of Ca2+ into target cells treated with PFN or attacked by CTLs suggests that PFN does indeed form plasma membrane pores.11 However, at PFN sublytic concentrations that mimic physiologic conditions of apoptosis induction, the pores that form in the plasma membrane might be too small to allow Gzms to pass through. In fact, small dyes that ought to be able to pass through PFN-sized pores do not disseminate into the cytosol after PFN treatment.11,12 Moreover, Gzms do not directly enter the cytoplasm, as they would if they entered via cell membrane pores, but concentrate first in early endosomal antigen-1 (EEA-1)–staining endosomes.11,13 Therefore an alternate model postulates that PFN functions at the endosomal membrane to release endocytosed Gzms.11,13
Although PFN greatly enhances cellular uptake of Gzms,11 a clear mechanism to explain the increased endocytosis of Gzms is lacking. The Ca2+ influx induced in cells treated with sublytic PFN is transient, lasting at most 5 minutes.11 This suggests that PFN forms small pores in the plasma membrane, but they are rapidly removed or repaired. We previously showed that the PFN-mediated increase in intracellular Ca2+ triggers a stereotypic damaged-membrane response (called cellular “wound healing”), where intracellular vesicles, including endosomes and lysosomes, are rapidly mobilized to donate their membranes to reseal the damaged plasma membrane.11,14,15 This repair response helps preserve the integrity of the cell membrane and allows the targeted cell to avoid immediate necrosis and undergo the slower process of programmed cell death.16 Although the wound healing response might facilitate membrane repair, it does not explain how PFN enhances Gzm uptake. However, a recent study showed that the bacterial pore-forming protein, streptolysin-O (SLO), which can substitute for PFN for intracellular delivery of Gzms, activates Ca2+-dependent, dynamin (Dyn)–independent endocytosis of damaged membrane to facilitate membrane repair.17 If endocytosis occurs as part of the plasma membrane repair response (which PFN also activates11 ), it might provide a mechanism for Gzm and PFN coendocytosis in target cells.
The aim of this study was to investigate how PFN enhances GzmB uptake. For the first time, we visualize PFN within target cells. We find that PFN activates clathrin- and Dyn-dependent endocytosis to remove PFN from the plasma membrane and enhance GzmB uptake. Both PFN and GzmB are endocytosed into giant EEA-1+ endosomes that form after PFN treatment. When endocytosis is inhibited, sublytic PFN or SLO becomes lytic, causing necrosis. Consequently, inhibiting clathrin or Dyn pathways shifts the balance of target cell death by GzmB and PFN from apoptosis to necrosis. Our results suggest that the rapid induction of endocytosis to remove damaged cell membrane is an important component of the wound healing response used by cytotoxic cells to induce apoptosis.
Methods
Additional methods can be found in supplementary online material (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Treatment with PFN, ionomycin, SLO, and GzmB
Native human PFN (hPFN) and GzmB were purified from NK-YT cells and native rat PFN (rPFN) or GzmB was purified from RNK16 cells as described.18,19 Animal use was approved by the Animal Care and Use Committee of the Immune Disease Institute and Harvard Medical School. Ionomycin was from Sigma-Aldrich and purified SLO, from BioAcademia Inc. Cells were washed and equilibrated 5 minutes in Hanks balanced salt solution with 10mM Hepes (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.5, 4mM CaCl2, 0.4% bovine serum albumin before adding PFN, ionomycin, SLO, and/or GzmB, diluted in PFN buffer (Hanks balanced salt solution, 10mM Hepes, pH 7.5). Sublytic PFN, ionomycin, and SLO concentrations were determined independently for each experiment at the concentration required to induce 5% to 15% propidium iodide (PI) uptake (PI 2 μg/mL in 10mM Hepes, pH 7.5, 140mM NaCl, 2.5mM CaCl2 buffer; Sigma-Aldrich) measured 20 minutes later by flow cytometry (FACSCalibur; BD Biosciences).18,19
Immunostaining of EEA-1, GzmB, and PFN
HeLa cells were grown on rat collagen-coated glass coverslips (Sigma-Aldrich) and treated with sublytic hPFN and/or GzmB. After the indicated time, cells were fixed for 20 minutes in phosphate-buffered saline (PBS)/2% paraformaldehyde, washed, and incubated 20 minutes in PBS/50mM NH4Cl. Cells were then washed with PBS and permeabilized for 5 minutes in permeabilization buffer (PBS/0.2% Triton X-100). After 2 washes in PBS, coverslips were placed in blocking solution (PBS/10% fetal calf serum) for 30 minutes, washed once in PBS, and incubated for 1 hour at room temperature with the indicated primary antibodies (mouse anti–human PFN, clone Pf80/164 [Mabtech Inc]; goat anti–EEA-1, clone N19 [Santa Cruz Biotechnology Inc]; mouse anti–human GzmB, clone GB11 [Caltag Laboratories]) in incubation buffer (PBS/0.05% Triton X-100). Cells were then washed 3 times with incubation buffer and incubated for 1 hour at room temperature with donkey AlexaFluor 488– and/or 647–conjugated secondary antibodies (Molecular Probes) in incubation buffer containing 5% normal donkey serum (Sigma-Aldrich). Cells were then washed 3 times in PBS and mounted in Vectashield mounting medium containing 4,6 diamidino-2-phenylindole (DAPI; Vector Laboratories) before epifluorescence or spinning disk confocal imaging, as indicated (see supplemental Methods for additional information about microscope configurations).
Measurement of membrane-associated AP-2
Measurement of membrane-associated adaptor protein 2 (AP-2) puncta was performed as described previously.20,21 All particle tracks were individually validated. Calculation of lifetime, maximal intensity distributions, and total number of puncta was performed using an image analysis application developed using Matlab 7 (Mathworks).21 The percentage increase in AP-2–mediated endocytosis after treatment was calculated as: ([sum of maximum intensity of membrane-associated AP-2 spots after treatment/sum of maximum intensity of membrane-associated AP-2 spots before treatment] − 1) × 100.
Inhibition of endocytosis
siRNAs for green fluorescent protein (GFP), μ2-adaptin (AP-2μ2), clathrin heavy chain (CHC),22 dynamin 2 (Dyn2; ON-TARGETplus siRNA; Dharmacon), and flotillin-1 (Santa Cruz Biotechnology) were transfected into HeLa cells using Oligofectamine (100nM final siRNA concentration; Invitrogen) according to the manufacturer's protocol. Cells were tested for knockdown 48 hours later by immunoblot probed with anti–AP-2 (clone AP50), anti–flotillin-1 (clone 18; BD Transduction Laboratories), anti-CHC (clone X22; Affinity BioReagents) mouse monoclonal Abs, anti-Dyn2 (Calbiochem), or anti–α-tubulin (Affinity BioReagents) rabbit polyclonal Abs. In some experiments, HeLa cells were pretreated for 30 minutes before adding PFN and GzmB with 80μM Dynasore, a cell-permeable inhibitor of Dyn,23 in 0.8% DMSO or 300mM sucrose (Sigma-Aldrich) in cell buffer without bovine serum albumin. Potassium depletion was performed as described.24
Apoptosis and necrosis assays
To assess apoptosis, cells incubated for 2 hours at 37°C with buffer or sublytic rPFN and/or 200nM native human GzmB were analyzed for caspase activation by flow cytometry using M30-fluorescein isothiocyanate–conjugated monoclonal antibody staining according to the manufacturer's protocol (M30 CytoDEATH; Roche) to detect a caspase-cleavage product of cytokeratin 18.25 PFN-induced necrosis was assessed by flow cytometry 15 minutes after loading by PI staining.
Results
Perforin and granzymes are endocytosed into outsized early endosomes
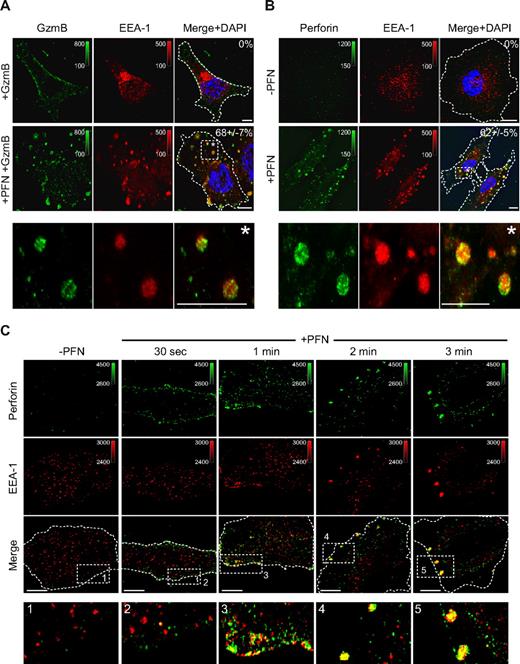
To examine the effect of PFN on GzmB internalization, we incubated HeLa cells with native human GzmB (hGzmB) in the presence or absence of sublytic concentrations of native human PFN (hPFN). The sublytic concentration is the concentration that delivers Gzms to induce apoptosis without excessive necrosis.11,18 As previously reported,11 hPFN greatly enhanced hGzmB uptake. Both hPFN and hGzmB were taken up within 5 minutes into large EEA-1+ intracellular vesicles that form after PFN treatment (Figure 1A-B). We termed these structures gigantosomes, because they are approximately an order of magnitude larger in diameter than other endosomes.11 Because both anti–human PFN and GzmB antibodies were from mice, we were unable to costain hPFN and hGzmB in the same cells. However, staining with affinity-purified polyclonal antisera to purified rat NK-cell GzmB (rGzmB) and rPFN, showed that rPFN and rGzmB colocalize in EEA-1+ vesicles when HeLa cells were loaded with sublytic native rat PFN and GzmB (supplemental Figure 1A-D). The enlargement of early endosomes was also selective, because when cells were analyzed 2 minutes after adding hPFN, EEA-1 vesicles increased in size, but Lamp-1 staining vesicles (a marker of late endosomes/lysosomes) remained approximately the same size (supplemental Figure 2A). Endocytosis of PFN was rapid (Figure 1C). Thirty seconds after adding sublytic hPFN to HeLa cells, PFN immunostaining was mostly at the plasma membrane and only few EEA-1+ endosomes stained for PFN. However, after 1 minute, plasma membrane–associated PFN partitioned into EEA-1+ vesicles that evolved into gigantosomes within approximately 2 to 3 minutes.
PFN and GzmB are rapidly endocytosed into EEA-1+ outsized early endosomes. (A-B) Within 5 minutes of treatment with sublytic native hPFN and hGzmB, large intracellular and membrane bound endosomes (gigantosomes) coimmunostain for GzmB (A) or PFN (B) and the early endosomal marker EEA-1. Images were acquired by wide-field fluorescence microscopy and deconvolved using iterative deconvolution. Representative Z stack series projections from 3 independent experiments are shown. Percentage of cells with GzmB or PFN staining enlarged endosomes is indicated (mean ± SD). (C) HeLa cells were exposed to sublytic hPFN for indicated times and stained for PFN and EEA-1. PFN is first detected at the plasma membrane and then endocytosed into EEA-1+ endosomes before forming gigantosomes. Representative confocal sections from 3 independent experiments are shown. PFN or GzmB was detected using AlexaFluor 488–conjugated secondary antibody and EEA-1 using AlexaFluor 647–conjugated secondary antibody. Color bars and associated numbers indicate fluorescence intensity levels. Scale bars represent 10 μm. Dashed lines indicate plasma membrane. Magnification and other image acquisition data are in supplemental Methods.
PFN and GzmB are rapidly endocytosed into EEA-1+ outsized early endosomes. (A-B) Within 5 minutes of treatment with sublytic native hPFN and hGzmB, large intracellular and membrane bound endosomes (gigantosomes) coimmunostain for GzmB (A) or PFN (B) and the early endosomal marker EEA-1. Images were acquired by wide-field fluorescence microscopy and deconvolved using iterative deconvolution. Representative Z stack series projections from 3 independent experiments are shown. Percentage of cells with GzmB or PFN staining enlarged endosomes is indicated (mean ± SD). (C) HeLa cells were exposed to sublytic hPFN for indicated times and stained for PFN and EEA-1. PFN is first detected at the plasma membrane and then endocytosed into EEA-1+ endosomes before forming gigantosomes. Representative confocal sections from 3 independent experiments are shown. PFN or GzmB was detected using AlexaFluor 488–conjugated secondary antibody and EEA-1 using AlexaFluor 647–conjugated secondary antibody. Color bars and associated numbers indicate fluorescence intensity levels. Scale bars represent 10 μm. Dashed lines indicate plasma membrane. Magnification and other image acquisition data are in supplemental Methods.
Sublytic hPFN also greatly enhanced the uptake of fluorescent 70-kDa cationic dextran into large intracellular vesicles. Treatment of cells labeled with a fluorescent lipophilic dye (FM4-64) with sublytic rPFN or hPFN also induced rapid redistribution of the plasma membrane–associated dye to intracellular membranes (supplemental Figure 2B). However, uptake of transferrin (Tf), which binds to its receptor at the cell surface and is rapidly internalized by clathrin-mediated endocytosis in the absence of PFN, was not enhanced or accelerated by hPFN treatment (supplemental Figure 2C-D). However, Tf concentrated into gigantosomes, and Tf-containing gigantosomes costained with PFN (supplemental Figure 2E). Therefore, PFN activates rapid endocytosis of Gzms as well as other cell membrane–associated molecules into gigantosomes. Moreover, molecules that are normally rapidly endocytosed independently of PFN (such as Tf) localize into large endosomes when PFN is present. In the process, PFN is also removed from the plasma membrane to the gigantosome.
Cytotoxic T-lymphocyte degranulation triggers gigantosome formation and PFN endocytosis in target cells
To determine whether PFN-containing gigantosomes also form in the physiologically relevant setting of CTL attack, we looked for gigantosome formation by live cell microscopy when HeLa cells expressing enhanced green fluorescent protein (EGFP)–EEA-1 were treated with HeLa cell–specific CTLs. When cells were coincubated with the Ca2+ chelator EGTA (ethyleneglycoltetraacetic acid) to allow effector/target conjugate formation but block cytotoxic granule exocytosis, the distribution of EGFP–EEA-1 was unchanged. However when Ca2+ was added to trigger CTL degranulation, large EEA-1+ vesicles were seen within a few minutes near the immunologic synapse in the target cell, suggesting a localized effect of PFN (Figure 2A, supplemental Videos 1-2). Gigantosomes were also generated in target cells when concanavalin A–coated HeLa cells were mixed with lymphokine-activated killer (LAK) cells (Figure 2B). Moreover, PFN colocalized with EEA-1 in the target cells. Therefore, PFN-containing EEA-1+ gigantosomes form in target cells attacked by human T cells.
Large endosomes (gigantosomes) form in target cells within minutes of triggering CTL degranulation. (A) Large EEA-1+ endosomes form in a target cell after CTL degranulation. EGFP–EEA-1–transfected HeLa target cells were incubated with specific CTLs in the presence of EGTA to allow conjugate formation. After 2 minutes, CaCl2 was added to induce CTL degranulation (bottom; images from supplemental Videos 1-2). Enlarged endosomes form in the target cell within minutes after CTL degranulation, although the size of early endosomes does not change in the absence of calcium (top). Data (deconvolved wide-field images) are representative of 3 independent experiments. (B) Large endosomes formed in target cells after CTL attack contain PFN. Concanavalin A–coated HeLa cells were incubated with LAK cells in the presence of EGTA to allow conjugate formation, and then buffer (top) or CaCl2 (bottom) was added to induce cytotoxic granule exocytosis 5 minutes before fixation. Data depicted (deconvolved wide-field 3-dimensional images followed by Z projection) are representative of 2 independent experiments. PFN signal was detected using AlexaFluor 488–conjugated secondary antibody and EEA-1 using AlexaFluor 647–conjugated secondary antibody. Color bars and associated numbers indicate fluorescence intensity levels. Scale bars represent 10 μm. Dashed lines indicate plasma membrane.
Large endosomes (gigantosomes) form in target cells within minutes of triggering CTL degranulation. (A) Large EEA-1+ endosomes form in a target cell after CTL degranulation. EGFP–EEA-1–transfected HeLa target cells were incubated with specific CTLs in the presence of EGTA to allow conjugate formation. After 2 minutes, CaCl2 was added to induce CTL degranulation (bottom; images from supplemental Videos 1-2). Enlarged endosomes form in the target cell within minutes after CTL degranulation, although the size of early endosomes does not change in the absence of calcium (top). Data (deconvolved wide-field images) are representative of 3 independent experiments. (B) Large endosomes formed in target cells after CTL attack contain PFN. Concanavalin A–coated HeLa cells were incubated with LAK cells in the presence of EGTA to allow conjugate formation, and then buffer (top) or CaCl2 (bottom) was added to induce cytotoxic granule exocytosis 5 minutes before fixation. Data depicted (deconvolved wide-field 3-dimensional images followed by Z projection) are representative of 2 independent experiments. PFN signal was detected using AlexaFluor 488–conjugated secondary antibody and EEA-1 using AlexaFluor 647–conjugated secondary antibody. Color bars and associated numbers indicate fluorescence intensity levels. Scale bars represent 10 μm. Dashed lines indicate plasma membrane.
Clathrin associates with gigantosomes
Our next goal was to define the endocytic pathway triggered by PFN. Endocytosis can be mediated by both clathrin-dependent and clathrin-independent pathways, including endocytosis via caveolin or flotillin vesicles, phagocytosis, macropinocytosis, and transendocytosis.26 Because clathrin-mediated endocytosis is a major endocytic pathway, we used both fixed and live cell imaging to examine whether PFN-induced gigantosomes associate with clathrin. Gigantosomes formed after sublytic rPFN treatment stain for EEA-1 and clathrin heavy chain (CHC), but do not stain for caveolin-1, flotillin-1 (involved in clathrin-independent endocytosis), or Lamp-1 (CD107a; supplemental Figure 3A). To examine gigantosome formation without potential fixation artifacts, HeLa cells, stably expressing EGFP–clathrin light chain (CLC) and transiently transfected with mRFP–EEA-1, were treated with sublytic rPFN and imaged by live cell confocal microscopy (Figure 3, supplemental Figure 3B). After adding PFN, early endosomes rapidly enlarged to form vesicles that partially immunostained with clathrin. Indeed, 3-dimensional capture images showed that clathrin partially coated the gigantosomes (supplemental Videos 3-4). The accumulation of clathrin in gigantosomes suggests that clathrin might be involved in gigantosome formation.
Gigantosomes are coated with clathrin. HeLa cells stably expressing EGFP-CLC (green) and transfected with mRFP–EEA-1 (red) were analyzed by live 3-dimensional confocal capture 10 minutes after sublytic rPFN treatment. In the presence of rPFN, gigantosomes stained with EEA-1 (red), colocalize with clathrin (green) as indicated. One representative optical section obtained from sequential 0.1-μm optical sections (supplemental Videos 3-4) is shown for each condition. Most of the gigantosomes (red) are coated with clathrin (green). Data are representative of 5 independent experiments. Color bars and associated numbers indicate fluorescence intensity levels. Scale bars represent 10 μm. Dashed lines indicate plasma membrane.
Gigantosomes are coated with clathrin. HeLa cells stably expressing EGFP-CLC (green) and transfected with mRFP–EEA-1 (red) were analyzed by live 3-dimensional confocal capture 10 minutes after sublytic rPFN treatment. In the presence of rPFN, gigantosomes stained with EEA-1 (red), colocalize with clathrin (green) as indicated. One representative optical section obtained from sequential 0.1-μm optical sections (supplemental Videos 3-4) is shown for each condition. Most of the gigantosomes (red) are coated with clathrin (green). Data are representative of 5 independent experiments. Color bars and associated numbers indicate fluorescence intensity levels. Scale bars represent 10 μm. Dashed lines indicate plasma membrane.
PFN treatment enhances clathrin-coated pit formation and clathrin-mediated endocytosis
Because gigantosomes associate with clathrin, we wanted to know whether PFN or 2 other agents known to trigger cellular wound healing, ionomycin11 and SLO,17 might enhance clathrin-mediated endocytosis. As previously described, sublytic concentrations of rPFN, SLO, and ionomycin activate wound healing, without necrosis, as confirmed by detecting externalization of the lumenal lysosomal marker Lamp-1, but not PI uptake (supplemental Figure 4A-C).11,27 Gigantosomes also formed after sublytic ionomycin or SLO treatment (supplemental Figure 4D), suggesting that gigantosome formation may be a general feature of the response to increased intracellular Ca2+, which activates both homotypic and heterotypic fusion of membrane-delimited vesicles.11,17 Both sublytic rPFN and SLO enhanced uptake of GzmB labeled with AlexaFluor 488 (A488-GzmB), but even the highest concentration of ionomycin did not (as previously reported11 ; Figure 4A). To determine whether rPFN- and SLO-mediated enhanced uptake of GzmB might be secondary to enhanced clathrin-mediated endocytosis, we analyzed the effect of these 3 membrane-perturbing agents on the rate of clathrin-dependent endocytosis using spinning disc confocal microscopy. The number, maximum fluorescence intensity (proportional to the size of coated vesicles), and lifetime of new clathrin-coated pits (CCPs) forming at the plasma membrane, identified as AP-2 clathrin adaptor spots,20,21 were quantified before and after adding sublytic rPFN, SLO, or ionomycin to HeLa cells stably expressing an EGFP-tagged σ2 subunit of the clathrin adaptor AP-2 complex (EGFP–AP-2σ2; Figure 4B-E; supplemental Videos 5-7). Sublytic rPFN and SLO significantly increased both the number of new plasma membrane–associated AP-2 puncta and their total intensity, whereas ionomycin (5μM) did not. Moreover, a slightly higher, but still sublytic, concentration (7μM) of ionomycin led to a rapid and dramatic loss of all membrane-associated AP-2 within approximately 2 minutes (supplemental Figure 4E), suggesting disruption of CCP formation. In addition, the majority of AP-2 spots newly formed in the presence of rPFN and SLO disappeared quickly from the visual field (lifetime ∼50-200 seconds), suggesting clathrin-coated vesicles formed normally after treatment with PFN or SLO and were transported deeper in the cytosol. The percentage increase in AP-2/clathrin-mediated endocytosis was calculated from the change in the total fluorescence intensity of new membrane-associated AP-2 puncta formed in the same cell before and after treatment. Sublytic rPFN and SLO significantly increased AP-2–mediated endocytosis by approximately 110% (± 38%) and 65% (± 7%), respectively, but the slight increase with ionomycin (∼14% ± 13%) was not significant (Figure 4E). To understand the reason for the differing effects of PFN, SLO, and ionomycin on clathrin-mediated endocytosis, we measured the Ca2+ flux induced by these 3 agents. Sublytic concentrations of all 3 led to increased intracellular Ca2+ (Figure 4F). However, the increase in intracellular Ca2+ was transient in cells treated with sublytic rPFN or SLO, but sustained and greater after 5μM ionomycin. Similar data were obtained after treatment with 1μM to 10μM ionomycin (not shown). Therefore, a transient cytosolic Ca2+ rise seems to be required to trigger enhanced endocytosis, whereas a sustained increase in intracellular Ca2+, as produced by ionomycin, may disrupt clathrin-mediated endocytosis, as has been reported.28
Perforin increases clathrin-mediated endocytosis. (A) Within 7 minutes of treatment, sublytic rPFN and SLO activate uptake of A488-GzmB, whereas ionomycin, even at the highest lytic concentration, does not. Mean fluorescence intensity (mean ± SD) from 3 independent experiments is indicated. (B) rPFN and SLO increase the rate of AP-2–dependent endocytosis, but ionomycin does not. HeLa cells stably expressing EGFP–AP-2σ2 were used for spatial and temporal analysis of AP-2 at CCPs. Cells were imaged every 10 seconds by spinning disk confocal microscopy before and after addition of sublytic rPFN, ionomycin, or SLO. The maximum fluorescence intensity and lifetime of new membrane-associated AP-2 spots were measured 400 seconds before and after treatment. Representative data from one cell treated with rPFN, ionomycin, or SLO (supplemental Videos 3-5) are shown. (C-D) Sublytic rPFN and SLO significantly increase the number of new AP-2 spots associated with the plasma membrane and the total intensity of membrane-associated AP-2 molecules, whereas ionomycin (5μM) only slightly increases membrane-associated AP-2. Data depicted were obtained from 3 different cells and 3 independent experiments. Data depicted in panel B correspond to cell no. 3 for each treatment. (E) The percentage increase of AP-2–mediated endocytosis after treatment (mean ± SD) was calculated. (F) Intracellular Ca2+ increases after sublytic rPFN, SLO, and ionomycin treatment. Calcium influx was measured by FlexStation III (Molecular Devices) in HeLa cells stained with Fura-2/AM at 5-second intervals after adding PFN, SLO, or ionomycin. At sublytic concentrations, rPFN and SLO induce a transient Ca2+ flux, whereas ionomycin induces a sustained and global rise of intracellular Ca2+. Data are representative of 3 independent experiments.
Perforin increases clathrin-mediated endocytosis. (A) Within 7 minutes of treatment, sublytic rPFN and SLO activate uptake of A488-GzmB, whereas ionomycin, even at the highest lytic concentration, does not. Mean fluorescence intensity (mean ± SD) from 3 independent experiments is indicated. (B) rPFN and SLO increase the rate of AP-2–dependent endocytosis, but ionomycin does not. HeLa cells stably expressing EGFP–AP-2σ2 were used for spatial and temporal analysis of AP-2 at CCPs. Cells were imaged every 10 seconds by spinning disk confocal microscopy before and after addition of sublytic rPFN, ionomycin, or SLO. The maximum fluorescence intensity and lifetime of new membrane-associated AP-2 spots were measured 400 seconds before and after treatment. Representative data from one cell treated with rPFN, ionomycin, or SLO (supplemental Videos 3-5) are shown. (C-D) Sublytic rPFN and SLO significantly increase the number of new AP-2 spots associated with the plasma membrane and the total intensity of membrane-associated AP-2 molecules, whereas ionomycin (5μM) only slightly increases membrane-associated AP-2. Data depicted were obtained from 3 different cells and 3 independent experiments. Data depicted in panel B correspond to cell no. 3 for each treatment. (E) The percentage increase of AP-2–mediated endocytosis after treatment (mean ± SD) was calculated. (F) Intracellular Ca2+ increases after sublytic rPFN, SLO, and ionomycin treatment. Calcium influx was measured by FlexStation III (Molecular Devices) in HeLa cells stained with Fura-2/AM at 5-second intervals after adding PFN, SLO, or ionomycin. At sublytic concentrations, rPFN and SLO induce a transient Ca2+ flux, whereas ionomycin induces a sustained and global rise of intracellular Ca2+. Data are representative of 3 independent experiments.
Inhibiting dynamin- and clathrin-mediated endocytosis increases PFN-induced necrosis
Because endocytosis removes PFN from the plasma membrane, we hypothesized that inhibiting endocytosis might increase necrotic cell death caused by persistence of PFN pores in the plasma membrane. Moreover, because PFN increases AP-2–mediated endocytosis, we hypothesized that PFN is removed from the plasma membrane by a clathrin-dependent mechanism that also relies on Dyn2, a ubiquitously expressed GTPase that plays an essential role in vesicle scission during both clathrin-mediated and clathrin-independent endocytosis.29 (The other Dyn isoform [Dyn1] is not expressed in HeLa cells.) To investigate whether inhibiting endocytosis enhances PFN-induced necrosis, PI uptake was measured 15 minutes after adding PFN to HeLa cells pretreated with inhibitors of Dyn and clathrin-dependent endocytosis. Pretreatment with hypertonic sucrose, potassium depletion (which interfere with the clathrin pathway by dispersing clathrin lattices on the plasma membrane30 ), or Dynasore (a small molecule Dyn2 inhibitor23 ) increased necrosis induced by sublytic and lytic rPFN concentrations (supplemental Figure 5A-B). Similarly, hypertonic sucrose and Dynasore increased necrosis by SLO (supplemental Figure 5C-D). However, because some of these inhibitors are not specific,31 we confirmed our data by knocking down key genes involved in Dyn/clathrin-dependent endocytosis by transfecting siRNAs to clathrin heavy chain (CHC), the μ2 subunit of AP-2 adaptor (AP-2μ2)22 or Dyn2 (supplemental Figure 5E). EGFP siRNAs were used as an irrelevant control and flotillin-1 siRNAs, as a negative control for clathrin-independent endocytosis. As expected, Tf uptake was decreased in cells knocked down for CHC, AP-2μ2, or Dyn2, but not in cells treated with flotillin-1 or GFP siRNAs (not shown). HeLa cells transfected with Dyn2, AP-2μ2, and CHC siRNAs were more prone to necrosis induced by sublytic or lytic rPFN than cells transfected with flotillin-1 or control siRNAs (Figure 5A-C). These experiments confirm that Dyn/clathrin-dependent endocytosis preserves membrane integrity, likely by removing PFN (and SLO) pores from the plasma membrane.
Inhibiting clathrin-mediated endocytosis increases PFN-induced necrosis. Inhibition of Dyn/clathrin-dependent endocytosis increases necrotic cell death after sublytic rPFN treatment. HeLa cells were transfected with siRNAs for Dyn2, AP-2μ2, and CHC or pretreated with Dynasore (80μM), an inhibitor of Dyn GTPase function, and then incubated for 15 minutes with sublytic rPFN. GFP (ctrl) or Flotillin-1 siRNAs were used as negative controls. Necrosis was evaluated immediately by flow cytometric measurement of PI uptake. Representative histograms after sublytic rPFN treatment (A), and mean ± SD from 3 independent experiments with sublytic (B) or different doses of rPFN (C) are shown. *P < .03, #P < .001, relative to control siRNA–treated cells.
Inhibiting clathrin-mediated endocytosis increases PFN-induced necrosis. Inhibition of Dyn/clathrin-dependent endocytosis increases necrotic cell death after sublytic rPFN treatment. HeLa cells were transfected with siRNAs for Dyn2, AP-2μ2, and CHC or pretreated with Dynasore (80μM), an inhibitor of Dyn GTPase function, and then incubated for 15 minutes with sublytic rPFN. GFP (ctrl) or Flotillin-1 siRNAs were used as negative controls. Necrosis was evaluated immediately by flow cytometric measurement of PI uptake. Representative histograms after sublytic rPFN treatment (A), and mean ± SD from 3 independent experiments with sublytic (B) or different doses of rPFN (C) are shown. *P < .03, #P < .001, relative to control siRNA–treated cells.
Inhibiting dynamin and clathrin decreases PFN-induced GzmB uptake
Because PFN and GzmB are endocytosed into the same EEA-1+ compartment, we hypothesized that GzmB internalization is also clathrin and Dyn2 dependent. Previous studies have come to contradictory conclusions about whether PFN-mediated endocytosis of GzmB is Dyn2 dependent.32,33 To investigate whether GzmB uptake is Dyn/clathrin dependent, we again used chemical inhibitors and siRNAs to inhibit Dyn/clathrin endocytosis. Pretreatment with hypertonic sucrose or Dynasore strongly decreased A448-GzmB internalization after sublytic rPFN treatment (Figure 6A-B). Similarly, transfection of CHC, AP-2μ2, and Dyn2, but not flotillin-1 or control, siRNAs inhibited rPFN-facilitated A488-GzmB uptake as assessed by either flow cytometry or fluorescence microscopy (Figure 6C-D). Therefore, PFN-induced uptake of GzmB requires Dyn2 and clathrin. Moreover, because inhibiting PFN-activated endocytosis increases PFN-induced necrosis probably by increasing the number of PFN pores persisting at the cell surface, but decreases GzmB uptake, it is unlikely that GzmB enters the cell by plasma membrane PFN pores.
Clathrin-mediated endocytosis is required for PFN-mediated GzmB internalization. (A-B) Inhibition of Dyn/clathrin-mediated endocytosis by pretreatment with hypertonic sucrose (300mM) or Dynasore (80μM) decreases sublytic rPFN-induced A488-GzmB internalization. (C) Cells transfected with Dyn2, AP-2μ2, or CHC siRNAs show reduced GzmB internalization, compared with control cells treated with GFP (ctrl) or Flotillin-1 siRNAs, 5 minutes after incubation with sublytic rPFN and A488-GzmB. Graphs are representative of 3 independent experiments and mean fluorescence intensity (MFI; mean ± SD) is indicated. (D) Concentration of A488-GzmB (green) in the nucleus of target cells 20 minutes after sublytic rPFN, and A488-GzmB incubation is seen in cells treated with flotillin-1 or GFP (Ctrl) siRNAs but not in cells treated with CHC siRNA. Numbers indicate the percentage of cells with nuclear GzmB (mean ± SD from 3 independent experiments). GzmB signal was detected using AlexaFluor 488–conjugated secondary antibody and EEA-1, using AlexaFluor 647–conjugated secondary antibody. Color bars and associated numbers indicate fluorescence intensity levels. Dashed lines indicate nuclei. Scale bar represents 10 μm.
Clathrin-mediated endocytosis is required for PFN-mediated GzmB internalization. (A-B) Inhibition of Dyn/clathrin-mediated endocytosis by pretreatment with hypertonic sucrose (300mM) or Dynasore (80μM) decreases sublytic rPFN-induced A488-GzmB internalization. (C) Cells transfected with Dyn2, AP-2μ2, or CHC siRNAs show reduced GzmB internalization, compared with control cells treated with GFP (ctrl) or Flotillin-1 siRNAs, 5 minutes after incubation with sublytic rPFN and A488-GzmB. Graphs are representative of 3 independent experiments and mean fluorescence intensity (MFI; mean ± SD) is indicated. (D) Concentration of A488-GzmB (green) in the nucleus of target cells 20 minutes after sublytic rPFN, and A488-GzmB incubation is seen in cells treated with flotillin-1 or GFP (Ctrl) siRNAs but not in cells treated with CHC siRNA. Numbers indicate the percentage of cells with nuclear GzmB (mean ± SD from 3 independent experiments). GzmB signal was detected using AlexaFluor 488–conjugated secondary antibody and EEA-1, using AlexaFluor 647–conjugated secondary antibody. Color bars and associated numbers indicate fluorescence intensity levels. Dashed lines indicate nuclei. Scale bar represents 10 μm.
Inhibiting dynamin- and clathrin-mediated endocytosis decreases PFN and GzmB-induced apoptosis
We previously showed that when the plasma membrane wound healing response is inhibited by chelating intracellular Ca2+, cells treated with PFN and GzmB are more likely to die by necrosis than by apoptosis.11 We suspected that a similar shift in the balance between apoptosis and necrosis would occur when endocytosis is inhibited. To test this idea, we compared caspase activation by measuring cytokeratin 18 cleavage in HeLa cells pretreated or not with hypertonic sucrose or Dynasore. Cells treated with these inhibitors were significantly less likely to die by apoptosis after rPFN and hGzmB treatment (Figure 7A-B). Similarly, HeLa cells transfected with Dyn2, AP-2μ2, or CHC siRNAs were significantly less likely to undergo apoptosis than cells transfected with control or flotillin-1 siRNAs, when apoptosis was measured either by caspase activation (Figure 7C-D) or by counting apoptotic nuclei after DAPI staining (supplemental Figure 6A). Because SLO has been extensively used to substitute for PFN to deliver GzmB to target cells, we also tested whether inhibition of Dyn- and clathrin-mediated endocytosis impairs apoptosis by SLO/GzmB. Although a previous report suggested that SLO-induced endocytosis of dextran is Dyn independent,17 we found that SLO- and GzmB-mediated apoptosis in HeLa cells was significantly decreased by hypertonic sucrose or Dynasore treatment (supplemental Figure 6B-C), suggesting that apoptosis caused by GzmB and PFN (or SLO) is indeed Dyn2 and clathrin dependent. When endocytosis was inhibited, PFN-mediated necrosis increased, whereas PFN delivery of GzmB to induce apoptosis was inhibited. To confirm these data, we next measured CTL-induced cell death by 51Cr release assay, which does not distinguish between apoptosis and necrosis, in HeLa cells transfected with Dyn2, AP-2μ2, or CHC siRNAs. The overall amount of cell death in Dyn2, AP-2μ2, or CHC siRNA-treated cells was comparable with that of control cells (Figure 7E). Therefore clathrin- and Dyn-dependent endocytosis does not affect whether a cell dies, but does influence how it dies. Taken together, these experiments strongly suggest that PFN-mediated GzmB delivery into target cells is Dyn2 and clathrin dependent and that endocytosis of PFN helps maintain membrane integrity to protect cells from necrosis and allow them to undergo the slower process of apoptosis.16
Inhibition of clathrin-mediated endocytosis reduces PFN- and GzmB-mediated apoptosis, but does not alter the extent of CTL-mediated death. (A-B) Chemical inhibitors of clathrin-mediated endocytosis decrease PFN- and GzmB-induced apoptosis. HeLa cells were preincubated with or without hypertonic sucrose or Dynasore before treatment with buffer or sublytic rPFN and/or GzmB. Apoptosis was measured 2 hours later by caspase activation by labeling with M30 monoclonal antibody. Representative flow cytometric histogram (A) and mean ± SD from 3 independent experiments (B) are depicted. **P < .005 relative to control siRNA–treated cells. (C-D) Specific inhibition of Dyn/clathrin-mediated endocytosis with siRNAs decreases PFN- and GzmB-induced apoptosis. HeLa cells were transfected with indicated siRNAs before treatment with rPFN and/or GzmB and apoptosis was measured as in panels A and B. Representative flow cytometric histogram (C) and mean ± SD from 3 independent experiments (D) are shown. **P < .005 relative to control siRNA–transfected cells. (E) Inhibition of Dyn/clathrin-mediated endocytosis does not interfere with overall CTL-induced cell death evaluated by 51Cr release assay. HeLa cells transfected with indicated siRNAs were incubated for 4 hours with specific CTLs. The data (mean ± SD) were obtained from 2 independent experiments performed in triplicate.
Inhibition of clathrin-mediated endocytosis reduces PFN- and GzmB-mediated apoptosis, but does not alter the extent of CTL-mediated death. (A-B) Chemical inhibitors of clathrin-mediated endocytosis decrease PFN- and GzmB-induced apoptosis. HeLa cells were preincubated with or without hypertonic sucrose or Dynasore before treatment with buffer or sublytic rPFN and/or GzmB. Apoptosis was measured 2 hours later by caspase activation by labeling with M30 monoclonal antibody. Representative flow cytometric histogram (A) and mean ± SD from 3 independent experiments (B) are depicted. **P < .005 relative to control siRNA–treated cells. (C-D) Specific inhibition of Dyn/clathrin-mediated endocytosis with siRNAs decreases PFN- and GzmB-induced apoptosis. HeLa cells were transfected with indicated siRNAs before treatment with rPFN and/or GzmB and apoptosis was measured as in panels A and B. Representative flow cytometric histogram (C) and mean ± SD from 3 independent experiments (D) are shown. **P < .005 relative to control siRNA–transfected cells. (E) Inhibition of Dyn/clathrin-mediated endocytosis does not interfere with overall CTL-induced cell death evaluated by 51Cr release assay. HeLa cells transfected with indicated siRNAs were incubated for 4 hours with specific CTLs. The data (mean ± SD) were obtained from 2 independent experiments performed in triplicate.
Discussion
PFN creates pores in the target cell plasma membrane, transiently allowing Ca2+ into the cell. The Ca2+ influx triggers a wounded membrane repair response, where lysosomes and endosomes fuse to the plasma membrane to reseal the damaged membrane. The wound healing response protects target cells from necrosis and allows them to undergo the slower, adenosine triphosphate–dependent process of Gzm-mediated apoptosis.11 We now show that PFN also triggers the rapid clathrin- and Dyn-dependent endocytosis of GzmB and PFN into enlarged EEA-1+ early endosomes, which we term gigantosomes. Rapid and enhanced endocytosis serves 2 important functions: (1) to remove the membrane-damaging agent PFN from the membrane and (2) to internalize PFN and Gzms—the critical first step of PFN-mediated intracellular delivery of Gzms to initiate target cell apoptosis. For the first time, we have been able to detect PFN within target cells, both in cells treated with native PFN or in cells subjected to CTL attack. PFN concentrates with GzmB into gigantosomes. Therefore we postulate a 2-step model for PFN: first, multimerization at the plasma membrane to create small pores that trigger a wound healing response that increases endocytosis of Gzms and PFN into gigantosomes, and then disruption of the gigantosome membrane to release Gzms to the cytosol. Understanding the latter step is outside the scope of this study, but our model is that PFN multimerizes in the gigantosome membrane to form larger pores that release Gzms into the cytosol. Indeed, we see Gzms in the cytosol within approximately 15 to 20 minutes of adding Gzms and PFN and then Gzms concentrate in the nucleus and mitochondria (Figure 6D, data not shown). Although some results were demonstrated with rat and some with human PFN, we have found these sources of PFN to function indistinguishably in these assays. Moreover, many of these experiments were also performed by substituting GzmA for GzmB, with comparable results (not shown). Therefore, we expect our findings to apply to PFN delivery of all Gzms.
Internalization of Gzms does not require a cell-specific receptor,32,34,35 although binding to mannose receptors may enhance uptake.36-38 This means that PFN-mediated delivery can deliver Gzms to all cells, irrespective of their cell surface protein expression. Indeed, binding and uptake of GzmB via PFN is not blocked by trypsinizing cells, but is blocked by interfering with ionic interactions.37,39 PFN indiscriminately induces capture into gigantosomes of other cationic molecules, such as cationic dextrans, bound to the plasma membrane in a receptor-independent manner by charge, as well as receptor-bound molecules, such as Tf. Although many of our results were obtained by loading PFN and GzmB into target cells, we also verified their relevance by visualizing gigantosome formation and PFN colocalization in gigantosomes in target cells within minutes of CTL attack. It should be mentioned that GzmB can be endocytosed inefficiently and slowly in the absence of PFN.13,40 However unless coendocytosed with PFN, GzmB is unable to escape from endosomes to activate apoptosis.39 By a combination of knockdown and inhibitor experiments, we showed that PFN delivery of GzmB is clathrin- and Dyn-dependent. In fact, PFN treatment increases the recruitment of the AP-2 adaptor complex to the plasma membrane, which is essential for clathrin-mediated endocytosis.
When endocytosis is inhibited in target cells treated with GzmB and PFN, the balance between necrotic and apoptotic cell death shifts: PFN-mediated necrosis increases, whereas delivery of GzmB to induce apoptosis is inhibited. Therefore, the endocytic component of the plasma membrane repair response, which removes PFN from the plasma membrane, is required to direct immune-mediated cell death by CTLs and NK cells toward apoptosis and to prevent the inflammation that occurs in response to necrotic cells.16 This finding of a Dyn-dependent alteration in the balance between necrotic and apoptotic death explains the seemingly contradictory results of other groups. Trapani et al concluded that PFN delivery of Gzms is Dyn-independent based only on cell survival assays, which measure both apoptosis and necrosis,32 whereas Bleackley's group found that GzmB- and PFN-induced apoptosis, measured by caspase activation, is Dyn-dependent.33 Our findings that overall cell death is not altered by inhibiting Dyn2, but that apoptosis induction requires Dyn2, are consistent with both studies and explain how these seemingly inconsistent results both can hold.
Rapid endocytosis to remove membrane-damaging agents may be a general feature of the plasma-membrane repair response, because it is also seen with SLO, as previously reported17 and confirmed here. We also confirmed that SLO enhances clathrin-mediated endocytosis, as previously reported.17 However, unlike the previous report, we found that SLO-triggered endocytosis is also Dyn-dependent, based on experiments using a Dyn inhibitor and siRNAs. The Idone et al study17 based their conclusion that SLO-mediated endocytosis is Dyn-independent on experiments using cells expressing K44A Dyn2 and cholesterol extraction with methyl-β-cyclodextrin (MβCD) to conclude that SLO-mediated internalization depends on endocytic pathways specific for cholesterol-rich membrane domains. However, MβCD treatment can also perturb formation of clathrin-coated vesicles.31 Moreover, Dyn is also used for vesicle scission in clathrin-independent pathways.
Although both PFN and SLO activate the damaged plasma membrane repair response,11,41 increase clathrin-mediated endocytosis, and induce GzmB uptake into endosomes, ionomycin, a Ca2+ ionophore that triggers the first feature of cellular wound healing (fusion of lysosomes with the plasma membrane), does not induce GzmB endocytosis. A salient difference between ionomycin and SLO or PFN is that the rise in intracellular Ca2+ by ionomycin is higher and persists, whereas for SLO and PFN it is transient. A recent study showed that the plasma membrane repair response to SLO operates only when intracellular Ca2+ is in the range of 5μM to 10μM,42 suggesting that high intracellular Ca2+ after ionomycin treatment may prevent plasma membrane repair, especially endocytosis. Ionomycin also differs from the other agents by its mechanism of action. The Ca2+ flux induced by ionomycin originates from both mobilization of intracellular stores and an influx of extracellular Ca2+.43 Because ionomycin depletes Ca2+ from mitochondria and disrupts the mitochondrial transmembrane potential and adenosine triphosphate generation, it may deplete the cell of the energy needed for endocytosis.44 Ionomycin is known to lead to a dramatic loss of clathrin puncta and dissociation of endocytic adaptors from the plasma membrane.28 In fact, sublytic concentrations of ionomycin actually inhibit clathrin-mediated endocytosis.45
In our previous study, when we blocked vesicular exocytosis in the membrane repair response by chelating intracellular Ca2+, we also blocked the endocytic part of the response.11 Here, we selectively blocked endocytosis using chemical inhibitors and siRNAs directed against key endocytic molecules and showed that endocytosis activated during membrane repair is critical for avoiding necrosis and permitting PFN to deliver GzmB to activate apoptotic death. Finding a way to block vesicular exocytosis selectively would enable researchers to evaluate whether endocytosis is activated to remove the extra plasma membrane added during vesicular membrane fusion and to assess the relative contributions of these 2 repair processes in preventing necrosis during immune cell–mediated death. Because CTL degranulation and PFN membrane binding and multimerization are all Ca2+-dependent processes, the simplest maneuvers of chelating Ca2+, either within cells or in the extracellular fluid, have multiple effects on PFN function. Therefore, dissecting the contributions of different components of membrane repair caused by pore-forming proteins may be more straightforward by studying Ca2+-independent pore-forming proteins, such as SLO, rather than PFN.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Eric Marino for assistance with microscopy and Sonia Sharma for experimental assistance.
This work was supported by National Institutes of Health grant AI063430 (J.L.) and fellowships from the Swiss National Science Foundation (M.W.) and the Human Frontier Science Program Organization (E.B.).
National Institutes of Health
Authorship
Contribution: J.T. designed and performed experiments, analyzed data, and wrote the paper; D.K., S.S., D.M., M.W., and E.B. performed and helped analyze some experiments; and T.K. and J.L. conceived and supervised the project, helped design experiments, and coordinated the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Judy Lieberman, Immune Disease Institute, Harvard Medical School, Warren Alpert Bldg, Rm 255, 200 Longwood Ave, Boston, MA 02115; e-mail: lieberman@idi.harvard.edu.