Abstract
Mast cells (MCs) are currently receiving increased attention among the scientific community, largely because of the recent identification of crucial functions for MCs in a variety of disorders. However, it is in many cases not clear exactly how MCs contribute in the respective settings. MCs express extraordinarily high levels of a number of proteases of chymase, tryptase, and carboxypeptidase A type, and these are stored in high amounts as active enzymes in the MC secretory granules. Hence, MC degranulation leads to the massive release of fully active MC proteases, which probably have a major impact on any condition in which MC degranulation occurs. Indeed, the recent generation and evaluation of mouse strains lacking individual MC proteases have indicated crucial contributions of these to a number of different disorders. MC proteases may thus account for many of the effects ascribed to MCs and are currently emerging as promising candidates for treatment of MC-driven disease. In this review, we discuss these findings.
Introduction
Mast cells (MCs) are still mainly known for their harmful effects in connection with allergic reactions. However, this simplistic view is currently being extensively modified, and it is becoming more and more established that MCs have a complex array of functions and can contribute to a number of additional disorders.1 In addition to being detrimental, MCs also carry out a number of beneficial functions, most notably in connection with innate immune responses toward various pathogens.2 Moreover, recent studies indicate that MCs can play important roles in down-regulating adaptive immune responses.3,4 As a consequence of this progress, MCs are gaining a massively increased interest worldwide, and much effort is invested into investigations of the mechanisms by which MCs contribute to different disorders.
A striking morphologic feature of MCs is their abundance of electron-dense secretory granules, which contain large amounts of preformed compounds commonly referred to as “MC mediators.” These include biogenic amines (histamine and serotonin), certain preformed cytokines (most notably tumor necrosis factor), serglycin proteoglycans, various lysosomal enzymes, and a number of MC-specific proteases of chymase, tryptase, or carboxypeptidase A (MC-CPA) type.5-8 When MCs are induced to undergo degranulation, for example, through engagement of FcϵRI-bound IgE by polyvalent antigen, these mediators are thus released.9
The MC proteases are expressed at exceptionally high levels, with mRNA levels approaching and even exceeding those of classic housekeeping genes,10 and they are stored in remarkably high amounts. Indeed, it has been calculated that MC proteases may account for more than 25% of the total MC protein.11,12 Importantly, in contrast to, for example, the pancreatic digestive proteases, the MC proteases are stored in fully active form; and when MCs undergo degranulation, large amounts of enzymatically active proteases are thus released into the extracellular space and probably have a profound impact on any condition in which MC degranulation occurs (Figure 1). Indeed, a plethora of potential functions of the MC proteases have previously been outlined, based on various approaches.6 However, it is not until relatively recently that the in vivo functions of these enzymes have started to become unraveled through experimental approaches involving MC protease-deficient mice. In this review, we summarize these findings and discuss their implications.
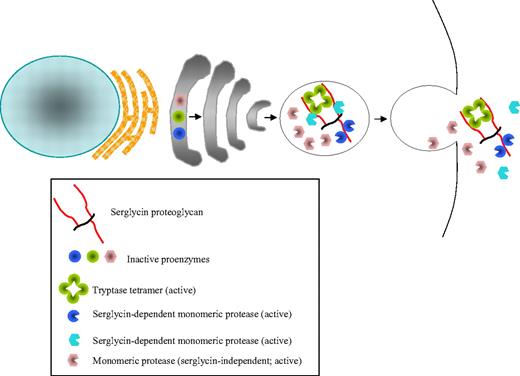
Storage and release of MC proteases. Mast cell (MC) proteases are synthesized as preproenzymes where both the signal (pre-) and activation (pro-) peptides are cleaved off intracellularly, a process in which dipeptidyl peptidase I/cathepsin C has a key role.13 Hence, enzymatically active proteases are stored in the MC granule. Major MC tryptases are tetrameric enzymes, whereas chymases and mast cell carboxypeptidase A (MC-CPA) are monomeric. Notably, for many MC proteases, the storage is strongly dependent on electrostatic interaction with serglycin proteoglycan, whereas others are stored independently of serglycin. After MC degranulation, MC proteases are released in complexes with serglycin. At exposure to extracellular pH, MC proteases may either be dissociated from serglycin or remain associated.
Storage and release of MC proteases. Mast cell (MC) proteases are synthesized as preproenzymes where both the signal (pre-) and activation (pro-) peptides are cleaved off intracellularly, a process in which dipeptidyl peptidase I/cathepsin C has a key role.13 Hence, enzymatically active proteases are stored in the MC granule. Major MC tryptases are tetrameric enzymes, whereas chymases and mast cell carboxypeptidase A (MC-CPA) are monomeric. Notably, for many MC proteases, the storage is strongly dependent on electrostatic interaction with serglycin proteoglycan, whereas others are stored independently of serglycin. After MC degranulation, MC proteases are released in complexes with serglycin. At exposure to extracellular pH, MC proteases may either be dissociated from serglycin or remain associated.
Substrate specificity, distribution, and storage
The term “MC proteases” usually refers to the proteases that are expressed specifically by MCs and are stored in their secretory granules, that is, chymases, tryptases, and MC-CPA. However, it should be pointed out that MCs express a number of additional, non–MC-specific, proteases, such as lysosomal cathepsins, granzymes, neurolysin, and, possibly, cathepsin G.6 Recently, the strictly MC-specific expression of the chymases has been used to generate mouse strains in which the Cre recombinase is expressed under the control of chymase promoters, strains that can be used to specifically inactivate genes of interest in MCs.14,15
Of the 3 classes of MC proteases, the tryptases and chymases are serine proteases, whereas MC-CPA is a Zn-dependent metalloprotease.6,16 Tryptases are denoted so because of their trypsin-like substrate specificity; that is, they cleave preferentially after Lys/Arg residues (Table 1). A unique feature of the secreted MC tryptases is that they are tetrameric, with all of the active sites facing a narrow central pore17 (Figure 1). Accordingly, their active sites are largely inaccessible for macromolecular protease inhibitors, and tryptase therefore resists inhibition by any of the proteinaceous protease inhibitors found in mammals. Further, this also limits the access to potential substrates.17 In contrast to the tryptases, chymases are monomeric and have chymotrypsin-like specificities; that is, they cleave preferentially after aromatic amino acid residues. MC-CPA is a monomeric exopeptidase, cleaving amino acid residues from the C-terminal end of peptides/proteins.16
The exact repertoire of expressed MC proteases varies among species and also among different MC subsets6-8 (Table 2). In humans, MCs are classified according to their protease content, with the MCT subclass expressing tryptase (α and β) only and the MCTC subclass expressing all types of MC proteases, that is, tryptase, chymase, and MC-CPA (Table 2). In mouse, MCs are divided into the connective tissue MC (CTMC) and mucosal MC (MMC) subtypes. CTMCs express predominantly 2 different chymases (the β-chymase mouse mast cell protease 4 [mMCP-4] and the α-chymase mMCP-5), whereas MMCs express predominantly 2 types of β-chymases (mMCP-1 and mMCP-2). CTMCs also express 2 tetrameric tryptases (mMCP-6 and mMCP-7) as well as MC-CPA (Table 2).
The highly efficient storage of the MC proteases is made possible through their tight packaging in complexes with serglycin proteoglycan, the latter consisting of a small protein core to which highly negatively charged glycosaminoglycans of chondroitin sulfate or heparin type are attached19 (Figure 1). Notably, the knockout of the serglycin gene resulted in an almost complete inability of MCs to store several MC proteases.20 Most probably, the storage-promoting function of serglycin is explained by the high extent of sulfation of its glycosaminoglycan components, with the sulfate groups engaging in electrostatic interactions with corresponding basic regions of the MC proteases. In support of this notion, targeting of N-deacetylase/N-sulfotransferase 2, an enzyme that is essential for the sulfation of heparin, caused similar storage defects as those imposed by serglycin deficiency.21,22 However, it should be pointed out that not all of the MC proteases depend on serglycin for storage: whereas mMCP-4, -5, -6, and MC-CPA20 are strictly serglycin-dependent, mMCP-2 storage is only partially dependent, and mMCP-1 and -7 storage is unaffected by the absence of serglycin.23 The most probable reason for the differential serglycin dependence of the various MC proteases lies within their exposure of surface basic charge. For example, mMCP-1 has a considerably lower net positive charge than mMCP-4 and -5, well explaining why mMCP-4 and -5 are strongly dependent on serglycin for storage whereas mMCP-1 is not.
Tryptase function
Human MCs express 2 main, enzymatically active tryptases that are secreted as tetramers during MC degranulation: α- and β-tryptases (β I- III). The murine tryptases (mMCP-6 and mMCP-7) are also tetrameric and most probably constitute the counterparts to human α- and β-tryptase. In addition, both human and mouse MCs express a monomeric, membrane-bound tryptase as well as a number of other trypsin-like serine proteases.6-8 To study the biologic function of MC tryptase, several different approaches have been used, including instillation of purified or recombinant tryptases and assessment of tryptase inhibitors in animal models for disease.6-8 Recently, a more precise evaluation of the biologic function of MC tryptase has been made possible through the generation of mouse strains deficient in mMCP-6, the murine tryptase that may constitute the closest homolog to human β-tryptase.24,25 In the mMCP-6−/− strain generated by Thakurdas et al,25 the targeting of the mMCP-6 gene also resulted in inactivation of mMCP-7 expression and, hence, this strain is double deficient in mMCP-6 and -7. In contrast, the mMCP-6−/− strain reported by Shin et al retained mMCP-7 expression.24 As a tool for studying mMCP-7 function, several mouse strains, including the C57BL/6 strain, are known to lack the expression of mMCP-7 because of spontaneous mutations at various positions of the mMCP-7 gene.26
Parasite infection
MCs are widely recognized as effector cells involved in clearance of diverse parasites, including Trichinella spiralis,27 although the precise effector mechanisms are poorly understood. Using the mMCP-6–deficient (but mMCP-7–sufficient) strain, Shin et al showed that MC tryptase contributes to the immune response toward T spiralis,24 and it was demonstrated that mMCP-6 had a role in eosinophil recruitment into infected skeletal muscle. Notably, the absence of mMCP-6 did not adversely affect the rate of parasite clearance, and there was no effect of mMCP-6 deficiency on the IgE response toward T spiralis. Thus, mMCP-6 contributes to the innate immune response toward T spiralis, although not being essential for combating infection.
Bacterial infection
A number of studies have shown that MCs are of primary importance in the defense toward bacterial infection,2 but the mechanisms by which MCs exert this function have not been completely clarified. Given the ability of mMCP-6 to recruit neutrophils,28,29 it may be a candidate for mediating MC-dependent effects during bacterial infection. Indeed, Thakurdas et al reported that mMCP-6 contributes to the defense toward intraperitoneal Klebsiella pneumoniae infection.25 mMCP-6 null animals displayed a significantly reduced survival rate, and it was also shown that the neutrophil recruitment was dependent on mMCP-6. Importantly, the defective neutrophil recruitment was only seen in the early phases of infection, in line with a primary role of MCs in the first-line innate defense toward bacterial insults.
Arthritis
In a hallmark study, Lee et al reported that MC-deficient mice are resistant to arthritis induced by administration of arthritogenic serum from K/BxN mice,30 although it should be emphasized that a role for MCs in experimentally induced arthritis has been questioned.31 A role for MCs in arthritis is also supported by clinical observations.32 In 2 recent studies, the possibility that tryptase could account for the MC-dependent contribution to arthritis has been investigated. In one of these, arthritis was passively induced using methyl-bovine serum albumin/interleukin-1β (IL-1β).33 The results indicated that, indeed, mMCP-6−/− mice developed less severe joint inflammation than did corresponding wild-type (WT) mice. Interestingly, mMCP-7 was shown to compensate for an absence of mMCP-6 and vice versa, indicating that mMCP-6 and -7 show a redundancy in this model.33
In a more recent study, the same authors assessed the mMCP-6–deficient mouse strains in the K/BxN model for arthritis. It was shown that mMCP-6−/− animals developed lower clinical scores, inflammation, and bone/cartilage erosion than did WT mice.34 Notably, the clinical scores and inflammatory parameters were affected to a relatively moderate extent compared with the effects seen in globally MC-deficient mice, suggesting that other MC-mediated mechanisms contribute to disease.
Together, these studies have clearly implicated MC tryptase in the pathology of arthritis. However, it should be pointed out that both of the models used involve passive induction of disease, whereas the involvement of tryptase in actively induced disease, for example, by immunization with collagen II, remains to be investigated.
Chymase function
To date, knockout strains deficient in mMCP-1, mMCP-4, and mMCP-5 have been generated (Table 3). Importantly, though, because only one MC chymase gene is expressed in humans (CMA1), it is critical to ascertain which of the murine chymases that is the closest homolog of human chymase to extrapolate functions into the human situation. Based on sequence similarity, mMCP-5 is the closest homolog to human chymase. However, an examination of the cleavage specificity of mMCP-5 revealed that it has elastase- rather than chymotrypsin-like substrate specificity.48 Thus, mMCP-5 cannot be considered as a functional homolog to human chymase. mMCP-1 has a tissue distribution different from that of human chymase, has markedly lower affinity for serglycin than does human chymase, and its substrate cleavage specificity is slightly different from that of human chymase.49 In addition, mMCP-2 has a tissue location that is different from that of human chymase; moreover, mMCP-2 has been shown to be virtually without enzymatic activity.50 mMCP-4, on the other hand, has a substrate specificity highly similar to that of human chymase51,52 ; in addition, mMCP-4 and human chymase have similar tissue distribution and serglycin-binding properties. It has also been demonstrated that mMCP-4 accounts for essentially all stored chymotrypsin-like activity in skin and peritoneum.38 Taken together, these facts indicate that, of the murine chymases, mMCP-4 could be considered as the closest functional homolog to human chymase. Therefore, the mMCP-4–deficient strain may be a relevant tool for studies aimed at clarifying the function of human chymase. However, it cannot be excluded that some of the corresponding functions of chymase in humans are executed by murine chymases other than mMCP-4 and that all of the murine chymases thus contribute to the total impact of chymase function. Therefore, to provide a comprehensive insight into the biologic function of MC chymase based on murine studies, it is important to discuss the function of additional murine chymases, that is, also mMCP-1, -2, and -5.
Parasite infection
In the first report of the mMCP-1 knockout, it was shown that the absence of mMCP-1 affected the kinetics of MMC recruitment after Nippostrongylus brasiliensis infection,53 and follow-up studies demonstrated that mMCP-1−/− mice display defective clearance of T spiralis,42 with the latter effect being accompanied by reduced enteropathy and cytokine (tumor necrosis factor) responses.43 These results are thus in concordance with previous studies showing that mice with global MC deficiency are more susceptible to T spiralis infection than are WT mice,54 and suggest that chymase partly accounts for the MC-dependent protection toward T spiralis infection. Although the underlying mechanism has not been fully established, it has been suggested that mMCP-1 may promote inflammation by cleaving cell-cell contact proteins, such as occludin and ZO-1.44,55,56 In line with such a notion, infusion of rats with rMCP-2, that is, a rat homolog of mMCP-1, causes increased epithelial permeability.57
Bacterial infection
Interesting insight into the function of chymase in bacterial disease came through a study where the role of interleukin-15 (IL-15) in regulating sepsis was studied.58 The authors reported that IL-15−/− mice showed a markedly higher survival rate in sepsis induced by cecal ligation and puncture compared with WT counterparts and that this effect was largely the result of IL-15 expressed in MCs. It was found that a main effect of IL-15 in MCs was to suppress the expression of mMCP-2, but not of other chymases58 ; collectively, these results suggest a role for chymase in regulating bacterial infection.
Arthritis
Because MCs have been implicated in rheumatoid arthritis,30,33 it was of interest to evaluate the contribution of chymase. Indeed, using a model where arthritis is induced actively by immunization with collagen II, mMCP-4−/− mice showed a reduced clinical score and fewer signs of tissue damage compared with WT controls. Moreover, the absence of mMCP-4 resulted in lower levels of anticollagen II IgG in plasma, suggesting that mMCP-4 indeed may contribute to the sensitization step.35 In line with these findings, previous in vitro–based experiments have suggested that purified chymase can enhance the production of IgE and IgG,59 and epidemiologic studies have suggested a connection between a chymase promoter polymorphism and IgE levels in atopic dermatitis.60
AAA
Animals lacking MCs altogether (C57BL/6-KitW-sh/W-sh) are largely protected in a model for abdominal aortic aneurysm (AAA) formation61 ; and when assessing the mMCP-4−/− mice in this model, the degree of AAA formation was markedly lower in the mMCP-4 null compared with WT mice.36 Strikingly, the extent of protection resulting from mMCP-4 deficiency was similar to that seen in C57BL/6-KitW-sh/W-sh mice, indicating that mMCP-4 is the main effector molecule in MC-mediated promotion of AAA formation.36 A dissection of the underlying mechanisms showed that mMCP-4 contributed to the inflammatory reaction seen in AAA and also that mMCP-4 has a role in inducing apoptosis, angiogenesis, and elastase degradation.36
Allergic airway inflammation
Because MCs are strongly implicated in asthma and in animal models of allergic airway inflammation, it may be expected that chymase, if anything, may have a pathogenic role. However, in a recent study, it was shown that mMCP-4 indeed protects toward extensive allergic airway inflammation in an ovalbumin-based model and that it protects toward bronchial hyper-reactivity.37 Evidence was also presented suggesting that mMCP-4 may exert this activity by limiting the thickening of the airway smooth muscle cell layer that accompanies allergic airway responses. These data thus indicate that, although the MC as such contributes to the pathology of allergic airway inflammation, one of the individual granule compounds may actually have an activity that counteracts the overall harmful impact of the MCs. Notably, a protective function for MC chymase in allergic airway responses is also supported by clinical observations in which chymase presence has been linked to preserved lung function in asthmatics.62
Ischemia reperfusion injury
It has been reported that mice lacking mMCP-5 show reduced ischemia reperfusion injury in skeletal muscle.41 Noteworthy, because the mMCP-5−/− animals also lack MC-CPA at the protein level,63 it cannot be ruled out that MC-CPA accounted at least partially for the effects reported. To fully evaluate the individual impact of mMCP-5, it would therefore be valuable to generate a mouse strain in which the active site of mMCP-5 has been mutated, a strategy that presumably would not compromise the storage of MC-CPA.
Tissue homeostasis
In the initial report of the mMCP-4 knockout, evidence was presented suggesting that the lack of chymase causes impaired fibronectin turnover38 ; and in a subsequent study, it was shown that mMCP-4−/− mice show an excessive accumulation of fibronectin in various tissues.39 Intriguingly, these findings were obtained using naive animals, suggesting that MC chymase can contribute to normal tissue homeostasis under conditions not associated with extensive MC degranulation. In line with a homeostatic function of chymase, it was recently shown that the absence of mMCP-4 also leads to a reduced basal intestinal permeability,40 an effect that at least partly could be explained by effects of mMCP-4 on epithelial cell migration.
MC-CPA function
In all investigated species, only one MC-CPA gene has been identified (Table 1). The tissue location and other properties of human and murine MC-CPA are similar.16 Hence, the interspecies variation that applies for the tryptase and chymase families is less of an issue for MC-CPA, and it is therefore probable that functions identified for murine MC-CPA are mimicked by corresponding functions of the human counterpart.
Two different mouse strains with impaired MC-CPA expression have been reported.45,47 In the original report, a knockout for MC-CPA was generated.47 An important finding was that the absence of MC-CPA resulted in a secondary loss of mMCP-5 protein. This finding, together with the secondary lack of MC-CPA protein in mMCP-5−/− MCs (see “Chymase function”) suggests that mMCP-5 and MC-CPA storage is strongly interdependent. Another finding was that the absence of MC-CPA resulted in altered granule-staining properties, the latter most probably being explained by effects on the storage of serglycin proteoglycan.47 Thus, it appears that MC-CPA has an important role in maintaining normal secretory granule homeostasis, apparently by imposing secondary effects on other compounds stored within the granule. However, because of these secondary effects, it is difficult to discriminate between those mediated by MC-CPA as opposed to those mediated by compounds that are dependent on MC-CPA for their proper storage. To circumvent this issue, a strain with a mutated MC-CPA active site (MC-CPA knock-in) has also been generated.45 This strain does not exhibit any secondary effects on mMCP-5 storage and is therefore a more relevant tool for studies of MC-CPA function.
Using the MC-CPA–deficient strains and using an shRNA-based approach, it has been shown that MC-CPA plays an important role in degradation of certain toxic peptides in vivo. It had previously been shown that MCs are of central importance for clearance of endothelin 1, an endogenous peptide released during bacterial infection, and that MC-dependent endothelin 1 degradation prevents its toxic effects.64 Using the MC-CPA knock-in strain, it was shown that MC-CPA was responsible for this effect.45 This indicates that MC-CPA may have a role in regulating sepsis by preventing excessive accumulation of toxic peptides. It has also been shown, first using an shRNA-based approach46 and subsequently using the MC-CPA knock-in strain,45 that MC-CPA is of central importance for degrading certain snake venom toxins, in this way preventing their lethal effects. Together, these findings suggest an important function for MC-CPA in regulating innate immunologic reactions toward external insults.
Mechanism of MC protease action
Although recent research efforts have implicated the MC proteases in various novel settings, there is still limited knowledge of their true in vivo substrates. Important insight into this critical issue could potentially be obtained by determining their exact cleavage specificities, using unbiased approaches, such as peptide phage display-based techniques.49,51,52,65-67 Sequences that are preferentially cleaved by a given protease can then be used to identify matching sequences in known proteins, inferring that the protein is a preferred substrate for the respective MC protease. However, the MC proteases are relatively promiscuous in terms of cleavage specificity, except for the stringent requirements at the amino acid residue N-terminal of the cleavage site (the P1 position), and it is therefore apparent that each of the MC proteases has the capacity to cleave a multitude of substrates and even can cleave the same substrate at multiple positions. Hence, this type of strategy has only provided partial insight into the identities of their in vivo substrates. To resolve this critical issue, it will therefore be necessary to use alternative strategies, for example, the use of proteomics to identify differentially processed proteins in WT versus MC protease-deficient animals.
Given the broad cleavage specificity of the MC proteases, it is highly probable that a single MC protease may cleave a number of different substrates during one isolated condition, depending on the available repertoires of potential substrates. Moreover, there is also the likelihood that the preferred substrate(s) will vary during different phases of a given pathologic condition. For example, during the early stages of an inflammatory reaction, proteins expressed by resident tissue cells may be preferentially cleaved; whereas, in a later stage of the process, compounds secreted by extravasated inflammatory cells may be the preferred substrates (Figure 2). Clearly, a firm identification of the in vivo substrates for the MC proteases will therefore be a substantial challenge.
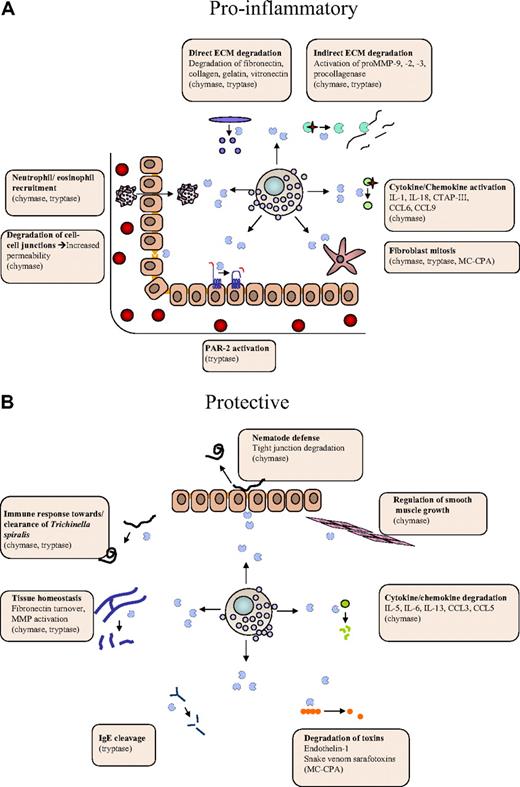
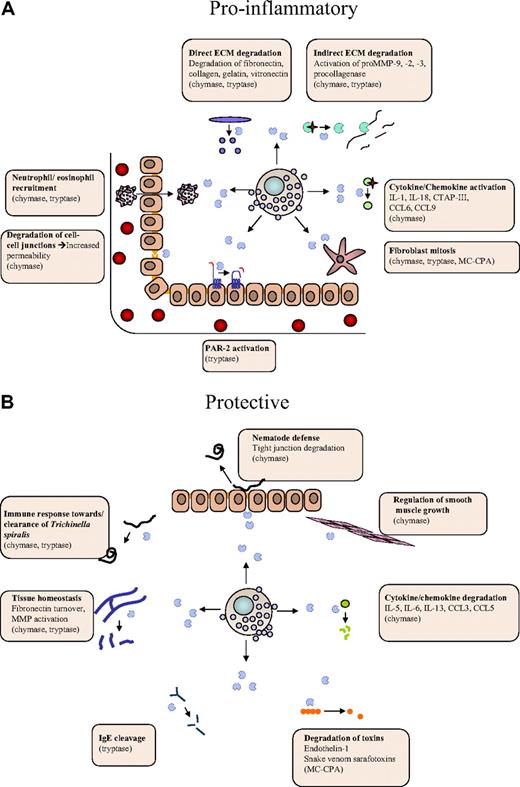
Dual roles of MC proteases in the regulation of inflammatory processes. MC proteases may affect inflammation at different levels, either by promoting inflammatory processes (A) or by offering protection (B). (A) Proinflammatory activities include (1) activating cleavages of proinflammatory cytokines/chemokines, (2) degradation of endothelial cell-cell contacts, (3) activation of extracellular matrix (ECM)–degrading enzymes, (4) recruitment of eosinophils/neutrophils, and (5) effects on gene expression (eg, via cleavage of protease-activated receptor 2). (B) Protective activities include (1) degradation of proinflammatory cytokines/chemokines, (2) degradation of toxic peptides, and (3) suppression of smooth muscle cell expansion.
Dual roles of MC proteases in the regulation of inflammatory processes. MC proteases may affect inflammation at different levels, either by promoting inflammatory processes (A) or by offering protection (B). (A) Proinflammatory activities include (1) activating cleavages of proinflammatory cytokines/chemokines, (2) degradation of endothelial cell-cell contacts, (3) activation of extracellular matrix (ECM)–degrading enzymes, (4) recruitment of eosinophils/neutrophils, and (5) effects on gene expression (eg, via cleavage of protease-activated receptor 2). (B) Protective activities include (1) degradation of proinflammatory cytokines/chemokines, (2) degradation of toxic peptides, and (3) suppression of smooth muscle cell expansion.
So far, efforts aimed at clarifying the function of MC proteases have predominantly focused on the contribution of individual MC proteases, without evaluating the possibility that the different MC proteases may have cooperative activities. For example, it cannot be excluded that different MC proteases may act on the same substrate but cleave it at distinct sites, potentially leading to a more efficient cleavage/degradation and enhanced biologic impact. To address this issue, it will therefore be important to evaluate mouse strains deficient in multiple MC proteases.
Activation/inactivation of inflammatory compounds
Previous studies have identified a wide array of potential substrates for the various MC proteases, mostly derived from in vitro–based strategies, but also through in vivo experimentation. A survey of identified MC substrates reveals that they fall into distinct categories. Interestingly, a large number of them are proinflammatory compounds; and in many cases, MC protease–catalyzed cleavages are activating, as exemplified by the cleavage of proIL-1β,68 proIL-18,69 CTAP-III,70 CCL6, CCL9, CCL15, and CCL2371 by chymase (Figure 2A). In other cases, MC protease–catalyzed cleavage leads to degradation and destroyed activity, as exemplified by the degradation of CCL3, CCL5,71 IL-6, and IL-1372 by chymase, the degradation of IgE by tryptase,73 and the degradation of endothelin-1 by MC-CPA45 (Figure 2B). Hence, MC proteases may have the ability both to promote and dampen an inflammatory reaction, depending on the availability of cleavable substrates during the given condition. Clearly, because MC proteases are mainly implicated as proinflammatory agents, activating cleavages may be a dominating function. However, as discussed in “Tryptase function” and “Chymase function,” MC proteases have in some cases been shown to have protective/anti-inflammatory functions, and we may thus envisage that such activities can be attributed to degradation of proinflammatory substances.
Effects on ECM
Another plausible scenario is that MC proteases affect the inflammatory process by modifying the extracellular matrix (ECM), either by direct effects on ECM components or indirectly by regulating the activities of ECM-processing enzymes (Figure 2). Direct effects on the ECM include tryptase-catalyzed degradation of type VI collagen,74 denatured collagen (gelatin),75 as well as chymase-catalyzed degradation of vitronectin76 and procollagen.77 Moreover, both tryptase and chymase have been shown to degrade fibronectin38,78 ; and importantly, there is even in vivo evidence supporting that fibronectin is a chymase substrate.39 One notable example of indirect effects on the ECM is the ability of chymase to convert pro-matrix metalloprotease 9 (proMMP-9) into active form, a notion that is supported both by in vitro79 and in vivo39 evidence. It has also been shown that MC proteases can activate yet other MMPs.80-82 Potentially, MC protease–mediated ECM modification could lead to a more efficient influx of inflammatory cells into the tissue and to effects on cellular adhesion to the ECM. It is also noteworthy that degradation of the ECM can unleash proinflammatory cleavage products, such as fibronectin fragments or chemokines/growth factor bound to proteoglycans present in the ECM. Finally, MC proteases may indirectly promote ECM accumulation by acting as mitogens for fibroblasts83,84 (Figure 2).
Effects on cell surface proteins
Another mode by which MC proteases could affect inflammation is to enhance capillary permeability by degrading cell-cell contact components.44,55,56 Further, MC proteases may regulate the local gene expression pattern, as exemplified by the ability of tryptase to induce chemokine secretion.34 It is also well known that tryptase can cleave and thereby activate protease-activated receptor 2,85-87 a notion that is also supported by in vivo evidence.88
Therapeutic potential of MC protease inhibitors
An obvious extension of the findings reviewed here is the possible use of MC protease inhibitors as therapeutic agents. Generally, proteases are attractive targets for inhibition, considering their defined active sites and the relative ease to design low molecular weight, synthetic inhibitors with high selectivity.89 Because the MC proteases are cell-type specific, their inhibition will only affect activities mediated by MCs, with a low risk of side effects because of interference with actions of other cell types. This is in contrast to many other proteases being evaluated as potential drug targets, for example, the MMPs, which are expressed by many cell types. Moreover because the MC proteases under normal/unprovoked conditions are sequestered within the MC granule, MC protease inhibitors may be designed to selectively target MC proteases at the site of a pathologic condition, that is, where MC degranulation has occurred.
Tryptase inhibitors
During last decade, much effort has been invested into the development of MC protease inhibitors. After the implication of tryptase in allergic asthma,90-92 tryptase inhibitors were evaluated in models of asthma and were proved to be effective,93 although a subsequent clinical trial was only moderately successful.94 However, it should be noted that the first-generation tryptase inhibitor used in the clinical trial was rather unselective, of low potency, and extremely slow-acting.95 Hence, the failure of the clinical trial may have been related to these circumstances, and it cannot be excluded that a more favorable outcome may have been reached using the much more efficient and selective tryptase inhibitors that are available today.96-98 Based on the recent findings described in this article, we may also foresee that tryptase inhibitors may be effective for treatment of arthritis. Indeed, because chymase also appears to have a pathogenic role in arthritis, a combination of tryptase and chymase inhibitors may constitute an attractive, future therapeutic regimen.
Chymase inhibitors
A large number of chymase inhibitors, with various potency and selectivity, have recently been developed6,99 and assessed for ability to ameliorate experimentally induced disease. In particular, chymase inhibitors have been assessed in various models for cardiovascular disease, with the background being the strong implication for chymase in angiotensin I conversion in vivo.100 Indeed, chymase inhibitors have in many cases proved to be effective. For example, it has been shown that chymase inhibitors reduce AAA lesions.101 This finding, along with the smaller degree of AAA formation in mMCP-4−/− mice,36 clearly indicates that chymase inhibition may be effective in prevention of aneurysms. Chymase inhibitors are also effective in models for myocardial infarction,102,103 intimal hyperplasia after balloon injury,104 Big endothelin-1 conversion,105 and ventricular remodeling induced by intermittent hypoxia.106 In addition, chymase inhibitors have anti-inflammatory functions, for example, by reducing the eosinophilia during N brasiliensis infection107 and by preventing eosinophil accumulation in a model for atopic dermatitis.108 Together, this development strongly implicates chymase as a promising therapeutic target in variety of clinical settings, and we anticipate that this notion will be evaluated through clinical trials in the near future. However, it is important to use MC protease inhibitors with caution because they may interfere with beneficial functions of MC proteases, for example, in connection with bacterial and parasite infection.
MC proteases as biomarkers
Currently, MC tryptase is frequently used as biomarker both for systemic anaphylaxis and mastocytosis,109 and it has also been suggested that MC-CPA can serve as a biomarker for anaphylaxis.110 Based on the recent implication of MC proteases in additional types of diseases, we anticipate that MC proteases may be evaluated as biomarkers in number of novel settings. For example, it was recently shown that plasma levels of chymase correlated with AAA growth rate, implicating chymase as a potential biomarker for cardiovascular disease.36
In conclusion, as described in this review article, it is now becoming clear that the MC proteases may account for many of the detrimental and beneficial activities that have been attributed to MCs. However, many important questions remain to be answered. For example, there is still limited knowledge concerning the downstream effects of MC protease action: that is, which substrates are cleaved by the MC proteases in vivo and how do MC protease-catalyzed cleavages of these substrates lead to (or protect from) disease? Certainly, a major future direction will therefore be to address these issues. Another major direction will be to further evaluate the clinical applications of these findings, that is, whether MC proteases can be used as targets for therapy. Clearly, we anticipate that the near future will reveal an even more detailed picture of how the MC proteases contribute to inflammatory disease. We think that this knowledge will enhance our understanding of the inflammatory process and lead to novel therapeutic regimens for inflammatory disease.
Acknowledgments
This work was supported by the Swedish Research Council, Formas, the Vårdal Foundation, King Gustaf V's 80-year Anniversary Fund, the Swedish Society of Medicine, Åke Wiberg Foundation, Torsten and Ragnar Söderberg Foundation, and the Swedish Cancer Foundation.
Authorship
Contribution: G.P. conceived the article and wrote it; E.R. and I.W. contributed to the writing and designed figures; and S.W. contributed to the writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gunnar Pejler, Swedish University of Agricultural Sciences, Department of Anatomy, Physiology and Biochemistry, BMC, Box 575, 75123 Uppsala, Sweden; e-mail: Gunnar.Pejler@afb.slu.se.
References
Author notes
E.R. and I.W. contributed equally to this study.