Abstract
Approximately 280 000 children are born with sickle cell anemia (SCA) in Africa annually, yet few survive beyond childhood. Falciparum malaria is considered a significant cause of this mortality. We conducted a 5-year prospective surveillance study for malaria parasitemia, clinical malaria, and severe malarial anemia (SMA) in Dar-es-Salaam, Tanzania, between 2004 and 2009. We recorded 10 491 visits to the outpatient clinic among 1808 patients with SCA and 773 visits among 679 patients without SCA. Similarly, we recorded 691 hospital admissions among 497 patients with SCA and 2017 in patients without SCA. Overall, the prevalence of parasitemia was lower in patients with SCA than in patients without SCA both at clinic (0.7% vs 1.6%; OR, 0.53; 95% CI, 0.32-0.86; P = .008) and during hospitalization (3.0% vs 5.6%; OR, 0.46; 95% CI, 0.25-0.94; P = .01). Furthermore, patients with SCA had higher rates of malaria during hospitalization than at clinic, the ORs being 4.29 (95% CI, 2.63-7.01; P < .001) for parasitemia, 17.66 (95% CI, 5.92-52.71; P < .001) for clinical malaria, and 21.11 (95% CI, 8.46-52.67; P < .001) for SMA. Although malaria was rare among patients with SCA, parasitemia during hospitalization was associated with both severe anemia and death. Effective treatment for malaria during severe illness episodes and further studies to determine the role chemoprophylaxis are required.
Introduction
The global annual birth prevalence of sickle cell disease is 300 000 children,1 the predominant genotype being homozygous hemoglobin SS (HbSS) sickle cell anemia (SCA). Up to 70% of these births occur in sub-Saharan Africa where recent reports suggest that 50% to 80% of affected children die annually.2 Malaria is thought to be a major cause of severe morbidity and death3 ; thus, antimalarial chemoprophylaxis is recommended in patients with SCA.4
Recently, there have been 3 important developments in the fields of malaria and SCA that prompted us to review the role of malaria in SCA. First, effective interventions against malaria have become available, in the form of insecticide-treated nets (ITNs)5 and artemisinin-based combination therapies.6 Second, changes in the epidemiology of malaria have been reported, with reductions in both transmission rates and the incidence of disease in some parts of Africa.7-9 Finally, increasing evidence elucidating protection against malaria by HbS particularly in heterozygous carriers (HbAS) has been published.10-12 Surprisingly, however, descriptions of the role of malaria as a cause of morbidity and mortality in patients with SCA remain limited. In this study we followed a cohort of patients with SCA in Tanzania between 2004 and 2009 and investigated the burden of malaria by documenting malaria events both at the outpatient clinic and during hospitalization.
Methods
The study was conducted at Muhimbili National Hospital (MNH), in Dar-es-Salaam, Tanzania. This is the largest and most densely populated region in Tanzania, with a population of 2.5 million and a population density of 1793 persons per square kilometer.13 Malaria is endemic, the prevalence of Plasmodium falciparum malaria parasitemia, ranging from 0.8% to 20%,14,15 with a lower prevalence in urban compared with rural areas (6.8% vs 19.9%).16 During the period of the study, first-line therapy for clinical symptoms of malaria changed from monotherapy with chloroquine to sulfadoxine pyrimethamine, with the current recommendation being artemisinin-based combination therapy, artemether lumefantrine.17 Ethical approval was obtained from Muhimbili University of Health and Allied Sciences (reference MU/RP/AEC/VOL XI/33).
Study populations
SCA populations.
Patients with SCA were recruited both from the preexisting hospital outpatient clinic and from the wards of MNH. All patients with SCA were provided information about SCA, malaria, and the study both verbally and with printed leaflets in the local language, Kiswahili. Advice was given about the general management of SCA and procedures for seeking care in the event of acute illness events such as fever, pain, or symptomatic anemia. Each patient was then invited to join the study. Patients who agreed either signed a consent form or provided a thumbprint if they could not write, in accordance with the Declaration of Helsinki. For children, consent was requested from the parent or guardian. After informed consent, detailed histories and examinations were recorded onto standardized proformas. Investigations included a full blood count, biochemical analysis, and detection of malaria parasitemia. Because patients with SCA are generally perceived to be at risk of malaria mortality, any parasitemia, irrespective of density or absence of symptoms, was treated following recommended protocols: chloroquine, sulfadoxine pyrimethamine, or artemether lumefantrine for uncomplicated malaria and quinine for severe malaria.4,17 Patients were scheduled to attend the outpatient department (OPD) clinic at 3-month intervals, when standardized clinical and laboratory data were recorded. During episodes of acute illness, patients were encouraged to contact either a study physician or their nearest health facility, following the referral procedures through the public health care system. The decision to admit patients with SCA rested with the attending clinician in the hospital casualty department, following criteria set by MNH. Patients with SCA admitted to MNH were identified through active daily ward surveillance. Clinical and laboratory data were collected at admission. Additional investigations were performed as clinically indicated. Patients suspected to have SCA who had not been recruited into the cohort were also identified during hospitalization and recruited into the study.
Non-SCA populations.
We collected data from 2 non-SCA populations: (1) persons invited for SCA screening at the OPD clinic, including relatives and friends of patients with SCA, during the study period and (2) patients without SCA who were admitted to the pediatric wards of MNH between July 2006 and July 2008.
Laboratory methods
Sickle phenotyping into HbAA, HbAS, and HbSS states was done by alkaline hemoglobin electrophoresis (Helena, Sunderland, Tyne & Wear) and confirmed by high-performance liquid chromatography (Variant analyzer; Bio-Rad). Full blood count s were performed with the use of an automated cell counter (Pentra 60; Horiba ABX;). Reticulocytes were counted following standard methods with new methylene blue staining. Biochemical tests were performed with the use of a chemistry analyzer (Roche Cobas Mira or Abbott Architect). All persons were investigated for malaria at all visits. Malaria parasitemia was confirmed with the use of rapid diagnostic tests (Parahit, Span Diagnostics; or Paracheck, Orchid Biomedical Systems) and/or Giemsa-stained thick blood films following standard methods. P falciparum densities were assessed by counting the number of asexual-stage parasites per 200 white blood cells (WBCs) and expressed as parasites per microliter whole blood. For patients with SCA we used 14.8 × 1012/μL, which is the mean WBC count in this study population. For the non-SCA population we used a WBC count of 7.1 × 1012/μL, which is the reference value in the healthy population.18
Statistical methods
All data were checked for consistency before double entry onto a database written in MySQLv5.0 (Sun Microsystems Inc). STATA Version 10.0 (Stata Corporation) was used for analysis. Malaria events were analyzed according to 3 definitions: (1) parasitemia, a positive blood film, a positive rapid diagnostic test irrespective of clinical status, or both; (2) clinical malaria, a positive test in the presence of fever (an axillary temperature of > 37.5°C); and (3) severe malarial anemia (SMA), a Hb less than 5 g/dL in the presence of malaria parasitemia. For the purposes of this analysis, we did not follow the definition for SMA laid down by the World Health Organization,19 which includes a threshold parasite density of more than 10 000 parasites/μL. This is because the presence of Hb less than 5 g/dL is an indication of severe, life-threatening illness even when the parasite density does not exceed the threshold laid down by the World Health Organization. Descriptive data were summarized as means or proportions, whereas between-group comparisons were analyzed with logistic regression, results being presented as odds ratios (ORs) with 95% confidence intervals (95% CIs). Multivariable logistic regression included all variables that were significantly associated (P < .05) on univariate analysis.
Patients entered the SCA cohort on the day they were first seen at the hospital, whether at the OPD clinic or during hospitalization. Persons (with SCA and without SCA) who were seen in the casualty department but were not hospitalized were not included in the study. Incidence rates for malaria events were calculated for the whole study period, combining events at OPD clinic and during hospitalization. The rates were calculated by the ratio of relevant malaria events divided by the number of person-years of exposure (PYOs) and were presented as the number of events per 100 PYOs. The 95% CIs were calculated by using the normal approximation to the binomial distribution. The age-specific incidence of malaria was calculated by analyzing the frequency of events in each person, excluding episodes that were clustered within a person.
Results
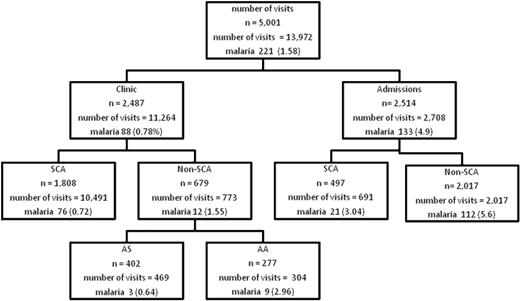
The analysis period was from March 24, 2004, to March 23, 2009. The OPD clinic data were derived from 10 491 visits among 1808 patients with SCA (median age, 11 years; range, 4 months to 47 years) and 773 visits from among 679 persons without SCA (median age, 12.8 years; range, 3 months to 64 years), whereas the inpatient data were derived from 691 admission events in 497 patients with SCA (median age, 10.8 years; range, 5 months to 43 years). Control data were collected from 2017 patients without SCA aged 6 months to 10 years who were admitted between 2006 and 2008. The prevalence of malaria in the various subject groups is summarized in Figure 1.
Flow of persons and visits in the study with prevalence of malaria parasitemia. SCA indicates sickle cell anemia; AS and AA are the hemoglobin phenotypes. Clinic refers to events at outpatient clinic visits; admissions refer to events during hospitalization. Number of visits refers to all visits; n is number of persons. Malaria refers to malaria parasitemia. The number after malaria is the number of episodes of malaria parasitemia, figures in parentheses is the prevalence per visit (not per person). Note that persons without SCA (non-SCA) were not part of the prospective surveillance and were seen only once.
Flow of persons and visits in the study with prevalence of malaria parasitemia. SCA indicates sickle cell anemia; AS and AA are the hemoglobin phenotypes. Clinic refers to events at outpatient clinic visits; admissions refer to events during hospitalization. Number of visits refers to all visits; n is number of persons. Malaria refers to malaria parasitemia. The number after malaria is the number of episodes of malaria parasitemia, figures in parentheses is the prevalence per visit (not per person). Note that persons without SCA (non-SCA) were not part of the prospective surveillance and were seen only once.
Malaria in persons with SCA and without SCA
The overall prevalence of malaria parasitemia at the OPD clinic was 88 of (0.78%) 11 264 (Figure 1). The prevalence of parasitemia was lower in persons with SCA than in persons without SCA at the OPD clinic (0.72% vs 1.55%; OR. 0.53; 95% CI, 0.32-0.86); P = .008). Further, the prevalence was significantly lower both in subjects with HbAS (0.64%; OR, 0.21; 95% CI, 0.06-0.79; P = .02) and subjects with HbSS (0.72%; OR, 0.24; 95% CI, 0.12-0.48; P < .001) than in subjects with HbAA (2.96%).
Malaria in SCA at clinic versus during hospitalization
The prevalence of parasitemia was lower in persons with SCA than in persons without SCA during hospitalization (3.0 vs 5.6; OR, 0.46; 95% CI, 0.25-0.94; P = .01). The events in patients with SCA both at the outpatient clinic and during hospitalization are shown in Table 1. The prevalence of parasitemia, malaria, and SMA were all higher during hospitalization than at the outpatient clinic. Among patients with SCA visiting the OPD clinic, subjects were only clinically ill (with either fever or severe anemia associated with parasitemia) on 14 (0.13%) of 10 466 visits. Six patients (0.06% of the total visits) had fever alone, 8 (0.08%) had severe anemia alone, and 4 (0.04%) had both fever and severe anemia. During hospitalization, 11 (1.59%) of 691 patients had either clinical malaria or SMA, 7 (1.03%) of 682 patients had clinical malaria alone, 11 (1.71%) of 644 patients had SMA, and 4 (0.58%) of 691 patients had both clinical malaria and SMA.
Clinical features associated with malaria in patients with SCA attending the OPD clinic
The features associated with parasitemia in patients with SCA are summarized in Table 2. During OPD clinic visits, factors that were significantly associated with parasitemia on univariate analysis included fever, higher oxygen saturation, spleen and liver enlargement, high WBC count and mean corpuscular volume, and low Hb. On multivariate analysis, high WBC count was the only feature that remained significantly associated (OR, 3.29; 95% CI, 1.5-7.18; P = .003), whereas fever (OR, 3.3; 95% CI, 0.97-11.29; P = .057) was associated with borderline significance.
Clinical features associated with malaria in patients with SCA during hospitalization
The presenting symptoms in patients with SCA during hospitalization occurred with the following frequencies: pain was reported in 523 of (75.9%) 689 admissions, fever in 407 (59.2%) of 688 admissions, symptoms of anemia in 144 of (20.9%) 687 admissions, and jaundice in 107 (16.3%) of 657 admissions. Fever (axillary temperature ≥ 37.5°C on examination) was documented at 204 hospitalization events (29.9%). Parasitemia was detected in 21 admissions (3.0%), with only 1 patient testing positive during more than one admission event.
The factors associated with malaria parasitemia during hospitalization on univariate analysis are summarized in Table 2. At admission, patients with SCA with parasitemia were older, had lower Hb concentrations, and had higher mean corpuscular volumes, reticulocyte counts, and plasma aspartate transaminase (AST) concentrations than patients without parasitemia. The risk of death during hospitalization was higher in patients with parasitemia (OR, 4.9; 95% CI, 1.04-23.20; P = .04). The factors that were independently associated with parasitemia were severe anemia (OR, 3.46; 95% CI, 1.27-9.45; P = .02) and high AST level (OR, 3.41; 94% CI, 1.23-9.45; P = .02; Table 3).
Incidence of malaria in SCA
During the 5 years of study, we recorded 3827 PYO. The incidence rate of parasitemia was 2.53 (95% CI, 2.06-3.09), malaria was 0.37 (95% CI. 0.20-0.61), and SMA was 0.50 (95% CI, 0.30-0.78) episodes per 100 PYO. Malaria events stratified into 2 age bands (older and younger than 5 years), excluding events clustered in persons, is shown in Table 4. Compared with persons older than 5 years, children younger than 5 years had a lower prevalence of parasitemia (OR, 0.57; 95% CI, 0.29-1.08; P = .08) but a higher prevalence of malaria (OR, 2.96; 95% CI, 1.02-8.57; P = .05) and SMA (OR, 2.29; 95% CI, 0.89-5.88; P = .08), with malaria reaching statistical significance.
Discussion
This is the first detailed report to have focused on the importance of malaria as a cause of morbidity and mortality in patients living with SCA. Malaria is widely considered a major cause of illness and death in patients living with SCA in sub-Saharan Africa,3,20 but the evidence to support this has been conflicting. Some investigators have reported an association between malaria and admission to hospital, anemic crises,21-23 and mortality24 ; however, these studies included relatively small numbers of patients with SCA and were conducted in areas of high malaria transmission where the finding of parasitemia cannot be taken as proof that malaria was the cause of the clinical presentation. Conversely, there is more compelling evidence to suggest that patients with SCA are protected from malaria, both in terms of a lower prevalence of malaria infection and a lower parasite density.25-29 Our cohort of patients living with SCA in Dar-es-Salaam provided an opportunity to study this question in more detail; although chloroquine is recommended for prophylaxis, resistance rates are high and may not provide adequate protection. Ethical concerns about conducting an observational study of subjects with SCA, generally considered to be at risk of malaria, in a group who were essentially not on effective chemoprophylaxis, was countered because the malaria prevalence in Dar-es-Salaam is generally low (0.8%-14%)14-16 and interventions such as ITNs and prompt, effective treatment with artemisinin-based combination therapy are available.
The most striking finding in our study was the lower prevalence of malaria parasitemia in patients with SCA than in patients without SCA, both at the outpatient clinic and during hospitalization. A degree of malaria resistance in patients with SCA seems plausible, given that protection is unequivocal in heterozygotes (HbAS).10,30 Our observation raises the question of whether, if HbS protects the heterozygote persons against malaria, patients with SCA, who have even higher levels of HbS, might be even better protected. A dose-dependent effect is certainly seen in HbC, another hemoglobinopathy, commonly found in West Africa, in which both heterozygous (HbAC) and homozygous (HbCC) persons are protected against malaria,31 with protection considerably greater in HbCC.32
The incidence of malaria parasitemia in our study was 2.53 events per 100 PYO. The incidence of parasitemia in children younger than 5 years was lower than in children older than 5 years, a finding that may be due to protection of younger children by ITNs, provided free in Tanzania to children of this age group.16
The spectrum of malarial disease includes asymptomatic infection, febrile episodes, and severe illness (anemia and cerebral complications). The likelihood of a child progressing from one stage to the next in a malaria endemic area is as follows: parasitemia (50% of each year); clinical febrile (twice per year), and severe disease (anemia or cerebral malaria; 3% per year).33 Our findings show a similar trend, with fewer episodes of malaria compared with parasitemia and younger children having a significantly higher prevalence of malaria (P = .05). However, in this study more patients had SMA (1.0%) than malaria (0.75%), most likely because of preexisting hemoglobinopathy.
We found that malaria events occurred at higher rates in patients with SCA during hospitalization than at the outpatient clinic. This suggests that, although patients with SCA are at lower risk of malaria than persons without SCA, protection is not complete, and malaria is an important factor that needs to be considered during hospitalization. We acknowledge that this finding may simply reflect the fact that patients with malaria are more likely to have severe illness and to be hospitalized. However, the aim of the study was not to determine whether malaria was the cause of hospitalization but to look for an association between malaria parasitemia and clinical events, including during hospitalization. Parasitemia was significantly associated with mortality during hospitalization (9.5% vs 2.2%; OR, 4.9; P = .04) and was independently associated with severe anemia and high AST levels. Therefore, the consequences of malaria in SCA appear to be severe during acute illness, as previously suggested by Molineaux et al.24 These findings highlight the importance of prompt and effective management of malaria in patients with SCA during hospitalization, even though such patients appear to benefit from a degree of protection from the disease.
Our study has several limitations. First, the overall prevalence of malaria parasitemia at the OPD clinic was only 0.78%, which is considerably lower than the 3.9% reported in a recent survey of health facilities conducted in Dar-es-Salaam.14 Therefore, it is necessary to validate these results in areas with higher and more intense malaria transmission. The second limitation is that, because this study was conducted in a referral hospital, it was open to several potential biases relating to the treatment-seeking behavior of patients with SCA and the perception of their health care providers. There is a possibility that some events may have been missed as a result of treatment at casualty, other health care facilities, or at home. This would apply to both persons with SCA and persons without SCA. Furthermore, although we did not change protocols of referral and management in the hospital, SCA is known to have a high risk of mortality, and, because patients were both part of a cohort study and were encouraged to present early in the event of an intercurrent illness, this may have introduced a bias toward higher rates of presentation and hospitalization in patients with SCA. A stronger study design might have included a prospective cohort study that reported careful monitoring of SCA and control subjects that used well-defined criteria for medical consultation, diagnosis of asymptomatic malarial parasitemia and acute malaria infection. Nevertheless, at the outset of our study, we did not make any assumptions about malaria and did not alter the current practice of referral and hospitalization to observe the actual practice in the community. Given existing attitudes to malaria in patients with SCA, it would have been difficult to design a comparative cohort study of this sort without close monitoring of effective antimalarial chemoprophylaxis.
In summary, in our study, conducted in an area of low but perennial malarial transmission; we found that malaria was less common in subjects with SCA than in subjects without SCA both at the outpatient clinic and during hospitalization. This raises the question of whether, and under what circumstances, malaria prophylaxis is required in patients with SCA. Further studies are required to establish the role of malaria as a cause of ill health in such patients under conditions of higher malarial transmission. Nevertheless, we also found that malaria was significantly associated with severe anemia and death in hospitalized patients with SCA; therefore, we recommend that malaria should be promptly and effectively diagnosed and treated in such patients. Evidence is needed, ideally in the form of randomized clinical trials, about the most appropriate approach to the prevention and treatment of malaria in patients living with SCA in malaria-endemic environments.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank patients and staff of MNH and MUHAS; we also thank Bob Snow and Fenella Kirkham for commenting on the manuscript.
This work was supported by the Wellcome Trust, UK (JKM 072064, TNW 076934, project grant 080025, strategic award 084538) and Kenya Medical Research Institute (KEMRI) Center for Geographic Medicine Research (Coast).
National Institutes of Health
Authorship
Contribution: J. Makani designed the research, collected data, analyzed the results, and wrote the paper; K. Marsh designed the research and wrote the paper; G.F. and T.N.W. reviewed and analyzed the results and contributed to writing the paper; S.E.C. and C.R.N. reviewed the results and contributed to writing the paper; A.N.K., S.E.C., K. Mwamtemi, J.K., P.M., S.R., E.M., J. Mgaya, and K.P. collected data; and J.O., E.O., and D.M. managed the data and contributed to the analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Julie Makani, Department of Haematology and Blood Transfusion, Muhimbili University of Health and Allied Sciences, P O Box 65001, Dar-es-Salaam, Tanzania; e-mail: jmakani@muhas.ac.tz.