Abstract
Trisomy of human chromosome 21 (Hsa21) results in Down syndrome (DS), a disorder that affects many aspects of physiology, including hematopoiesis. DS children have greatly increased rates of acute lymphoblastic leukemia and acute megakaryoblastic leukemia (AMKL); DS newborns present with transient myeloproliferative disorder (TMD), a preleukemic form of AMKL. TMD and DS-AMKL almost always carry an acquired mutation in GATA1 resulting in exclusive synthesis of a truncated protein (GATA1s), suggesting that both trisomy 21 and GATA1 mutations are required for leukemogenesis. To gain further understanding of how Hsa21 contributes to hematopoietic abnormalities, we examined the Tc1 mouse model of DS, which carries an almost complete freely segregating copy of Hsa21, and is the most complete model of DS available. We show that although Tc1 mice do not develop leukemia, they have macrocytic anemia and increased extramedullary hematopoiesis. Introduction of GATA1s into Tc1 mice resulted in a synergistic increase in megakaryopoiesis, but did not result in leukemia or a TMD-like phenotype, demonstrating that GATA1s and trisomy of approximately 80% of Hsa21 perturb megakaryopoiesis but are insufficient to induce leukemia.
Introduction
Down syndrome (DS) results from trisomy of human chromosome 21 (Hsa21, trisomy 21) and is the most common chromosomal disorder in liveborn humans.1 Persons with DS have an increased risk of developing several hematologic defects, including macrocytosis, neutrophilia, thrombocytopenia, and polycythemia.2-5 In addition, they also have an increased incidence of both acute lymphoblastic leukemia (ALL) and acute megakaryoblastic leukemia (AMKL) in childhood.6 Approximately 10% of DS newborns are born with a transient myeloproliferative disorder (TMD), and, of these, 20% to 30% progress to AMKL by 4 years of age.7,8 Both diseases are characterized by the presence of a clonal population of megakaryoblasts in the blood. However, whereas TMD is a spontaneously regressing neoplasia, AMKL is life threatening and requires chemotherapy.
An important step in understanding the origins of these diseases was the discovery that almost all cases of TMD and AMKL have acquired and clonal mutations in exon 2 of the X-linked GATA1 gene, which codes for the GATA1 transcription factor.9-14 The effect of these mutations is to prevent expression of full-length GATA1 from the normal ATG initiation codon, forcing translation to initiate at an ATG codon in exon 3, thus resulting in the synthesis of a truncated GATA1 protein termed GATA1 short (GATA1s). Examination of GATA1 mutations shows that in any one person, the mutation found in AMKL cells is the same as the mutation seen first in TMD, thus leading to the proposal that TMD is a preleukemic disease that can eventually develop into AMKL, presumably as a result of further mutations.10,15 Interestingly, although GATA1 mutations are found in all cases of DS-AMKL, no such mutations are seen in non–DS-AMKL.13,14 Taken together, these observations indicate that the oncogenic transformation in both TMD and DS-AMKL requires the cooperation of at least 2 genetic abnormalities: trisomy 21 and stereotypical mutations in GATA1 leading to elimination of full-length GATA1 and exclusive synthesis of GATA1s.
Recent studies have shown that there is an increase in megakaryocytic and erythroid progenitors in the fetal liver of DS fetuses compared with euploid controls.16-18 Importantly, these studies were carried out on fetal liver samples in which there were no detectable mutations in GATA1. This implies that trisomy 21 alone is sufficient to cause an expansion of megakaryocytic and erythroid progenitors, and suggests that this may be the mechanism by which trisomy 21 contributes to the establishment of TMD and AMKL.
GATA1 is a transcription factor essential for the development of multiple hematopoietic lineages, including megakaryocytes, erythrocytes, eosinophils, and mast cells, with loss of GATA1 resulting in embryonic lethality due to severe anemia.19 Analysis of a family segregating a germline mutation of GATA1, which mimics the mutation found in DS-AMKL, thereby eliminating synthesis of wild-type GATA1 but leaving production of GATA1s intact, showed that this resulted in macrocytic anemia and neutropenia but not leukemia.20 An analogous mutation in mice did not cause leukemia, but instead resulted in hyperproliferative megakaryocytic progenitors in the fetal liver.21 Interestingly, this effect was seen only in mouse fetal liver cells between embryonic day 9.5 (e9.5) and e16.5 of gestation, after which the abnormality disappeared, suggesting there may be a transient fetal liver progenitor, which is uniquely susceptible to the effects of GATA1s.
These studies have led to a hypothesis explaining the involvement of trisomy 21 and the GATA1s mutation in the generation of TMD and AMKL. Trisomy 21 is proposed to cause an expansion of megakaryocytic and erythroid progenitors in the fetal liver. If these cells then acquire a mutation in GATA1 leading to exclusive synthesis of GATA1s, this leads to a selective expansion of megakaryocytic progenitors resulting in TMD at birth.21,22 The transient nature of TMD is proposed to be a consequence of the transient existence of a megakaryocytic progenitor sensitive to the effects of GATA1s.21 Further mutations would then be needed to transform the original TMD preleukemic cells into AMKL.
Several mouse models of DS have been developed to provide experimental systems in which the pathology of DS phenotypes can be investigated, and as tools that can be used to identify dosage-sensitive genes that contribute to specific phenotypes, and thus gain an understanding of mechanisms underlying the syndrome.1 In principle, these should also be useful in studies to understand the contribution of trisomy 21 to TMD, AMKL, and ALL. There are approximately 324 recognized genes on Hsa21, and their mouse orthologues are split over 3 chromosomes: mouse chromosome 16 (Mmu16), Mmu17, and Mmu10.1 Two recent studies have reported analysis of the hematopoietic system in 2 different DS mouse models. The Ts65Dn mouse strain is trisomic for 143 gene orthologues of Hsa21 located on Mmu16 (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).23,24 These mice have macrocytosis with decreased red blood cell (RBC) counts and develop a myeloproliferative disorder (MPD) with features including thrombocytosis, megakaryocyte hyperplasia, myelofibrosis, and extramedullary hematopoiesis.25 The Ts1Cje mouse model of DS is trisomic for 94 genes on Mmu16, covering approximately two-thirds of the trisomic region in Ts65Dn mice (supplemental Table 1).24,26 This model displays some similar hematopoietic defects to those present in the Ts65Dn model, including the presence of macrocytic anemia, but does not develop an MPD.27 These results suggest that 1 or more of the triplicated genes in Ts1Cje mice are responsible for the phenotypes shared between the 2 models, such as the macrocytic anemia. In contrast, the MPD seen in Ts65Dn must require 3 copies of 1 or more of the 49 genes triplicated in Ts65Dn but not Ts1Cje mice. Notably, neither of these mouse strains develops TMD, AMKL, or ALL, perhaps because they are not trisomic for the relevant Hsa21 orthologues.
In this study, we analyzed the hematopoietic system of the Tc1 mouse model of DS. This mouse strain contains an almost complete, freely segregating copy of Hsa21, and is trisomic for approximately 269 genes, including almost all of the genes whose orthologues are located on Mmu10 and Mmu17 (supplemental Table 1).28 Thus, the Tc1 strain represents the most complete model of DS generated to date. Tc1 mice display features similar to those found in DS, including alterations in learning and memory, as well as defects in cerebellar neuronal number, heart development, and mandible size.28-30 We show that Tc1 mice have a macrocytic anemia that persists for at least a year, but do not develop TMD or leukemia. Furthermore, older Tc1 mice show extensive extramedullary hematopoiesis characterized by splenomegaly and increased megakaryopoiesis and erythropoiesis in the spleen. Analysis of Tc1 mice crossed to a Gata1 mutant expressing only GATA1s showed that the 2 mutations synergistically increased megakaryopoiesis. However, these double mutants also did not develop TMD or leukemia, suggesting that either one or more Hsa21 genes that are required in 3 copies for TMD and AMKL are not present in the Tc1 mouse strain, or that further oncogenic mutations are required to induce disease.
Methods
Mice
The Tc(Hsa21)1TybEmcf (Tc1) mouse strain containing a freely segregating copy of human chromosome 21 (Hsa21)28 was maintained by crossing Tc1 female mice to male B6129S8F1/Nimr mice, which were F1 progeny generated from a cross of C57BL/6J female and 129S8/SvEv male mice. All analysis was carried out using male Tc1 mice and their male euploid littermates as wild-type controls. Mice bearing the Gata1Δe2 mutation21 were also maintained on the B6129S8F1/Nimr background and intercrossed with Tc1 mice. For analysis, we used males from this colony whose genotypes were Gata1+/Y (wild-type, Wt), Gata1+/Y;Tc1 (Tc1), Gata1Δe2/Y (Gata1s), and Gata1Δe2/Y;Tc1 (Tc1Gata1s). All mice were housed at the MRC National Institute for Medical Research, and all animal experimentation was carried out with approval from the United Kingdom Home Office.
Blood cell counts
Blood was collected using ethylenediaminetetraacetic acid–coated micropipettes (Drummond Scientific), either from the tail vein of adult mice or from the heart of newborn mice (< 24 hours after birth) that had been lethally anesthetized using isoflurane. Blood (5 μL) was diluted in 45 μL of phosphate-buffered saline (PBS), and complete blood counts (CBCs) were performed in triplicate using a Vetscan HMII (Abaxis). Blood smears were stained with Wright-Giemsa stain according to standard protocols.
Histology
Tissues were fixed in Bouin fixative, embedded in paraffin, sectioned, and stained with hematoxylin and eosin using standard protocols. For immunohistochemistry, sections were deparaffinized in xylene and rehydrated. After antigen retrieval in citrate buffer, samples were stained using standard procedures. Slides were stained with antibodies specific for integrin αIIb (CD41) or Ter119 (Santa Cruz Biotechnology) and Vectastain Elite ABC kits (Vector Laboratories). Slides were developed using 3,3′-diaminobenzidine substrate system (D3939; Sigma-Aldrich) and counterstained with Harris hematoxylin solution. Stained sections were dehydrated and photographed using an Axioplan 2 Imaging microscope with an Axiocam HRc camera (Zeiss). Megakaryocytes were identified using standard morphologic criteria on hematoxylin and eosin–stained sections of spleen and bone marrow; the number of megakaryocytes in 6 representative high-power fields (HPFs; ×40) was counted and expressed as the mean number of megakaryocytes per HPF.
Cytospins
A suspension of 105 bone marrow cells in PBS was centrifuged onto glass slides for 10 minutes at 100g using a Shandon Cytospin 4 (Thermo Electron Corporation). Slides were air dried, stained for 5 minutes with May-Grünwald stain, rinsed in PBS, stained for 15 minutes with Giemsa stain, rinsed in PBS, and air dried.
Flow cytometry
Single-cell suspensions of bone marrow and spleen cells were prepared and red cells lysed in ACK lysis buffer (150mM NH4Cl, 1mM KHCO3, 1μM ethylenediaminetetraacetic acid). Cells were stained according to standard flow cytometric procedures using the following antibodies: biotinylated anti-Ter119 followed by streptavidin-peridinin-chlorophyll-protein complex, anti–CD19-allophycocyanin, and anti–CD3ϵ-phycoerythrin (BD Pharmingen). For myeloid progenitor analysis, bone marrow and spleen samples were first depleted of mature hematopoietic cells by staining with biotinylated antibodies to “lineage” markers CD3ϵ, CD4, CD8, B220, Gr1, Ter119, CD19, immunoglobulin M, and interleukin-7 receptor α (IL-7Rα) and then using streptavidin-coupled Dynal beads (Invitrogen) to remove stained cells. The remaining cells were incubated with streptavidin–peridinin-chlorophyll-protein complex for 30 minutes at 4°C, followed by anti-CD34–fluorescein isothiocyanate (eBioscience), anti–FcγR-phycoerythrin, anti–Sca1-phycoerythrin–Texas red, and anti–cKit-allophycocyanin (BD Pharmingen), overnight at 4°C. Cells were washed and analyzed on an LSRII cytometer (BD Biosciences). Cell numbers were calculated for a whole spleen and for bone marrow from 2 femurs and 2 tibiae.
Colony assays
Single-cell suspensions were prepared from bone marrow, spleen, or fetal liver in Iscove modified Dulbecco medium containing 5% fetal calf serum, and erythrocytes were lysed in ACK lysis buffer. Colony assays were performed by plating cells in either MethoCult or MegaCult-C (StemCell Technologies) with appropriate cytokines according to the manufacturer's instructions. For erythroid colony-forming units (CFU-Es), cells were plated in M3334 MethoCult already containing erythropoietin and supplemented with stem cell factor (50 ng/mL); for granulocyte-macrophage colony-forming units (CFU-GMs), cells were plated in M3234 MethoCult supplemented with granulocyte-macrophage colony-stimulating factor (1 ng/mL), Flt3 ligand (5 ng/mL), stem cell factor (50 ng/mL), and thrombopoietin (5 ng/mL); and for megakaryocyte colony-forming units (CFU-MKs), assays were carried out using MegaCult-C supplemented with thrombopoietin (50 ng/mL), interleukin-6 (IL-6, 20 ng/mL), and interleukin-3 (IL-3, 10 ng/mL). Cells were cultured at 37°C and 5% CO2. For CFU-E and CFU-GM assays, colonies were enumerated after 11 days of culture. For CFU-MK assays, cells were cultured for 7 days, and then fixed and stained for acetylcholinesterase with acetylthiocholineiodide (Sigma-Aldrich) according to the manufacturer's instructions for MegaCult-C (StemCell Technologies). Only colonies staining positive for acetylcholinesterase were counted.
Statistical analysis
Significance of differences was calculated using the Mann-Whitney test.
Results
Tc1 mice develop a persistent macrocytic anemia
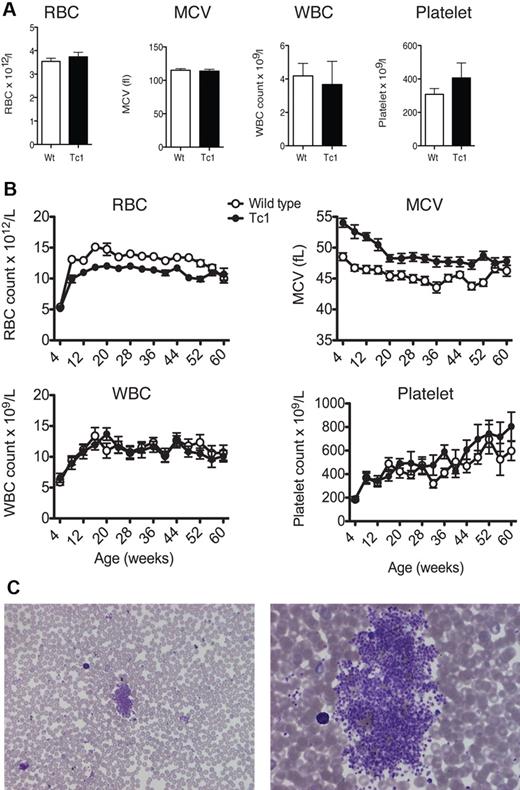
To determine whether Tc1 mice develop hematologic abnormalities, including TMD or leukemia, we measured peripheral complete blood counts (CBCs) in Tc1 and wild-type littermate control mice. Because TMD is a preleukemic disorder that presents at birth, we measured CBCs in newborn Tc1 mice (aged less than 24 hours) but found no abnormalities within their blood in terms of counts of red and white blood cells and platelets and we detected no change in the mean corpuscular volume (MCV; Figure 1A). Analysis of Wright-Giemsa–stained blood smears showed no evidence of increased blasts compared with wild-type mice (not shown). To monitor Tc1 mice for the possible development of leukemia, we measured CBCs at monthly intervals on 15 Tc1 mice and 15 wild-type littermate controls up to 60 weeks of age. Tc1 mice did not have any significant differences in their white blood cell count at any age examined (Figure 1B), and once again analysis of blood smears showed no blasts. However, after 8 weeks of age, Tc1 mice developed decreased red blood cell counts, which remained significantly lower than wild-type controls up to 56 weeks of age. These mice also had an increased MCV, which presented from the age of 4 weeks and continued until 52 weeks of age (Figure 1A), indicating an increase in erythrocyte size.
Tc1 mice present with macrocytic anemia throughout life. (A) Graphs of mean (± SEM) red blood cell (RBC), white blood cell (WBC), and platelet counts and mean corpuscular volume (MCV) of neonate (< 1 day old) Tc1 and wild-type (Wt) mice (n = 6). (B) Graphs show mean (± SEM) RBC, WBC, and platelet counts and MCV as a function of age of Tc1 and wild-type mice (n = 15). Tc1 mice show decreased RBC counts from 8 to 52 weeks of age and increased MCV from 4 to 52 weeks of age (P < .05). (C) Low-power (left) and high-power (right) views of Wright-Giemsa–stained blood smears from Tc1 mice aged 64 weeks showing clouds of platelets.
Tc1 mice present with macrocytic anemia throughout life. (A) Graphs of mean (± SEM) red blood cell (RBC), white blood cell (WBC), and platelet counts and mean corpuscular volume (MCV) of neonate (< 1 day old) Tc1 and wild-type (Wt) mice (n = 6). (B) Graphs show mean (± SEM) RBC, WBC, and platelet counts and MCV as a function of age of Tc1 and wild-type mice (n = 15). Tc1 mice show decreased RBC counts from 8 to 52 weeks of age and increased MCV from 4 to 52 weeks of age (P < .05). (C) Low-power (left) and high-power (right) views of Wright-Giemsa–stained blood smears from Tc1 mice aged 64 weeks showing clouds of platelets.
Although automated analysis of platelet counts showed no significant changes in Tc1 mice (Figure 1B), we noted that in blood smears from Tc1 mice there were frequent platelet “clouds,” which were never seen in wild-type mice (Figure 1C). Because these would not have been detected by the automated analyzer, it is likely that the real platelet counts were increased in Tc1 mice relative to wild-type controls. These data show that Tc1 mice do not develop TMD or leukemia, but present with a macrocytic anemia beginning a few weeks after birth, which is similar to that reported previously in the Ts65Dn and Ts1Cje mouse models of DS.25,27 They may also have thrombocytosis, similar to that reported for Ts65Dn mice.25
Tc1 mice develop splenomegaly with an infiltration of megakaryocytic and erythroid cells
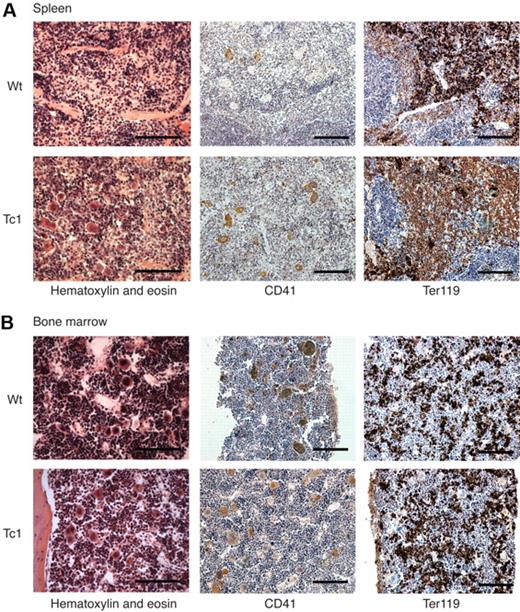
To further investigate the hematopoietic system of Tc1 mice and examine the reason for the peripheral blood abnormalities, mice were killed after 15 months of age and their bone marrow and spleen examined. Of 11 Tc1 mice, 6 were found to have developed splenomegaly, which was not observed in any wild-type control mice, and the average weight of Tc1 spleens was significantly higher than that of wild-type littermate controls (Figure 2A-B). This propensity for splenomegaly was accompanied by increased total cell counts in spleens of Tc1 mice aged 15 months (Figure 2C). Histologic examination of Tc1 spleens showed a disrupted red and white pulp structure, and an infiltration of both CD41+ megakaryocytes and Ter119+ erythroid cells throughout the Tc1 spleens (Figure 3A). Quantitative analysis confirmed a large and significant increase in both megakaryocytes and Ter119+ erythroid cells in the spleens of Tc1 mice aged 15 months, but no change in the number of CD3+ T cells or CD19+ B cells (Figure 2D-E and supplemental Figure 1B). To establish whether Tc1 mice also presented with splenomegaly at a younger age, mice were analyzed at 2 months and 6 months of age; however, no evidence of splenomegaly was found in any of the mice examined, and there was no increase in cellularity and in the number of Ter119+, CD3+, or CD19+ cells (Figure 2C,E, supplemental Figure 1A, and data not shown). These data suggest that the development of splenomegaly and extramedullary hematopoiesis in Tc1 mice initiates between 6 and 15 months of age.
Splenomegaly and extramedullary hematopoiesis in Tc1 mice older than 15 months. (A) Photograph shows appearance of an enlarged spleen found in a Tc1 mouse aged 15 months, alongside a spleen from a wild-type (Wt) age-matched control mouse. Size marker indicates 1 cm. (B) Graph shows weights of spleens taken from mice aged 15 months or older. Horizontal bar indicates the mean. (C) Graphs showing mean (± SEM) total number of cells in the spleen and bone marrow of mice of the indicated genotypes and ages (n = 6-11). (D) Graphs showing mean (± SEM) number of megakaryocytes per microscope field (40× objective) in mice aged older than 15 months (n = 5). (E) Histograms of flow cytometric analysis of surface levels of Ter119 expression on spleen and bone marrow cells from a Wt mouse at 2 months of age. Numbers indicate percentage of cells falling into the indicated markers. Graphs show mean (± SEM) numbers of Ter119+ cells in the spleens and bone marrow of mice of the indicated ages and genotypes (2 months, n = 11 [Wt], n = 10 [Tc1]; > 15 months, n = 7 [Wt], n = 11 [Tc1]). *P < .05, **P < .01.
Splenomegaly and extramedullary hematopoiesis in Tc1 mice older than 15 months. (A) Photograph shows appearance of an enlarged spleen found in a Tc1 mouse aged 15 months, alongside a spleen from a wild-type (Wt) age-matched control mouse. Size marker indicates 1 cm. (B) Graph shows weights of spleens taken from mice aged 15 months or older. Horizontal bar indicates the mean. (C) Graphs showing mean (± SEM) total number of cells in the spleen and bone marrow of mice of the indicated genotypes and ages (n = 6-11). (D) Graphs showing mean (± SEM) number of megakaryocytes per microscope field (40× objective) in mice aged older than 15 months (n = 5). (E) Histograms of flow cytometric analysis of surface levels of Ter119 expression on spleen and bone marrow cells from a Wt mouse at 2 months of age. Numbers indicate percentage of cells falling into the indicated markers. Graphs show mean (± SEM) numbers of Ter119+ cells in the spleens and bone marrow of mice of the indicated ages and genotypes (2 months, n = 11 [Wt], n = 10 [Tc1]; > 15 months, n = 7 [Wt], n = 11 [Tc1]). *P < .05, **P < .01.
Increased megakaryopoiesis in Tc1 mice older than 15 months. (A) Photomicrographs of sections of spleens from a Tc1 mouse with an enlarged spleen and a wild-type (Wt) control mouse, both aged 15 months. Sections were stained with hematoxylin and eosin, or with antibodies to CD41 or Ter119. The enlarged Tc1 spleen shows an increased number of megakaryocytes seen both in the hematoxylin and eosin and anti-CD41 stains. (B) Bone marrow sections from Wt and Tc1 mice stained as in panel A. Scale bars in panels A and B indicate 100 μm.
Increased megakaryopoiesis in Tc1 mice older than 15 months. (A) Photomicrographs of sections of spleens from a Tc1 mouse with an enlarged spleen and a wild-type (Wt) control mouse, both aged 15 months. Sections were stained with hematoxylin and eosin, or with antibodies to CD41 or Ter119. The enlarged Tc1 spleen shows an increased number of megakaryocytes seen both in the hematoxylin and eosin and anti-CD41 stains. (B) Bone marrow sections from Wt and Tc1 mice stained as in panel A. Scale bars in panels A and B indicate 100 μm.
We carried out a similar analysis of the bone marrow of Tc1 mice aged either 2 or 15 months. Total bone marrow cellularity and the number of megakaryocytes and Ter119+, CD3+, and CD19+ cells was unchanged (Figure 2C-E and supplemental Figure 1C-D). Histology of sections of bone marrow confirmed a largely normal structure with apparently similar numbers of CD41+ megakaryocytes and Ter119+ erythroid cells (Figure 3B), and there was no evidence of myelofibrosis, as assessed in sections stained with hematoxylin and eosin or for reticulin (Figure 3B and data not shown).
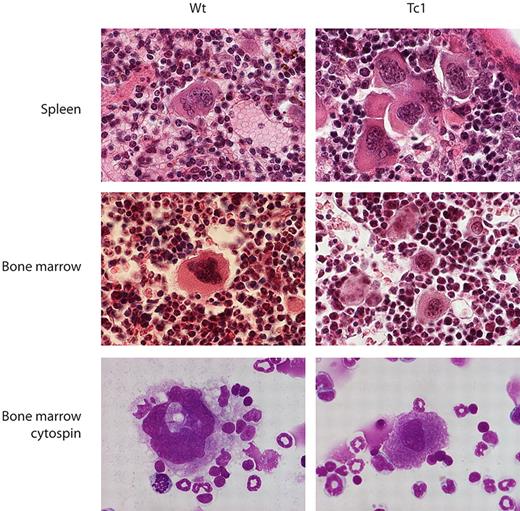
More detailed examination showed that occasional, morphologically normal megakaryocytes were seen in spleen sections from wild-type mice, whereas spleen sections from Tc1 mice contained increased numbers of smaller, more immature megakaryocytes, often present in small clusters (Figure 4). Similarly, bone marrow sections and cytospins from Tc1 mice contained slightly increased numbers of megakaryocytes that tended to be smaller and left-shifted in comparison with the predominantly mature, terminally differentiated megakaryocytes seen in wild-type bone marrow sections and cytospins, and there was no other evidence of myelodysplasia (Figure 4).
Megakaryocytes in Tc1 mice are less mature. Photomicrographs of sections of spleen and bone marrow stained with hematoxylin and eosin and cytospins of bone marrow cells stained with May-Grünwald and Giemsa stains, from Wt and Tc1 mice aged 15 months.
Megakaryocytes in Tc1 mice are less mature. Photomicrographs of sections of spleen and bone marrow stained with hematoxylin and eosin and cytospins of bone marrow cells stained with May-Grünwald and Giemsa stains, from Wt and Tc1 mice aged 15 months.
Increased myeloid progenitors in the spleen of aged Tc1 mice
To determine whether the increased numbers of megakaryocytes and erythroid cells in the enlarged spleens from Tc1 mice were due to changes in the stem cell or progenitor compartments, we measured the frequencies of these in the spleen and bone marrow of Tc1 and control mice aged 2 months and in mice aged older than 15 months (Figure 5A-E). No differences were observed in the frequency of Lineage−Sca1+cKit+ (LSK) cells in Tc1 mice compared with wild-type controls in either the spleen or bone marrow at either 2 months or more than 15 months of age. The LSK compartment includes the most primitive hematopoietic stem cells as well as more committed multilineage progenitors. Similarly, there were no significant changes in the frequency of common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs), and megakaryocyte-erythroid progenitors (MEPs) in either spleen or bone marrow at either age.
Analysis of hematopoietic progenitors in Tc1 mice. (A) Two-dimensional contour plots of bone marrow cells showing flow cytometric analysis used to identify hematopoietic progenitors. Numbers indicate percentage of cells falling into the gates. Lineage− cells were separated by expression of cKit and Sca1, and the earliest progenitors were identified as Lineage−Sca1+cKit+ (LSK). Lineage−Sca1−cKit+ cells were further subdivided by expression of FcγR and CD34 into common myeloid progenitors (CMPs, CD34+FcγR−), the granulocyte-macrophage progenitors (GMPs, CD34+FcγR+), and the megakaryocyte-erythroid progenitor (MEPs, CD34−FcγR−). Note that before flow cytometric analysis, Lineage+ cells had been depleted, to enrich for Lineage− cells, thus explaining the apparently high percentage of Lineage− cells. (B-D) Graphs showing mean (± SEM) percentage of hematopoietic progenitors in the spleen (B-C) and bone marrow (D-E) of Tc1 and wild-type mice aged 2 months (B,D) and older than 15 months (C,E; 2 months, n = 8 [Wt], n = 13 [Tc1]; > 15 months, n = 7 [Wt], n = 11 [Tc1]).
Analysis of hematopoietic progenitors in Tc1 mice. (A) Two-dimensional contour plots of bone marrow cells showing flow cytometric analysis used to identify hematopoietic progenitors. Numbers indicate percentage of cells falling into the gates. Lineage− cells were separated by expression of cKit and Sca1, and the earliest progenitors were identified as Lineage−Sca1+cKit+ (LSK). Lineage−Sca1−cKit+ cells were further subdivided by expression of FcγR and CD34 into common myeloid progenitors (CMPs, CD34+FcγR−), the granulocyte-macrophage progenitors (GMPs, CD34+FcγR+), and the megakaryocyte-erythroid progenitor (MEPs, CD34−FcγR−). Note that before flow cytometric analysis, Lineage+ cells had been depleted, to enrich for Lineage− cells, thus explaining the apparently high percentage of Lineage− cells. (B-D) Graphs showing mean (± SEM) percentage of hematopoietic progenitors in the spleen (B-C) and bone marrow (D-E) of Tc1 and wild-type mice aged 2 months (B,D) and older than 15 months (C,E; 2 months, n = 8 [Wt], n = 13 [Tc1]; > 15 months, n = 7 [Wt], n = 11 [Tc1]).
We extended our analysis of hematopoietic progenitors in Tc1 mice using in vitro colony-forming assays. No significant differences were seen in the number of megakaryocyte-, erythroid-, or granulocyte-macrophage colony-forming units (CFU-MKs, CFU-Es, and CFU-GMs, respectively) in either the spleens or bone marrow from Tc1 mice aged 2 months compared with wild-type controls (Figure 6A,C). In contrast, there was a significant increase in the frequency of CFU-GMs in the spleens of Tc1 mice aged older than 15 months (Figure 6B). The frequency of CFU-MKs and CFU-Es was also increased in the spleens of these aged Tc1 mice, although the differences did not reach significance (P = .06 and P = .17, respectively). Finally, there were no differences in any of these progenitor populations within the bone marrow of the aged Tc1 mice (Figure 6D). Taken together, the data presented here show that Tc1 mice aged older than 15 months show prominent extramedullary hematopoiesis characterized by splenomegaly and a large increase in the numbers of megakaryocytic and erythroid cells and their progenitors in the spleen.
Increased hematopoietic progenitor numbers in the spleen of Tc1 mice. Graphs showing mean (± SEM) frequency of CFU-MK, CFU-E, and CFU-GM progenitors in the spleen (A-B) or bone marrow (C-D) of Tc1 and wild-type mice aged 2 months (A,C) and older than 15 months (B,D; 2 months, n = ≥ 8 [Wt], ≥ 13 [Tc1]; > 15 months, n = 7 [Wt], 11 [Tc1]). *P < .05.
Increased hematopoietic progenitor numbers in the spleen of Tc1 mice. Graphs showing mean (± SEM) frequency of CFU-MK, CFU-E, and CFU-GM progenitors in the spleen (A-B) or bone marrow (C-D) of Tc1 and wild-type mice aged 2 months (A,C) and older than 15 months (B,D; 2 months, n = ≥ 8 [Wt], ≥ 13 [Tc1]; > 15 months, n = 7 [Wt], 11 [Tc1]). *P < .05.
Expression of GATA1s in Tc1 mice does not cause leukemia
Although the appearance of extramedullary hematopoiesis in older Tc1 mice showed that the increased gene dosage of Hsa21 genes leads to perturbations of the hematopoietic system, the mice did not develop either AMKL, ALL, or a TMD-like phenotype. Because AMKL in DS is almost always characterized by a GATA1 mutation, which eliminates synthesis of wild-type GATA1 protein, leaving only GATA1s, we asked whether introduction of an analogous mutation into Tc1 mice would lead to TMD or AMKL. The Gata1Δe2 mutation is a deletion in the Gata1 gene of exon 2, which contains the normal ATG initiation codon used to synthesize wild-type GATA1.21 In mice bearing this mutant allele, translation initiates at an ATG in exon 3, resulting in exclusive synthesis of GATA1s. We crossed this mutation with Tc1 mice to generate male mice with 4 distinct genotypes: Gata1+/Y (wild type, Wt), Gata1+/Y; Tc1 (Tc1), Gata1Δe2/Y (Gata1s), and Gata1Δe2/Y;Tc1 (Tc1Gata1s).
As before, to determine whether Tc1Gata1s mice develop hematologic abnormalities, including TMD and leukemia, we measured complete blood counts on 10 to 15 mice of each of the 4 genotypes (Wt, Tc1, Gata1s, and Tc1Gata1s) in both neonates and at monthly intervals up to 60 weeks of age (Figure 7A-B). In neonatal mice, there were no changes in the white blood cell count in any of the genotypes. However the Gata1s mutation caused reduced numbers of red blood cells, an increase in MCV, and increased platelet counts (Figure 7A). These changes were not exacerbated in the Tc1Gata1s double mutant. Analysis of blood smears did not show evidence of any blast cells. In adult mice, we also did not see any changes in white blood cell counts, but, similar to Tc1 mice, Tc1Gata1s mice have reduced numbers of red blood cells and an increased mean corpuscular volume at most time points, compared with wild-type mice (Figure 7B). There was no noticeable increase in the severity of this macrocytic anemia in Tc1Gata1s compared with Tc1 mice. Once again, blood smears did not reveal any blasts, but showed platelet clouds in Tc1Gata1s mice, suggesting that, like Tc1 mice, they also have elevated numbers of platelets in the blood (not shown).
Gata1s expression does not lead to leukemia in Tc1 mice. (A) Graphs show mean (± SEM) red blood cell (RBC), white blood cell (WBC), and platelet counts and mean corpuscular volume (MCV) in neonatal mice of the indicated genotypes (n = 12 [Wt, Tc1], n = 8 [Gata1s, Tc1Gata1s]). (B) Graphs show mean (± SEM) RBC, WBC, and platelet counts and MCV as a function of age in mice of the indicated genotypes (n = 10-15). (C) Graph showing mean (± SEM) splenic weights of 15-month-old mice of the indicated genotypes (n = 12 [Wt], n = 7 [Tc1, Gata1s], n = 9 [Tc1Gata1s]). (D-E) Mean (± SEM) frequencies of CFU-MKs, CFU-Es, and CFU-GMs in the liver of (D) e11.5 and (E) e16.5 fetuses of the indicated genotypes (D: n = 8 [Wt, Tc1Gata1s], n = 10 [Tc1], n = 6 [Gata1s]; E: n = 7 [Wt], n = 6 [Tc1], n = 8 [Gata1s], n = 9 [Tc1Gata1s]). In panels A and C through E, significant differences relative to wild-type are indicated: *P < .05, **P < .01, ***P < .001.
Gata1s expression does not lead to leukemia in Tc1 mice. (A) Graphs show mean (± SEM) red blood cell (RBC), white blood cell (WBC), and platelet counts and mean corpuscular volume (MCV) in neonatal mice of the indicated genotypes (n = 12 [Wt, Tc1], n = 8 [Gata1s, Tc1Gata1s]). (B) Graphs show mean (± SEM) RBC, WBC, and platelet counts and MCV as a function of age in mice of the indicated genotypes (n = 10-15). (C) Graph showing mean (± SEM) splenic weights of 15-month-old mice of the indicated genotypes (n = 12 [Wt], n = 7 [Tc1, Gata1s], n = 9 [Tc1Gata1s]). (D-E) Mean (± SEM) frequencies of CFU-MKs, CFU-Es, and CFU-GMs in the liver of (D) e11.5 and (E) e16.5 fetuses of the indicated genotypes (D: n = 8 [Wt, Tc1Gata1s], n = 10 [Tc1], n = 6 [Gata1s]; E: n = 7 [Wt], n = 6 [Tc1], n = 8 [Gata1s], n = 9 [Tc1Gata1s]). In panels A and C through E, significant differences relative to wild-type are indicated: *P < .05, **P < .01, ***P < .001.
Examination of mice aged 15 months or older showed that, like Tc1 mice, many of the Gata1s and Tc1Gata1s mice also developed splenomegaly (Figure 7C) with disrupted red and white pulp architecture, and an infiltration of CD41+ megakaryocytes (supplemental Figure 2). Quantitative analysis demonstrated that Gata1s mice had elevated numbers of megakaryocytes in the spleen, which were further increased in Tc1Gata1s mice (Figure 2D). Examination of bone marrow histology in Gata1s and Tc1Gata1s mice showed normal structure and no evidence of myelodysplasia or myelofibrosis (supplemental Figure 2 and data not shown). However, although there was no increase in megakaryocytes in the bone marrow of Gata1s mice, Tc1Gata1s mice had significantly increased numbers relative to both wild-type and Gata1s mice (Figure 2D). Taken together, these data show that although the Gata1s mutation does not induce TMD or leukemia in Tc1 mice, it synergizes with the Tc1 mutation, leading to increased numbers of megakaryocytes in the spleen and bone marrow.
Increased megakaryocytic progenitors in the Tc1Gata1s fetal liver
The Gata1s mice have previously been shown to have transiently increased hyperproliferative megakaryocytic progenitors (CFU-MKs), with a peak in fetal liver at embryonic day 11.5 (e11.5), followed by a rapid decrease with only a few hyperproliferative CFU-MKs detectable by e16.5.21 This perturbation has been proposed to underlie the contribution of Gata1s to both TMD and AMKL, with trisomy Hsa21 synergizing with Gata1s to drive proliferation of megakaryocytic progenitors within the fetal liver.
To examine whether the Tc1 mutation could influence megakaryocytic or other myeloid progenitors, we measured the frequency of CFU-MKs, CFU-Es, and CFU-GMs in e11.5 and e16.5 livers from wild-type, Tc1, Gata1s, and Tc1Gata1s embryos. As previously reported, we saw a large increase in CFU-MK frequency in Gata1s e11.5 fetal livers compared with wild-type controls (Figure 7D). On its own, the Tc1 genotype did not affect the frequency of CFU-MKs, and did not have any additive effect with the Gata1s mutation, such that the frequency of CFU-MKs was similarly elevated in both Gata1s and Tc1Gata1s fetal livers. Similarly, e11.5 Tc1 embryos showed no change in CFU-E and CFU-GM frequencies, whereas both Gata1s and Tc1Gata1s embryos had increased frequencies of these progenitors (Figure 7D). Once again, the Tc1Gata1s embryos were not affected to a greater extent than Gata1s. By e16.5, the frequency of all these progenitors had decreased substantially, with no significant increases in the frequencies of any progenitors in either Tc1 or Gata1s embryos (Figure 7E). However, Tc1Gata1s embryos still had elevated frequencies of CFU-MKs, demonstrating a synergistic interaction between Tc1 and Gata1s mutations that causes the prolonged persistence of an increased frequency of CFU-MKs.
Discussion
Adult, but not neonatal, Tc1 mice developed extramedullary hematopoiesis characterized by increased megakaryopoiesis and erythropoiesis and increased numbers of splenic myeloid progenitors (CFU-GMs). In addition, the Tc1 mice also developed macrocytic anemia by 8 weeks of age, which persisted for at least a year, and had some evidence of thrombocytosis. Although there was no evidence for TMD or leukemia in Tc1 mice either at birth or later in life, we note that the changes seen in the megakaryocytic and erythroid lineages may nonetheless be related to the perturbations, which result in TMD and AMKL in human DS.
Comparison of Tc1 mice with 2 other mouse models of Down syndrome (Ts65Dn and Ts1Cje) shows both similarities and differences (supplemental Table 2).25,27 All 3 mouse strains have a macrocytic anemia, suggesting that this phenotype may be caused by increased gene dosage of one or more genes that are trisomic in all 3 strains. In contrast, only Tc1 and Ts65Dn mice have thrombocytosis and an increase in CD41+ splenocytes. Furthermore, only Tc1 mice have been reported to have splenomegaly, and only Ts65Dn mice show an increase in LSK and GMP progenitors in the bone marrow. These phenotypic differences could reflect the distinct sets of trisomic genes in these 3 mouse models (supplemental Table 1). Alternatively, some of the differences could be due to genetic background, because all 3 models were analyzed on different backgrounds.
The Tc1 model is trisomic for several genes that have been proposed to play an important role in DS leukemias, including the ETS family transcription factors ERG, ETS2, and GABPA (supplemental Table 1).8 A recent study has shown that overexpression of ERG leads to a megakaryoblastic leukemia in mice.31 Because Tc1 mice have 3 copies of ERG (2 mouse and 1 human), but no leukemia, we conclude that a 50% increase in the gene dosage of ERG is insufficient to induce leukemia. Nonetheless, this 50% increase in ERG dosage may contribute to the perturbations of megakaryocytic and erythroid lineages seen in Tc1 mice. Overexpression of ETS2 leads to increased expansion of megakaryocytes during in vitro culture of fetal liver progenitors,32 and thus increased dosage of this gene could also contribute to the increase in megakaryopoiesis in Tc1 mice. The RUNX1 gene has been suggested to be a dosage-sensitive gene on Hsa21, which contributes to DS-AMKL, because the RUNX1 protein cooperates with GATA1 during megakaryocyte differentiation.33 However, overexpression of RUNX1 cannot be responsible for the hematopoietic alterations found in Tc1 mice, because this gene is partially disrupted in the Hsa21 found in Tc1 mice (supplemental Table 1, and E.M.C.F. and V.L.J.T., unpublished results, December 2009). We note that a similar conclusion was reached in studies where a cross of the Ts65Dn mouse to mice heterozygous for a null allele of Runx1 reduced the Runx1 gene dosage from 3 to 2, but did not affect the myeloproliferative disorder in Ts65Dn mice.25 Finally, overexpression of microRNA-155, which is located on Hsa21, leads to splenomegaly and a myeloproliferative disorder characterized by increased numbers of megakaryocytes and erythroid progenitors in the spleen.34 The Hsa21 in Tc1 mice includes microRNA-155, and thus an increased dosage of this gene may contribute to the splenomegaly and extramedullary hematopoiesis in this strain.
Because we did not observe leukemia in Tc1 mice, we crossed the strain to mice bearing the Gata1Δe2 mutation to force the expression of Gata1s, and thereby potentially increase the chance of observing disease. We observed a synergistic interaction between the Tc1 and Gata1s mutations, resulting in increased CFU-MKs in the fetal liver, and increased numbers of megakaryocytes in the spleen and bone marrow of old mice. However, the combination of the Gata1s mutation with the Tc1 strain still did not result in detectable TMD or leukemia. We conclude that Tc1 mice may not be trisomic for 1 or more genes, which are required in 3 copies for the development of leukemia. Such genes would be in the 17% of Hsa21 genes not present in Tc1 mice (supplemental Table 1). Possible candidate genes include CXADR, SON, and GCFC, all of which are up-regulated in DS-AMKL compared with non–DS-AMKL,35 but are not trisomic in Tc1 mice. This possibility will need to be tested with more complete models of DS, trisomic for all Hsa21 genes, when they become available. Alternatively, development of AMKL or ALL in the Tc1 mouse model may require additional mutations, whose frequency in the mouse is not high enough to generate detectable rates of disease. For example, mutations in JAK2, JAK3, FLT3, and TP53 have been reported in DS-AMKL, and mutations in JAK2 have been found in DS-ALL.8 This could be tested by ectopically expressing such mutant genes in either Tc1 or Tc1Gata1s mice. Finally, it remains conceivable that it may not be possible to model TMD, DS-AMKL, or DS-ALL in the mouse because of subtle differences in the transcriptional networks regulating hematopoiesis in humans and mice.
In summary, the Tc1 mouse strain shows hematopoietic abnormalities in the megakaryocytic and erythroid lineages, which may underlie the TMD and AMKL found in human DS. Further studies are needed to identify the specific dosage-sensitive genes on Hsa21 that contribute to this pathology.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Matilda Haas and David Roper for photography, Elena Grigorieva for histology, and Hannah Boyes, Michelle Dodd, and Biological Services at National Institute for Medical Research for animal husbandry.
This work was supported by the Leukemia Research Fund (grant no. 06003) and the Medical Research Council (program no. U117527252).
Authorship
Contribution: K.A.A. designed and performed research, analyzed and interpreted data, performed statistical analysis, and wrote the paper; A.S. and L.V. performed research, and analyzed and interpreted data; E.M.C.F., Z.L., and S.H.O. contributed vital reagents; D.N. designed research; I.R. analyzed and interpreted data; and V.L.J.T. designed research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Victor L. J. Tybulewicz, National Institute for Medical Research, The Ridgeway, Mill Hill, London, NW7 1AA, United Kingdom; e-mail: vtybule@nimr.mrc.ac.uk.

![Figure 2. Splenomegaly and extramedullary hematopoiesis in Tc1 mice older than 15 months. (A) Photograph shows appearance of an enlarged spleen found in a Tc1 mouse aged 15 months, alongside a spleen from a wild-type (Wt) age-matched control mouse. Size marker indicates 1 cm. (B) Graph shows weights of spleens taken from mice aged 15 months or older. Horizontal bar indicates the mean. (C) Graphs showing mean (± SEM) total number of cells in the spleen and bone marrow of mice of the indicated genotypes and ages (n = 6-11). (D) Graphs showing mean (± SEM) number of megakaryocytes per microscope field (40× objective) in mice aged older than 15 months (n = 5). (E) Histograms of flow cytometric analysis of surface levels of Ter119 expression on spleen and bone marrow cells from a Wt mouse at 2 months of age. Numbers indicate percentage of cells falling into the indicated markers. Graphs show mean (± SEM) numbers of Ter119+ cells in the spleens and bone marrow of mice of the indicated ages and genotypes (2 months, n = 11 [Wt], n = 10 [Tc1]; > 15 months, n = 7 [Wt], n = 11 [Tc1]). *P < .05, **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/14/10.1182_blood-2009-06-227629/4/m_zh89991051110002.jpeg?Expires=1769131115&Signature=LyOZDXLp8cpLpIz2lTxEMPGqP8B9Yi9KqzLvnQDwY4ru820ePn9so5rSC2n5wmUI6yfKQV8xrpf9Bqqo5E6Atm7O6zOvQ48TrtXaPiMp~gmLWsI5X2BmLynSxGlIK5SNYfD0lG4ACgdc5TC~meM~aMM6aKEmHVVD2wP1hrtgLMSilz0SdrvfrZBZaYwvS~-EeJN6C5ULAVONkwOHv-BrEKkvizumiAFR~tm65j7Gsiclqvq5V-e1s9yIlqtASh03K8QxZIfZAtOPCgGqJfqmr2h8KYv~iTdkvKMJCR3jvEAxn~6Vxo9l2UzLDXH-yeA6D9862TAKSqavy268J9cUHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Analysis of hematopoietic progenitors in Tc1 mice. (A) Two-dimensional contour plots of bone marrow cells showing flow cytometric analysis used to identify hematopoietic progenitors. Numbers indicate percentage of cells falling into the gates. Lineage− cells were separated by expression of cKit and Sca1, and the earliest progenitors were identified as Lineage−Sca1+cKit+ (LSK). Lineage−Sca1−cKit+ cells were further subdivided by expression of FcγR and CD34 into common myeloid progenitors (CMPs, CD34+FcγR−), the granulocyte-macrophage progenitors (GMPs, CD34+FcγR+), and the megakaryocyte-erythroid progenitor (MEPs, CD34−FcγR−). Note that before flow cytometric analysis, Lineage+ cells had been depleted, to enrich for Lineage− cells, thus explaining the apparently high percentage of Lineage− cells. (B-D) Graphs showing mean (± SEM) percentage of hematopoietic progenitors in the spleen (B-C) and bone marrow (D-E) of Tc1 and wild-type mice aged 2 months (B,D) and older than 15 months (C,E; 2 months, n = 8 [Wt], n = 13 [Tc1]; > 15 months, n = 7 [Wt], n = 11 [Tc1]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/14/10.1182_blood-2009-06-227629/4/m_zh89991051110005.jpeg?Expires=1769131115&Signature=Whw0C3LgCGORVhz-qlsEvaHdpAjKiiuVmLnYdbWKJ68YNWRcITo3JrYAmRmpExG088ZlvxHQofyKnFN9ghqoWT4GifsELHyfqv6ih9yl~-ys774K-VRW4y4INyP7qhV4j7-OQKqF6gLYpZCcX6-T-~SkDGk8LGjmaNW7N15S4GEeF~WPArHttGEPPHa80fVH4Dy0mLLePGB2A1Ab8PgYsm5Jq8K-Qwm9rAh~dtGOv6uMh27n7n~~1iE-Stuius3uOMATWqtOAHkYImzhfkNTwIlFm4ZhHlT3ldkPSkCBoR5LUR~fQoe-8lyb4B3R6Qzf4FmvTeNV9PzPbPwH8Trgdw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Increased hematopoietic progenitor numbers in the spleen of Tc1 mice. Graphs showing mean (± SEM) frequency of CFU-MK, CFU-E, and CFU-GM progenitors in the spleen (A-B) or bone marrow (C-D) of Tc1 and wild-type mice aged 2 months (A,C) and older than 15 months (B,D; 2 months, n = ≥ 8 [Wt], ≥ 13 [Tc1]; > 15 months, n = 7 [Wt], 11 [Tc1]). *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/14/10.1182_blood-2009-06-227629/4/m_zh89991051110006.jpeg?Expires=1769131115&Signature=enIrvqgl0zToqvrx5wlVr7oOUKxoNLg74TfS4ahRAF9iPa4m7JRNW5FqsFE7a~QI33ITuJhq9F~Tlu~Ivb~iHiIhms~6fwUGcJn38OmOQLTnBTNzUwPiABzLbmzVI7-iZCONN8bxg0i4AxAe-d~-QGGDtegm-m2KYwiPAT128XIM9pRapcanK8SZe~aGHAUdtQkkXJqhVK8zqsQQK08xdsHsevenvsNuELvaxfHcKmBpPwBSLlqUW~4-uV-75xfu7wQyfZ1wwTIeVoloMLc5EiTAUCFRPSsxewR3WOQAOuWfdHY6j4QPgbNN~MPjWCb1wnFX18TrZsK2z26neO1SMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Gata1s expression does not lead to leukemia in Tc1 mice. (A) Graphs show mean (± SEM) red blood cell (RBC), white blood cell (WBC), and platelet counts and mean corpuscular volume (MCV) in neonatal mice of the indicated genotypes (n = 12 [Wt, Tc1], n = 8 [Gata1s, Tc1Gata1s]). (B) Graphs show mean (± SEM) RBC, WBC, and platelet counts and MCV as a function of age in mice of the indicated genotypes (n = 10-15). (C) Graph showing mean (± SEM) splenic weights of 15-month-old mice of the indicated genotypes (n = 12 [Wt], n = 7 [Tc1, Gata1s], n = 9 [Tc1Gata1s]). (D-E) Mean (± SEM) frequencies of CFU-MKs, CFU-Es, and CFU-GMs in the liver of (D) e11.5 and (E) e16.5 fetuses of the indicated genotypes (D: n = 8 [Wt, Tc1Gata1s], n = 10 [Tc1], n = 6 [Gata1s]; E: n = 7 [Wt], n = 6 [Tc1], n = 8 [Gata1s], n = 9 [Tc1Gata1s]). In panels A and C through E, significant differences relative to wild-type are indicated: *P < .05, **P < .01, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/14/10.1182_blood-2009-06-227629/4/m_zh89991051110007.jpeg?Expires=1769131115&Signature=2iyVeZSGxQRJe9qxfZmz-1Bb-ikJIs~xU9jDZ3PukfjM7VJ6xh9-kArn089tUGF2r6DihYtqwlYTyd7GYxy5No8jWqZChhOrT~H~t2ZlvybVIMXC0FleJAgW0AC2peLQ8-keJoZ1tQIgDRqdW~gxSbcVChQKyhRPWl5hJ31J7VsD5WrpQ28xqd9gYW-8SAE4v197pqHZnraDwn7hy1L-RjFcs8MnwSveXL189PmK-f~z5SzYOB-TOyxaX6Jteh~8LYPU-rgPTT82TPMF5gyBNQ6YSwgYBYXq4sppynrRDJFciYDE4y4BJNVVLN12R8T~QFGaXo46Ea-359tAI8SoSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
