Abstract
We analyzed the involvement of Wiskott-Aldrich syndrome protein (WASp), a critical regulator of actin cytoskeleton remodeling, in the control of natural killer (NK)–cell migration. NK cells derived from patients with Wiskott-Aldrich syndrome/X-linked thrombocytopenia (WAS/XLT), carrying different mutations in the WASP coding gene, displayed reduced migration through intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), or endothelial cells in response to CXCL12/stromal cell–derived factor-1 and CX3CL1/fractalkine. Inhibition of WAS/XLT NK-cell migration was associated with reduced ability of these cells to up-regulate the expression of CD18 activation neoepitope and to adhere to ICAM-1 or VCAM-1 following chemokine stimulation. Moreover, chemokine receptor or β1 or β2 integrin engagement on NK cells rapidly resulted in Cdc42 activation and WASp tyrosine phosphorylation as well as in WASp association with Fyn and Pyk-2 tyrosine kinases. NK-cell pretreatment with wiskostatin, to prevent Cdc42/WASp association, impaired chemokine-induced NK-cell migration through ICAM-1 and β2 integrin activation-dependent neoepitope expression. These results show that the Cdc42/WASp pathway plays a crucial role in the regulation of NK-cell migration by acting as a critical component of the chemokine-induced inside-out signaling that regulates lymphocyte function–associated antigen-1 function and suggest that after integrin or chemokine receptor engagement WASp function is regulated by the coordinate action of both Cdc42 and tyrosine kinases.
Introduction
The Wiskott-Aldrich syndrome protein (WASp) is a multidomain member of the WASp/suppressor of cAMP receptor/WASP family verprolin-homologous protein family whose expression is restricted to hematopoietic cells. WASp has been identified as one of the main regulators of actin cytoskeletal dynamics.1,2 WASp was initially discovered as the product of the gene whose mutations are responsible for the Wiskott-Aldrich syndrome (WAS), a rare X-linked recessive primary immunodeficiency characterized by eczema, thrombocytopenia, and impaired cellular and humoral immunity.3-6 This syndrome is highly heterogeneous in terms of clinical severity with some cases showing an attenuated clinical and immunologic phenotype referred to as X-linked thrombocytopenia (XLT). Most of the patients with XLT have missense mutations in exons 1 and 2 of the WASP gene, leading to decreased but detectable levels of protein expression. In contrast, WASP mutations that lead to total absence of WASp are usually associated with a more severe clinical phenotype.
In resting cells, WASp is found in a closed, autoinhibited form in which the guanosine triphosphate (GTPase) binding domain is bound to the carboxyl-terminal verprolin homology, cofilin homology, acidic region.1,2,7 After cell activation, WASp interacts with the GTP-bound form of the small Rho GTPase Cdc42 by its GTPase binding domain; this association results in a conformational change that allows WASp interaction with the Arp2/3 complex, thus initiating actin polymerization. WASp also contains a proline-rich region that is responsible for its association with several tyrosine kinases and adaptor proteins. Moreover, the N-terminal located at ENA-Vasp homology 1 domain binds to the WASp-interacting protein, known to modulate WASp functions. The ability of WASp to interact with and activate the Arp2/3 complex is regulated in a cooperative manner by the GTP-bound form of Cdc42 and the phosphatidylinositol 4,5 bisphosphate. Moreover, it has been reported that WASp functional activation is also enhanced by its tyrosine phosphorylation after ligation of immune or adhesion receptors.8-11 Because of its ability to induce cytoskeletal remodeling, WASp has been implicated in the regulation of many cellular functions, including T-cell activation and proliferation, phagocytosis, cell migration, and chemotaxis.1,2 Moreover, we and others have reported that patients with WAS or XLT phenotype are impaired in both natural and CD16-mediated natural killer (NK)–cell cytotoxicity as a result of disturbed cytoskeletal reorganization.12,13
NK cells are a CD3−CD16+CD56+ lymphocyte subpopulation that plays an important role in the early phase of immune responses against viruses, parasites, and microbial pathogens by exhibiting cytotoxic functions and secreting a number of cytokines and chemokines.14,15
NK cells are mainly found in the peripheral blood, but they are also present in several lymphoid and nonlymphoid organs such as spleen, lymph nodes, tonsils, liver, lungs, intestine, and uterus.14,16,17 During an inflammatory response, NK cells are rapidly recruited from blood and accumulate in the parenchyma of injured organs where they kill target cells and release inflammatory cytokines and chemokines, thus participating in the recruitment and activation of other leukocytes and in the modulation of immune cell functions.14,15,18-20
NK-cell recruitment from blood to inflamed tissues is a complex, multistep process regulated by a plethora of chemokines and adhesive molecules belonging to the selectin, integrin, and immunoglobulin families.21-24
Chemokines properly guide leukocyte recruitment and positioning into healthy or diseased tissues by interacting with 7-transmembrane-domain receptors and initiating a complex signaling cascade that governs leukocyte migration also through dynamic regulation of integrin adhesiveness.25-27 Despite the well-established role of chemokines in the regulation of integrin function, the chemokine-triggered intracellular signaling events that lead to up-regulation of integrin adhesive function have not yet been fully established. A role for phospholipase C signaling pathway, activation of small GTPases (Rap-1 and RhoA), and guanine nucleotide exchange factor (GEF) as well as the transient modulation of integrin conformation through the association with actin-binding proteins such as talin-1, α-actin, and L-plastin has been reported. Integrins too contribute to regulate migration by mediating adhesion and triggering outside-in signals.28,29
Thus, the propagation of migratory signals requires a fine remodeling of actin, myosin, and other cytoskeleton components to ensure the dynamic redistribution of both integrins and chemokine receptors and to provide a structural framework in which the signaling molecules activated by chemokine receptors and integrins redistribute and organize to efficiently control leukocyte migration and polarization.
Previous studies have shown that the immunologic disturbance observed in patients with WAS is at least in part attributable to defects in cell migration and tissue localization of macrophages, neutrophils, dendritic cells, T cells, and B cells, thus supporting a crucial role for WASp in the regulation of these functions.30-35 However, no evidence has been provided so far on the role of Cdc42/WASp signaling pathway in the control of human NK-cell migration or on its involvement in the regulation of chemokine receptor-induced integrin adhesive function.
In this study we demonstrated an impaired migratory ability of NK cells from patients carrying different mutations of the WASP gene, thus indicating a crucial role for WASp in the control of NK-cell migration. Moreover, we provide information on the molecular mechanisms involved in the regulation of WASp function during NK-cell migration as well as on the role of Cdc42/WASp pathway in the chemokine receptor inside-out signal leading to modulation of integrin adhesiveness.
Methods
Patients
Nine patients with molecularly defined WAS/XLT were included in this study. Informed consent was obtained from all subjects included in the study in accordance with the principles enunciated in the Declaration of Helsinki and according to protocols approved by the Department of Pediatrics of Spedali Civili of Brescia. The clinical phenotype was evaluated according to the score proposed by Zhu et al.4 Patients whose clinical score was less than or equal to 2 were classified as having XLT, whereas those with a score of 3 or greater were considered to have typical WAS.
To evaluate mutation in the WASP locus, genomic DNA was extracted from patient peripheral blood lymphocytes. Amplification of each of the 12 exons and flanking splice-sites at the WASP locus was performed as previously described.36 Mutation analysis was accomplished by single-strand conformation polymorphism or denaturing high-performance liquid chromatography and direct sequencing with the use of the ABI Prism 310 sequencer (Applied Biosystem).
WASp protein expression was analyzed by cytofluorimetric analysis using anti-WASp 5A5 monoclonal antibody (mAb) or by immunoblotting analysis as previously described.13
The clinical and molecular features of the patients are reported in Table 1.
Antibodies and reagents
The following mouse mAbs were used: anti-CD3, anti-CD56, anti-CD16, anti-WASp (5A5) were purchased from BD Biosciences; anti-CD56 (C218) was kindly provided by Dr A. Moretta (University of Genoa); anti-β2 (TS1/18), anti-β1 (TS2/16), and anti-α4 (HP2/1) integrin subunits were a generous gift of Dr F. Sanchez-Madrid (La Princesa Hospital, University of Madrid); the anti-β2 mAb (327C), recognizing the activation epitope of β2 integrin, was kindly provided by ICOS37 ; anti-phosphotyrosine (anti-pTyr; 4G10) and anti-Cdc42 (17-299) were purchased from Upstate Biotechnology Inc; anti-WASp (D-1) and anti-Fyn (clone 15) were purchased from Santa Cruz Biotechnology Inc.
Affinity-purified goat antisera against Pyk2 (C19 and N19) were purchased from Santa Cruz Biotechnology Inc; affinity-purified (Fab′)2 fragments of goat anti–mouse immunoglobulin unconjugated (GAM) and fluorescein isothiocyanate (FITC)–conjugated (GAM-FITC) were purchased from Cooper Biomedical Inc. Rabbit antisera against human WASp (H-250) or Fyn (FYN3) were purchased from Santa Cruz Biotechnology Inc. CXCL12/ stromal cell–derived factor-1 (SDF-1), CX3CL1/fractalkine, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) were purchased from R&D Systems.
Human NK-cell preparation
Patients' or healthy donors' peripheral blood mononuclear cells (PBMCs; 4 × 105 cells) were isolated by Lymphoprep (Nycomed AS) gradient centrifugation and then cocultured for 10 days with irradiated (30 Gy [3000 rad]) Epstein-Barr virus–transformed B-cell line RPMI 8866 (105 cells) at 37°C as previously described.13,38 On day 10, the cell population was routinely greater than 90% CD56+CD16+CD3−, as assessed by immunofluorescence and cytofluorimetric analysis. When purity was less than 90%, contaminant T cells were eliminated by immunomagnetic negative selection with anti-CD3 mAb, and the purity of the resulting NK-cell population was greater than 95%. For some experiments total PBMCs and freshly isolated highly purified NK cells obtained by magnetic-activated cell sorting human NK cell isolation kit (Miltenyi Biotec) were used.
Migration assay
Cell migration was measured with the use of a Transwell migration chamber (diameter insert, 6.5 mm; pore size, 5 μm; Costar Corporation). Highly purified cultured NK cells from either healthy donors or patients with WAS/XLT (W27, W28, W29, W19, W35) washed extensively and starved for 18 hours in complete medium (RPMI 1640 + 10% fetal calf serum), or freshly isolated highly purified NK cells from patient W2, were assayed for their ability to migrate through 5 μg/mL ICAM-1– or VCAM-1–precoated filters. As chemoattractant, CXCL12/SDF-1 or CX3CL1/fractalkine was added in the lower compartment at a concentration that gave the maximal response (10nM and 1nM, respectively). After 90 minutes at 37°C, the number of migrated cells was evaluated using the FACSCalibur cytofluorimeter (BD Biosciences); data are expressed as the mean plus or minus SD of migrated cell percentage obtained from 2 different determinations. In some experiments, migration assay was performed with NK cells pretreated for 60 minutes with different concentrations of wiskostatin (Calbiochem) that do not affect NK-cell viability.
For transendothelial migration assay, freshly isolated PBMCs from healthy donors or patients with WAS/XLT were allowed to migrate through a monolayer of tumor necrosis factor-α (TNFα)–activated human umbilical vein endothelial cells on transwell filters in response to the above-mentioned chemokines. After 60 minutes at 37°C, the number of migrated PBMCs was evaluated by fluorescence-activated cell sorting (FACS), and the CD56+CD3− NK-cell percentage was assessed by immunofluorescence and cytofluorimetric analysis. The percentage of migrated NK cells was calculated as follows: number of migrated NK cells/number of input NK cells × 100.
Cdc42 activation assay
NK cells were stimulated with mAb directed against β1, β2, or α4 integrin subunits, or with integrin ligands (VCAM-1, ICAM-1), or with chemokines (CXCL12/SDF-1 or CX3CL1/fractalkine) for different lengths of time at 37°C as previously reported.13 After stimulation, cells were lysed in 1% Triton X-100, 0.1% sodium deoxycholate, 1mM EDTA, 1mM EGTA, 150mM NaCl, 1mM phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, 2 μg/mL leupeptin, 100mM NaF, 1mM Na3VO4, 50mM Na4P2O7 in 50mM Tris, pH 7.5. Equal amounts of protein from cell lysates were then incubated with glutathione-S-transferase–p21-activated kinase (GST-PAK) fusion protein (kindly provided by Dr J.G. Collard, The Netherlands Cancer Institute) bound to glutathione-coupled Sepharose beads at 4°C for 30 minutes, and bound active GTP-Cdc42 molecules were analyzed by Western blotting with the use of an anti-Cdc42 mAb.
WASp tyrosine phosphorylation and tyrosine kinase coimmunoprecipitation
NK cells (4 × 107 cells/300 μL/tube) were stimulated for different lengths of time with saturating doses of the appropriate mAb followed by affinity-purified GAM secondary Ab, or with VCAM-1, ICAM-1, or chemokines (CXCL12/SDF-1, CX3CL1/fractalkine) at 37°C. Stimulation was stopped by adding ice-cold phosphate-buffered saline and pelleting the cells for 5 minutes at 500g. Cells were lysed for 30 minutes at 4°C in ice-cold lysis buffer containing 1% (vol/vol) Triton X-100, 1mM CaCl2, 1mM MgCl2, 0.1% NaN3, 1mM phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, 2 μg/mL leupeptin, 10mM NaF, 150mM NaCl, 10mM iodoacetamide, 1mM Na3VO4, in 50mM Tris, pH 7.5. Cell lysates were centrifuged at 15000g for 15 minutes at 4°C, and the supernatants were subjected to immunoprecipitation and immunoblotting analysis.
Chemokine-induced expression of β2 integrin activation neoepitope
PBMCs from patients with XLT or healthy donors were stimulated for different lengths of time with CXCL12/SDF-1 (10nM), CX3CL1/fractalkine (1nM), or control medium and simultaneously stained with anti-β2 (327C) mAb (ICOS) at 37°C. Cells were then incubated with GAM-FITC for 30 minutes at 4°C and for an additional 15 minutes with normal mouse serum, and then anti-CD3 and anti-CD56 fluorochrome-conjugated mAbs were added for an additional 30 minutes at 4°C. In some experiments, wiskostatin-pretreated (10μM for 60 minutes at 37°C) healthy donor NK cells were used. Stained cells were analyzed by flow cytofluorimeter. Histograms shown were obtained by applying a gate on CD56+CD3− NK cells. The ratio of mean fluorescence intensity of β2 neoepitope between stimulated and unstimulated NK cells is shown.
Results
Human NK-cell migration through ICAM-1, VCAM-1, or endothelial cells is impaired in patients with XLT and patients with WAS
The propagation of migratory signals elicited by chemokine receptors and integrins depends on a complex interplay between effector molecules able to regulate actin, myosin, and other cytoskeleton components necessary for the correct leukocyte polarization.29 The ability of WASp to regulate the Arp2/3 actin nucleating activity and to interact with many signaling molecules prompted us to evaluate its involvement in the control of NK-cell migration.
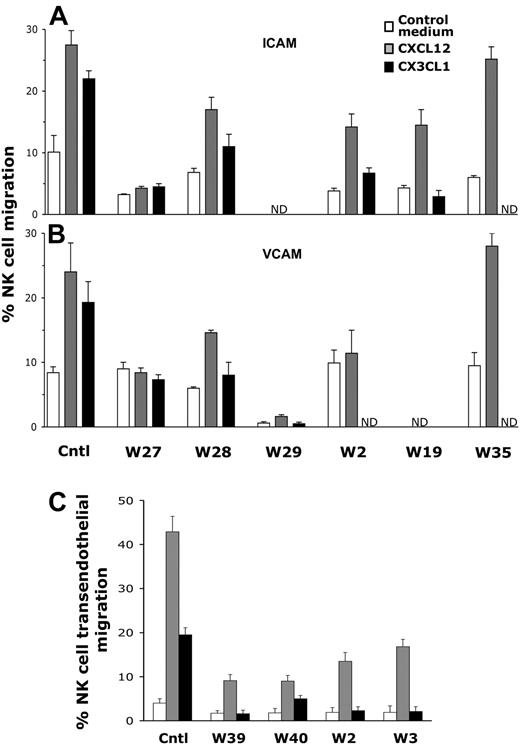
To address this question, we used NK cells derived from patients carrying different mutations of WASP, some of which are responsible for an attenuate form of WAS referred to as XLT, whereas 3 mutations were associated with the classical WAS phenotype (Table 1). Most patients with XLT had missense mutations within the exon 1 and 2, leading to decreased but detectable protein expression levels; by contrast, patients with classical WAS (W27, W39, W40) carried mutations that abrogate WASp expression. To evaluate NK-cell migration through ICAM-1 or VCAM-1, in vitro–expanded WAS/XLT NK cells, washed extensively and starved for 18 hours, or highly purified freshly isolated W2 NK cells obtained by immunomagnetic depletion were used. NK cells were then assayed for their ability to migrate through ICAM-1– or VCAM-1–precoated filters in response to chemokines such as CXCL12/SDF-1 or CX3CL1/fractalkine, known to drive NK-cell migration as previously described.39 As shown in Figure 1, CXCL12/SDF-1– or CX3CL1/fractalkine-induced NK-cell migration through both ICAM-1 (Figure 1A) or VCAM-1 (Figure 1B) was reduced in most patients with WASP mutations, regardless of the disease severity. However, migration of NK cells from 1 patient (W35) was comparable with that of healthy donors. The migratory ability of patient NK cells through ICAM-1 or VCAM-1 in response to CX3CL1/fractalkine or CXCL12/SDF-1 was differently inhibited; because migration in response to CX3CL1/fractalkine was almost completely abolished, whereas that elicited by CXCL12/SDF-1 was reduced approximately 50%. However, because the basal migration of some patient NK cells was lower than that of healthy donors, we also calculated the chemokine-induced fold-increase migration (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). On the basis of this data representation, we found that CX3CL1/fractalkine-induced WAS/XLT NK-cell migration through both ligands resulted in impaired compared with that of healthy donors; CXCL12/SDF-1–induced migration through ICAM-1 was clearly inhibited for W27 and only marginally affected for W28 NK cells, whereas that through VCAM-1 was strongly inhibited for W27, W29, and W2 and to a lesser extent for patient W28. CXCL12/SDF-1–induced NK cell chemotactic response through bovine serum albumin (BSA) used as control substrate was impaired in 3 (W27, W28, W29) of 6 patients (supplemental Figure 2).
NK-cell migration in response to chemokines is impaired in patients with XLT and patients with WAS. (A-B) Cultured NK cells from healthy donors or patients with WAS/XLT (W27, W28, W29, W19, W35) or highly purified freshly isolated W2 NK cells were assayed for their ability to migrate through ICAM-1 (5 μg/mL)–, or VCAM-1 (5 μg/mL)–precoated polycarbonate filters using CX3CL1/fractalkine (1nM) or CXCL12/SDF-1 (10nM) as chemoattractants. (C) Freshly isolated PBMCs from healthy donors or patients with WAS/XLT were allowed to migrate through a monolayer of TNFα (10 ng/mL)–activated endothelial cells on transwell filters in response to CXCL12/SDF-1 (1nM) or CX3CL1/fractalkine (1nM). After 1 hour of incubation, the number of migrated PBMCs was evaluated by FACS; the percentage of CD56+CD3− NK cells in input and migrated cells was assessed by immunofluorescence and FACS analysis. The percentage of migrated NK cells was calculated as follows: number of migrated NK cells/number of input NK cells × 100. Data from patients with WAS/XLT are expressed as the mean ± SD of the percentage of migrated cells obtained from 2 independent determinations. Control (Cntl) value represent the mean ± SD of the percentage of NK-cell migration obtained from 6 (A-B) or 4 healthy donors (C). ND indicates not determined. Statistical analysis performed by Student t test comparing the mean percentage of XLT/WAS NK-cell migration with that of control donors indicates that the inhibition of migration observed in all patients with WAS/XLT except for W35 is statistically significant (P < .02).
NK-cell migration in response to chemokines is impaired in patients with XLT and patients with WAS. (A-B) Cultured NK cells from healthy donors or patients with WAS/XLT (W27, W28, W29, W19, W35) or highly purified freshly isolated W2 NK cells were assayed for their ability to migrate through ICAM-1 (5 μg/mL)–, or VCAM-1 (5 μg/mL)–precoated polycarbonate filters using CX3CL1/fractalkine (1nM) or CXCL12/SDF-1 (10nM) as chemoattractants. (C) Freshly isolated PBMCs from healthy donors or patients with WAS/XLT were allowed to migrate through a monolayer of TNFα (10 ng/mL)–activated endothelial cells on transwell filters in response to CXCL12/SDF-1 (1nM) or CX3CL1/fractalkine (1nM). After 1 hour of incubation, the number of migrated PBMCs was evaluated by FACS; the percentage of CD56+CD3− NK cells in input and migrated cells was assessed by immunofluorescence and FACS analysis. The percentage of migrated NK cells was calculated as follows: number of migrated NK cells/number of input NK cells × 100. Data from patients with WAS/XLT are expressed as the mean ± SD of the percentage of migrated cells obtained from 2 independent determinations. Control (Cntl) value represent the mean ± SD of the percentage of NK-cell migration obtained from 6 (A-B) or 4 healthy donors (C). ND indicates not determined. Statistical analysis performed by Student t test comparing the mean percentage of XLT/WAS NK-cell migration with that of control donors indicates that the inhibition of migration observed in all patients with WAS/XLT except for W35 is statistically significant (P < .02).
To strengthen our observations, we also performed transendothelial migration assay using freshly isolated PBMCs from heathy donors or patients with WAS/XLT. As shown in Figure 1C and supplemental Figure 1C, in patients with WAS/XLT NK-cell migration through TNFα-activated endothelial cells was consistently reduced in response to both CXCL12/SDF-1 and CX3CL1/fractalkine.
The inhibition of NK-cell migration observed in patients with WAS/XLT was not attributable to differences in the chemokine receptor or β1 or β2 integrin expression because unstimulated NK cells from patients and healthy donors had similar levels of integrin and chemokine receptor expression (data not shown).
Altogether, these data show that WAS/XLT NK cells have a defective chemokine-induced migratory response as well as an impaired motility and clearly indicate a key role for WASp in the control of NK-cell transendothelial migration.
Integrin and chemokine receptor engagement on human NK cells results in Cdc42 activation and WASp tyrosine phosphorylation
The induction of WASp effector functions requires its release from the autoinhibitory structural form and has been mainly attributed to the cooperative action of the GTP-bound form of the Rho family GTPase Cdc42 and phosphatidylinositol 4,5 bisphosphate, as well as to WASp tyrosine phosphorylation.7-11,40,41
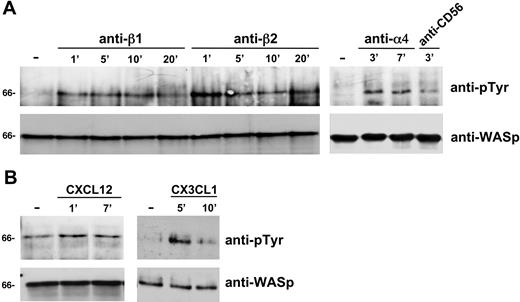
In an attempt to investigate whether Cdc42 could be involved in the regulation of WASp function during human NK-cell migration, we first analyzed Cdc42 activation on integrin or chemokine receptor engagement. To this purpose, NK cells were stimulated with anti-α4, anti-β1, or anti-β2 integrin mAb, or with VCAM-1 or ICAM-1 integrin natural ligands, or with CXCL12/SDF-1 or CX3CL1/fractalkine. Cell lysates from stimulated or unstimulated cells were then subjected to pull-down assay using a GST-PAK fusion protein that specifically binds to the active form of Cdc42. As shown in Figure 2, NK-cell stimulation with mAbs against α4, β1, or β2 integrin subunits (Figure 2A) or with VCAM-1 or ICAM-1 (Figure 2B) results in Cdc42 activation that was already evident at 30 seconds and still persisted at 30 minutes after stimulation. Similarly, NK-cell stimulation with CXCL12/SDF-1 or CX3CL1/fractalkine (Figure 2C) results in rapid activation of Cdc42 that was still evident at 30 minutes after stimulation. NK-cell stimulation with anti-CD56 mAb or BSA used as control did not significantly affect Cdc42 activation (Figure 2A-B).
Integrin or chemokine receptor engagement on human NK cells results in Cdc42 activation. Human NK cells were left untreated (−) or stimulated with GAM cross-linked anti-α4 (HP2/1), anti-β1 (TS2/16), anti-β2 (TS1/18), or anti-CD56 (C218) mAb (A) or with VCAM-1–, ICAM-1– or BSA-coated beads (B) or with CXCL12/SDF-1 or CX3CL1/fractalkine (C) for the indicated time periods at 37°C. Cell lysates were incubated with GST-PAK fusion protein, and bound active GTP-Cdc42 molecules were evaluated by Western blotting with anti-Cdc42 mAb (top). Cell lysates probed for total Cdc42 are shown as loading control (bottom). Sizes are indicated in kilodaltons. These results represent 1 of 3 independent experiments.
Integrin or chemokine receptor engagement on human NK cells results in Cdc42 activation. Human NK cells were left untreated (−) or stimulated with GAM cross-linked anti-α4 (HP2/1), anti-β1 (TS2/16), anti-β2 (TS1/18), or anti-CD56 (C218) mAb (A) or with VCAM-1–, ICAM-1– or BSA-coated beads (B) or with CXCL12/SDF-1 or CX3CL1/fractalkine (C) for the indicated time periods at 37°C. Cell lysates were incubated with GST-PAK fusion protein, and bound active GTP-Cdc42 molecules were evaluated by Western blotting with anti-Cdc42 mAb (top). Cell lysates probed for total Cdc42 are shown as loading control (bottom). Sizes are indicated in kilodaltons. These results represent 1 of 3 independent experiments.
We have previously reported that CD16 or β2 integrin ligation on human NK cells results in the induction of WASp tyrosine phosphorylation.13 Here, we analyzed whether WASp could undergo tyrosine phosphorylation on NK-cell stimulation through β1 or α4 integrin subunits or on chemokine receptor engagement. The data obtained show that, as for β2, also β1 and α4 integrin ligation on human NK cells (Figure 3A) as well as CXCL12/SDF-1 or CX3CL1/fractalkine NK-cell stimulation (Figure 3B) rapidly (1 minute) enhances WASp tyrosine phosphorylation. NK-cell treatment with anti-CD56 control mAb did not significantly affect the tyrosine phosphorylation status of WASp (Figure 3A).
Integrin or chemokine receptor engagement on human NK cells enhances WASp tyrosine phosphorylation status. Human NK cells were left untreated (-) or incubated with GAM cross-linked anti-β1 (TS2/16), anti-β2 (TS1/18), anti-α4 (HP2/1), or anti-CD56 (C218) mAb (A) or stimulated with CXCL12/SDF-1, CX3CL1/fractalkine (B) for the indicated time periods at 37°C. Cell lysates were then immunoprecipitated with anti-WASp mAb. The resulting immunocomplexes were resolved by 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and sequentially immunoblotted with anti-pTyr mAb (top) and anti-WASp antiserum (bottom). Sizes are indicated in kilodaltons. These results represent 1 of 3 independent experiments.
Integrin or chemokine receptor engagement on human NK cells enhances WASp tyrosine phosphorylation status. Human NK cells were left untreated (-) or incubated with GAM cross-linked anti-β1 (TS2/16), anti-β2 (TS1/18), anti-α4 (HP2/1), or anti-CD56 (C218) mAb (A) or stimulated with CXCL12/SDF-1, CX3CL1/fractalkine (B) for the indicated time periods at 37°C. Cell lysates were then immunoprecipitated with anti-WASp mAb. The resulting immunocomplexes were resolved by 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and sequentially immunoblotted with anti-pTyr mAb (top) and anti-WASp antiserum (bottom). Sizes are indicated in kilodaltons. These results represent 1 of 3 independent experiments.
These results show that both integrin and chemokine receptor engagement on NK cells rapidly stimulate Cdc42 activation and WASp tyrosine phosphorylation, thus suggesting that these signaling events couple WASp function to the chemokine-induced integrin-supported NK-cell migration.
Chemokine receptor or integrin ligation on human NK cells results in WASp association with Fyn and Pyk-2 tyrosine kinases
The ability of WASp to interact with many signaling molecules and the finding that integrin and chemokine receptor stimulation enhances its tyrosine phosphorylation status prompted us to identify the tyrosine kinase(s) that can associate with WASp after integrin or chemokine receptor engagement on human NK cells.
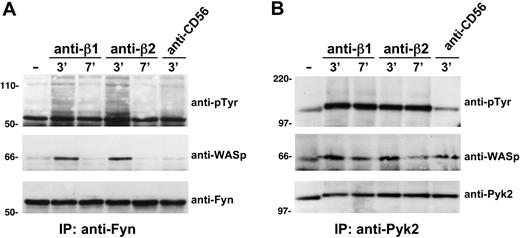
Because our previous evidence indicate that Pyk-2, a tyrosine kinase belonging to focal adhesion kinase family, plays a critical role in the control of NK-cell transendothelial migration,39 and WASp has been reported to bind to Pyk-2 in osteoclasts,42 we investigated whether Pyk-2 could interact with WASp. We also focused our attention on the Src family protein tyrosine kinase Fyn, based on its involvement in integrin and chemokine receptor signaling. Thus, Pyk-2 and Fyn were immunoprecipitated from lysates of β1 or β2 integrin–stimulated or unstimulated NK cells, and then the immunoprecipitates were analyzed by immunoblotting for the presence of WASp. As shown in Figure 4A and B, ligation of β1 or β2 integrins on NK cells results in Fyn and Pyk-2 tyrosine phosphorylation and significantly enhances their association with WASp. Similarly, when NKL cells, a human NK-cell line that displays many features of peripheral blood NK cells, were stimulated with CXCL12/SDF-1 or anti-β2 integrin mAb and cell lysates were immunoprecipitated with anti-WASp, WASp underwent tyrosine phosphorylation, and both integrin or chemokine receptor engagement stimulated its association with Fyn tyrosine kinase (supplemental Figure 3).
β1 or β2 integrin ligation on human NK cells enhances WASp association with Pyk2 and Fyn tyrosine kinases. Human NK cells were left untreated (−) or stimulated with GAM cross-linked anti-β1 (TS2/16), anti-β2 (TS1/18), or anti-CD56 (C218) mAb for the indicated time periods at 37°C. Cell lysates were then immunoprecipitated with anti-Fyn (A) or with anti-Pyk2 mAb (B). The resulting immunocomplexes were resolved by 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and sequentially immunoblotted with anti-pTyr mAb (top), anti-WASp antiserum (middle), anti-Fyn antiserum, or anti-Pyk2 mAb as loading controls (bottom). Sizes are indicated in kilodaltons. These results represent 1 of 3 independent experiments.
β1 or β2 integrin ligation on human NK cells enhances WASp association with Pyk2 and Fyn tyrosine kinases. Human NK cells were left untreated (−) or stimulated with GAM cross-linked anti-β1 (TS2/16), anti-β2 (TS1/18), or anti-CD56 (C218) mAb for the indicated time periods at 37°C. Cell lysates were then immunoprecipitated with anti-Fyn (A) or with anti-Pyk2 mAb (B). The resulting immunocomplexes were resolved by 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and sequentially immunoblotted with anti-pTyr mAb (top), anti-WASp antiserum (middle), anti-Fyn antiserum, or anti-Pyk2 mAb as loading controls (bottom). Sizes are indicated in kilodaltons. These results represent 1 of 3 independent experiments.
Taken together these results indicate that both Fyn and Pyk-2 tyrosine kinases can associate with WASp in human NK cells after integrin or chemokine receptor engagement, thus suggesting that these 2 kinases are involved in the regulation of WASp function.
Cdc42/WASp pathway is involved in the control of human NK-cell migration
To further investigate the involvement of Cdc42/WASp pathway in the control of NK-cell migration, healthy donor NK cells expanded in vitro were pretreated with different concentrations of wiskostatin, a pharmacologic inhibitor able to specifically prevent Cdc42 interaction with WASp43 and then were assayed for their migratory ability. Our results indicate that pretreatment of NK cells with wiskostatin inhibited in a dose-dependent manner CXCL12/SDF-1–or CX3CL1/fractalkine-induced migration through ICAM-1 (Figure 5). Similar results were obtained when migration assay was performed with wiskostatin-pretreated highly purified freshly isolated NK cells (data not shown).
Wiskostatin inhibits chemokine-induced integrin-dependent NK-cell migration. NK cells were preincubated for 1 hour with different concentration of Wiskostatin or vehicle (DMSO) and then assayed for their ability to migrate through ICAM-1 (5 μg/mL)–precoated polycarbonate filters using CXCL12/SDF1 (10nM) or CX3CL1/fractalkine (1nM) as chemoattractants. Data are expressed as the mean ± SD of the percentage of migrated cells obtained from 3 independent determinations. Statistical significance was evaluated by Student t test, *P < .02.
Wiskostatin inhibits chemokine-induced integrin-dependent NK-cell migration. NK cells were preincubated for 1 hour with different concentration of Wiskostatin or vehicle (DMSO) and then assayed for their ability to migrate through ICAM-1 (5 μg/mL)–precoated polycarbonate filters using CXCL12/SDF1 (10nM) or CX3CL1/fractalkine (1nM) as chemoattractants. Data are expressed as the mean ± SD of the percentage of migrated cells obtained from 3 independent determinations. Statistical significance was evaluated by Student t test, *P < .02.
Altogether these results clearly indicate a key role for Cdc42/WASp pathway in the control of human NK-cell migration.
Ability of chemokines to induce β2 neoepitope associated with lymphocyte function–associated antigen-1 high-affinity state involves Cdc42/WASp pathway activation and is impaired in NK cells from patients with XLT and patients with WAS
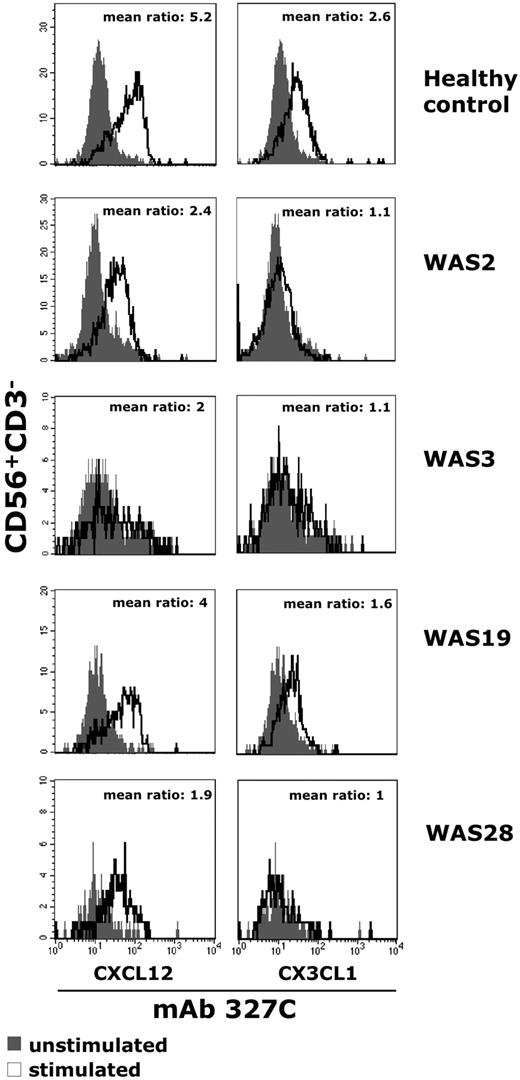
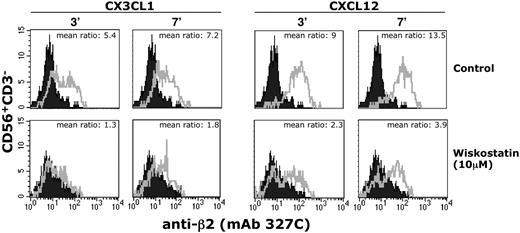
Chemokine receptor stimulation induces an array of inside-out signals leading to multiple conformational changes associated with the modulation of integrin adhesiveness.28,29 To understand whether the impaired migratory function of NK cells from patients with WAS was associated with defects in the chemokine receptor signaling responsible for the modulation of integrin adhesive function, we analyzed the ability of chemokines to induce the expression of a β2 integrin neoepitope in the regulatory I-like domain that is associated with the high-affinity state of lymphocyte function–associated antigen-1 (LFA-1). To this end, NK cells from patients with WAS/XLT were stimulated for different lengths of time with CXCL12/SDF-1 or CX3CL1/fractalkine and then were assayed by immunofluorescence and FACS analysis for the expression of the activation-dependent epitope of β2 integrin subunit with 327C mAb.37 The results obtained indicate that, differently from healthy donors, stimulation of WASP-mutated patient NK cells with CX3CL1/fractalkine fails to enhance the expression levels of this β2 neoepitope, whereas a marked (50%) reduction is observed in response to CXCL12/SDF-1 (Figure 6). Moreover, we found that the β2 neoepitope expression on CX3CL1/fractalkine- or CXCL12/SDF-1–stimulated healthy donor NK cells was almost completely inhibited by pretreatment with wiskostatin; this inhibition was already evident at 3 minutes and still persisted at 7 minutes after stimulation (Figure 7; Table 2). By contrast, wiskostatin pretreatment of NK cells resulted in a marginal down-modulation of chemokine-induced β2 surface expression similar to that observed in dimethyl sulfoxide (DMSO)–pretreated cells (supplemental Table 1). Finally, when wiskostatin-pretreated NK cells were subjected to drug removal, we found that chemokine stimulation was able to up-regulate the β2 neoepitope expression (supplemental Figure 4), thus indicating that the effect exerted by wiskostatin is reversible.
The ability of chemokine to induce LFA-1 high-affinity state is impaired in NK cells from patients with WAS. Freshly isolated peripheral blood NK cells from healthy donors or patients with WAS were left untreated or stimulated with CXCL12/SDF-1 (10nM) or CX3CL1/fractalkine (1nM) and simultaneously stained with the high-affinity reporter mAb 327C for 10 minutes at 37°C. Cells were then analyzed by flow cytometry. The ratio between the mean fluorescence intensity of stimulated (open profile) versus unstimulated (black profile) cells is shown. The results shown for healthy control are representative of 6 individual experiments.
The ability of chemokine to induce LFA-1 high-affinity state is impaired in NK cells from patients with WAS. Freshly isolated peripheral blood NK cells from healthy donors or patients with WAS were left untreated or stimulated with CXCL12/SDF-1 (10nM) or CX3CL1/fractalkine (1nM) and simultaneously stained with the high-affinity reporter mAb 327C for 10 minutes at 37°C. Cells were then analyzed by flow cytometry. The ratio between the mean fluorescence intensity of stimulated (open profile) versus unstimulated (black profile) cells is shown. The results shown for healthy control are representative of 6 individual experiments.
Wiskostatin inhibits chemokine-induced LFA-1 high-affinity state. Freshly isolated peripheral blood NK cells were preincubated for 1 hour with wiskostatin (10μM) or vehicle (DMSO) and then stimulated with CX3CL1/fractalkine (1nM) or CXCL12/SDF-1 (10nM) and simultaneously stained with the high-affinity reporter mAb 327C for the indicated time periods at 37°C. Stained cells were then analyzed by flow cytometry. The ratio between the mean fluorescence intensity of stimulated (open profile) versus unstimulated (black profile) cells is shown. These results represent 1 of 3 independent experiments.
Wiskostatin inhibits chemokine-induced LFA-1 high-affinity state. Freshly isolated peripheral blood NK cells were preincubated for 1 hour with wiskostatin (10μM) or vehicle (DMSO) and then stimulated with CX3CL1/fractalkine (1nM) or CXCL12/SDF-1 (10nM) and simultaneously stained with the high-affinity reporter mAb 327C for the indicated time periods at 37°C. Stained cells were then analyzed by flow cytometry. The ratio between the mean fluorescence intensity of stimulated (open profile) versus unstimulated (black profile) cells is shown. These results represent 1 of 3 independent experiments.
To further investigate the role of WASp in the chemokine-induced regulation of integrin adhesive function, healthy donor or WAS/XLT NK cells were assayed for their ability to adhere to ICAM-1 or VCAM-1. The results obtained indicate that both basal and chemokine-induced adhesion was severely impaired in WAS/XLT NK cells (supplemental Figure 5).
These data indicate that the defective NK-cell migration in patients with WAS/XLT is associated with impaired adhesiveness of these cells and their failure to up-regulate the high-affinity state of β2 integrins after chemokine stimulation and show a crucial role for Cdc42/WASp pathway in the chemokine-induced inside-out signals leading to modulation of integrin adhesive functions.
Discussion
In this study we show that the Cdc42/WASp pathway plays an important role in the control of NK-cell migration, acting as a critical regulator of the chemokine receptor–initiated inside-out signaling that normally leads to increased integrin adhesiveness.
The analysis of NK-cell migratory function in patients with different mutations in the WASP coding gene indicated that CXCL12/SDF-1– or CX3CL1/fractalkine-induced NK-cell migration through ICAM-1, VCAM-1, or endothelial cells was significantly impaired in most patients regardless of the clinical and immunologic phenotype, and despite that the chemokine receptor, CXCR4 and CX3CR1, or β1 or β2 integrin expression levels were similar in patients with WAS/XLT and in healthy donors.
We tend to rule out that the possibility that the reduced NK-cell migration in patients with WAS/XLT is determined by concurrent clinical problems or pharmacologic treatment, because patients included in this study were in good clinical conditions when tested, and the immunologic phenotype of patients with XLT was consistently identifiable.
Interestingly, the impaired NK-cell migration observed in patients W2 and W19 with XLT correlated with their reduced ability to form conjugates and to accumulate F-actin and with low levels of natural and antibody-dependent NK cell–mediated cytotoxic activity.13 Altogether these observations indicate that the WASP gene mutations found in patients W2 and W19 with XLT are clearly associated with impaired NK-cell migratory and cytolytic functions, and they strongly support the existence of a strict correlation between specific mutations and loss of WASp function.
Our data also indicate that the impairment of NK-cell migration in patients with WASP mutations correlates with a reduced ability of NK cells to up-regulate the expression of a β2 integrin activation–dependent epitope associated with a gain of integrin adhesive function after chemokine stimulation.37 This suggests that the reduced β2 neoepitope expression can contribute to the impaired WAS/XLT NK-cell migratory ability.
Integrin high-avidity state is required for NK cells as for other leukocytes to establish normal adhesive contacts with specific substrates and to initiate signaling cascades leading to an efficient migratory response. The signaling events involved in the up-regulation of integrin affinity/avidity state elicited by chemokine receptors have not yet been fully elucidated: in leukocytes, a role for a number of signaling molecules, such as phospholipase C, Rap-1, RhoA, phosphatidylinositol 3-kinase, protein kinase C-ζ, and the ARF–guanine nucleotide exchange factor cytohesin-1, has been shown.28,29 However, the involvement of WASp in the regulation of chemotactic factor–induced “inside-out” signaling leading to integrin clustering and high-avidity state has been poorly investigated, and controversial evidence has been provided. In murine T lymphocytes, WASp has been shown to be dispensable for the CXCL12/SDF-1–induced up-regulation of integrin adhesive function as well as for their chemotactic activity.44 In addition, WAS deficiency in both human and mouse neutrophils affects integrin clustering and adhesive functions. Nevertheless, neutrophil stimulation with formylmethionyl-leucyl-phenylalanine resulted in normal modulation of integrin affinity, thus suggesting that in neutrophils the G protein–coupled receptor–triggered integrin inside-outside signaling does not involve WASp function.45 By contrast, defects in the up-regulation of αIIbβ3 integrin affinity after stimulation of G protein–coupled receptor were described in platelets from patients with WAS.46 Moreover, evidence indicating a role for WASp-Arp2/3 complex in the regulation of β1 integrin–mediated adhesion following monocyte chemoattractant protein-1 stimulation of THP-1 monocytic cell line has been provided.47
In agreement with these latter observations, our results first indicate that WASp is implicated in the actin cytoskeleton reorganization required for integrin activation during chemokine-driven NK-cell migration.
WASp is organized into multimodular domains, some of which are able to bind to molecular components relevant for the induction of WASp effector function. Between the signaling molecules known to regulate WASp function, a crucial role is played by Cdc42. The binding of this small G protein to WASp causes the release of the intramolecular inhibitory interactions and the exposure of the verprolin homology, cofilin homology, acidic domain that allows WASp binding to Arp2/3 complex.7 Our observation on the rapid activation of Cdc42 by β1 or β2 integrin ligation or chemokines in human NK cells strongly suggests that Cdc42 is a critical upstream factor regulating WASp activity during NK-cell migration.
Further support to a role for Cdc42/WASp pathway in the regulation of NK-cell migration is given by our findings on the ability of wiskostatin, a pharmacologic compound that specifically prevents Cdc42 interaction with WASp,43 of inhibiting chemokine-induced NK-cell migration as well as up-regulation of β2 integrin neoepitope.
WASp functional activity can be also regulated by its tyrosine phosphorylation. Triggering of immunoreceptors such as the immunoglobulin E receptor on mast cells and the B-cell receptor on B cells, or the interaction of platelets with collagen through the GP VI, as well as β2 integrin engagement on NK cells was found to promote WASp tyrosine phosphorylation.8-11,13 To our knowledge, however, no evidence is available so far on the ability of chemokine or β1 integrin receptors to stimulate WASp tyrosine phosphorylation in primary leukocytes. Herein, we first demonstrated that chemokine stimulation or β1 integrin engagement on NK cells significantly enhanced WASp tyrosine phosphorylation.
WASp can interact with a number of cytoplasmic kinases that belong to both Src and Tec families as well as to the focal adhesion kinase family, and it is a specific substrate of Btk in B cells and of Lyn and Btk in a rat basophil leukemia.9,10 Evidence indicating that the Src kinase Hck can phosphorylate WASp at tyrosine 291 independently of Cdc42 and that this phosphorylation results in enhanced actin polymerization and filopodia formation have been also provided.40 Our findings indicate that WASp can associate with both the Src kinase Fyn and the focal adhesion kinase Pyk-2 following triggering of integrin and chemokine receptors on NK cells. We have previously reported a critical role for Pyk-2 in NK-cell migration.39 Herein, we provide evidence that Pyk-2 and Fyn, a Src kinase that binds to and activates Pyk-2,48,49 can associate with WASp, strongly suggesting that these tyrosine kinases cooperate with Cdc42 to couple chemokine or integrin receptors to WASp in human NK cells. Further studies are required to better clarify the contribution of Fyn and Pyk-2 tyrosine kinases versus Cdc42 in the regulation of WASp function during NK-cell migration.
As a whole, our findings indicate that similarly to other cells of the innate immune system, NK cells are highly dependent on WASp for normal migration and point out a crucial role of Cd42/WASp pathway in the chemokine-induced integrin-supported NK-cell transendothelial migration. Being that NK cells are one of the first lines of defense against microbial infections, the deficient NK-cell migratory function we found in patients with WAS/XLT may be of clinical relevance for the recurrences of viral infections exhibited by these patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr J. G. Collard for the GST-PAK fusion protein; ICOS Corporation, for the 327C mAb. We thank Dina Milana, Anna Maria Bressan, and Patrizia Birarelli for expert technical assistance. We thank Dr Fulvio Porta for clinical care of patients with WAS and Dr Daniele Moratto for the analysis of patient WASp protein expression. A special thanks to all patients who contributed to this study.
This work was supported by grants from the Italian Association for Cancer Research, Istituto Pasteur-Fondazione Cenci Bolognetti and Ministero dell'Istruzione, dell'Università e della Ricerca (Centri di Eccellenza BEMM and IDET, PRIN-MIUR, FIRB-MIUR, 60%); European Union Quality of Life (project QLG1-CT-1999-01090), EUROPID (QLG1-CT-2001-01395), and National Institutes of Health (grant P01-HL-059561-11A1).
National Institutes of Health
Authorship
Contribution: H.S., C.C., and S.M. performed experiments; H.S. and A.G. analyzed results; C.M., S.G., and Lucia D. Notarangelo were involved in clinical care of patients and performed mutation analysis; Luigi D. Notarangelo designed the research and contribute to the critical revision of the paper; and A.S. and A.G. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angela Gismondi, Department of Experimental Medicine, Sapienza University, Viale Regina Elena, 324, 00161 Rome, Italy; e-mail: angela.gismondi@uniroma1.it; or Angela Santoni, Department of Experimental Medicine, Sapienza University, Viale Regina Elena, 324, 00161 Rome, Italy; e-mail: angela.santoni@uniroma1.it.