Abstract
High-dose (200 mg/m2, MEL200) and intermediate-dose melphalan (100 mg/m2, MEL100) showed significant activity in myeloma. In a phase 3 study, 298 patients were randomly assigned to receive 2 autologous transplantations after conditioning with MEL200 or MEL100. Ninety-six of 149 (64%) completed MEL200 and 103 of 149 (69%) MEL100. Best response to MEL200 was: complete remission 22 of 149 (15%); partial remission 95 of 149 (64%), for an overall response rate of 79%. Best response to MEL100 was: complete remission 12 of 149 (8%); partial remission 95 of 149 (64%), for an overall response rate of 72%. Overall survival did not differ (P = .13); median progression-free survival (31.4 vs 26.2 months, P = .01), median time to progression (34.4 vs 27.0 months, P = .014) were longer in the MEL200. Treatment-related mortality was 3.1% in the MEL200 and 2.9% in the MEL100 group. Severe neutropenia and infections were marginally superior, whereas severe thrombocytopenia, mucositis, gastrointestinal adverse events, and the overall occurrence of at least 1 nonhematologic grade 3 or 4 adverse event were significantly higher in the MEL200 cohort. We conclude that MEL200 leads to longer remission duration and should be considered the standard conditioning regimen for autologous transplantation. This study was registered at www.clinicaltrials.gov as #NCT00950768.
Introduction
Several studies support the benefit of high-dose melphalan with stem cell rescue in patients with newly diagnosed multiple myeloma.1-3 Results from randomized trials are in favor of tandem autologous transplantation rather than only 1 procedure, even though a real benefit remains to be determined.4,5 Most conditioning regimens have been based on either melphalan alone or in combination with other agents. Melphalan at 200 mg/m2 (MEL200) is considered the standard dose for conditioning patients younger than 65 years.6 Older age and comorbidities may become limiting factors. In a retrospective case-matched study, 90 newly diagnosed patients treated with 2 courses of melphalan at 100 mg/m2 (MEL100) were compared with a control group of 90 pair mates, matched for serum β-microglobulin levels and Durie-Salmon clinical stage, and treated up-front with 2 MEL200 courses.7 Median progression-free survival (PFS) was significantly superior in the MEL200 group, but overall survival (OS) was not different. Moreover, hematologic and nonhematologic toxicities were significantly reduced in the MEL100 group. Here, we report a phase 3 clinical trial comparing 2 doses of melphalan, MEL200 versus MEL100, in newly diagnosed multiple myeloma. The underlying hypothesis was that rather than give 2 very intensive preparative regimens before autologous stem cell infusions, providing effective myeloma cell kill, a less toxic reduced-intensity conditioning would be better tolerated, especially in older patients, and equally effective.
Methods
Patients
From October 2001 to July 2006, 298 newly diagnosed myeloma patients younger than 65 years were enrolled in a prospective phase 3 trial (Figure 1). Patients were randomized before induction treatment either to receive 2 courses of MEL200 or 2 courses of MEL100 without any stratification for prognostic factors. Informed consent was obtained on enrollment. The protocol was approved by the institutional review boards of the participating centers according to the Declaration of Helsinki.
Inclusion criteria were: diagnosis of untreated Durie and Salmon stage8 IIA-IIIB, measurable multiple myeloma, and age younger than 65 years. Exclusion criteria were: prior treatment for myeloma; abnormal cardiac function, defined as systolic ejection fraction less than 50%; abnormal pulmonary spirometry test; serum bilirubin 2.5 times more than normal and alanine aminotransferase and/or aspartate aminotransferase 2 times more than normal; seropositivity for HIV, hepatitis C virus, or hepatitis B virus; and active nonhematologic malignancies.
Induction therapy, PBSC mobilization, and autografting
The initial treatment plan included induction chemotherapy with 2 courses of vincristine, 1 mg/m2 on day 1, adriamycin, 50 mg/m2 on day 1, and dexamethasone, 40 mg/d on days 1 to 4, administered 28 days apart, followed by peripheral blood stem cell (PBSC) mobilization and harvest after 1 or 2 cycles of cyclophosphamide, 4 g/m2, and granulocyte colony-stimulating factor, 10 μg/kg given intravenously or subcutaneously. After at least 1 month from PBSC collection, autografting consisted of MEL200 or MEL100 on day −2 and cryopreserved PBSC infusion on day 0. Patients received granulocyte colony-stimulating factor, 5 μg/kg, from day 3 until neutrophil count more than 1000/μL was achieved.
Supportive care and toxicity grading
After autografting, all patients received standard prophylaxis against bacterial and fungal infections, herpes simplex and varicella zoster virus reactivation, and Pneumocystis carinii. Cytomegalovirus (CMV) reactivation was monitored through levels of CMV antigenemia and/or serum CMV DNA levels and treated with ganciclovir or foscarnet as clinically indicated. Standard criteria (Common Toxicity Criteria, Version 3.0) were used for grading hematologic and nonhematologic toxicity.
Disease response
Response was evaluated before each treatment, monthly for the first 6 months after autografting, and at least every 3 months thereafter or as clinically indicated. Response criteria were defined according to the International Uniform Response Criteria for multiple myeloma.9 Complete remission (CR) required absence of serum monoclonal immunoglobulins and/or Bence Jones proteinuria by electrophoresis and immunofixation, less than 5% plasma cell infiltration in bone marrow aspirates, absence of soft tissue lesions, and no increase in size or number of osteolytic lesions. Very good partial remission (VGPR) was defined as detection of serum monoclonal immunoglobulins and/or Bence Jones proteinuria by immunofixation but not by electrophoresis or at least 90% reduction in serum monoclonal immunoglobulins or Bence Jones proteinuria with excretion lower than 100 mg/24 hours, and no increase in size or number of osteolytic lesions. Partial remission (PR) was defined as more than 50% reduction in the levels of serum monoclonal immunoglobulin, at least 90% reduction in 24-hour Bence Jones proteinuria, or excretion lower than 200 mg/24 hours, and no increase in size or number of lytic bone lesions. Patients with less than a PR were considered stable (SD). Progressive disease (PD) was considered an increase in serum monoclonal proteins or Bence Jones proteinuria of at least 25% in patients with at least PR, whereas disease relapse was considered as the reappearance of monoclonal proteins by immunofixation in case of previous CR.
Statistical analysis
Analyses were performed according to the intention-to-treat principle. Primary endpoints of the study were OS defined as the time from diagnosis until death from any cause. Secondary endpoints were PFS defined as the time from diagnosis until death from any cause or date of first relapse or progression; time to progression (TTP) defined as the time from the date of diagnosis to relapse or death from progression; incidence of gastrointestinal toxicity and infections; and treatment-related mortality defined as any death occurring within 60 days and attributable to therapy. Patients lost to follow-up or survivors who experienced no event were censored at the date of last contact. Moreover, subgroup analyses were performed in light of patient age (< 60 years, ≥ 60 years).
A sample size of 320 patients (160 per arm) was required to detect a 20% increase in OS at 5 years (from 40% to 60%) with an α error of 0.05 and a β error of 0.10, assuming an accrual of 36 months and a minimum follow-up of 24 months.
Proportions between groups were compared by the χ2 test or Fisher exact test. OS, PFS, and TTP were calculated according to the Kaplan-Meier method.10 Differences in OS, PFS, and TTP were tested with the 2-tailed log-rank test. Univariate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were estimated with the Cox proportional hazards model.11 Analyses of PFS and OS according to response were performed using the landmark analysis method (landmark point at 12 months). SAS 8.2 statistical software (SAS Institute) was used.
Results
Patients
Because of the declining enrollment, the steering committee decided to stop the trial in July 2006 after the first 298 randomized patients (93% of the planned sample size). Overall, 298 patients were randomized to 2 cohorts of 149 each at 31 Italian Divisions of Hematology and Bone Marrow Transplantation Units. Patient characteristics were evenly distributed in both groups (Table 1). Ninety-six of 149 (64%) completed the MEL200 arm, whereas in the 53 (36%) who did not complete it, main causes of early dropout were allografting (12 patients, 8%), consent withdrawal (10 patients, 7%), early adverse events (9 patients, 6%), early disease progression (8 patients, 5%), and poor PBSC collections (8 patients, 5%). A total of 103 of 149 (69%) completed the MEL100 arm, and main causes of early dropout in 46 (31%) were allografting (15 patients, 10%), early disease progression (9 patients, 6%), poor PBSC collection (9 patients, 6%), adverse events (5 patients, 6%), and consent withdrawal (4 patients, 3%; Figure 1).
Engraftment and response
Median numbers of CD34+ cells collected before transplantation in the MEL200 and MEL100 arms were 12 × 106/kg (range, 0-54 × 106) and 14 × 106/kg (range, 0-72 × 106), respectively. Median numbers of CD34+ cells infused in each arm were as follows: 5 × 106/kg (range, 2-12 × 106) and 5 × 106/kg (range, 2-10 × 106) patient body weight in the MEL200 group for the first and second transplantation, respectively; and 4 × 106/kg (range, 2-15 × 106) and 3 × 106/kg (range, 2-15 × 106) patient body weight in the MEL100 group for the first and second transplantation, respectively. In the MEL200 patients, best response to dexamethasone, adryamicin, and vincristine (DAV) was: at least VGPR 6 of 149 (4%), at least PR 50 of 149 (33%), SD 90 of 149 (60%), and PD 4 of 149 (3%). In the MEL100 patients, best response to DAV was: at least VGPR 3 of 149 (2%), at least PR 47 of 149 (31%), SD 91 of 149 (61%), and PD 7 (5%). Best response to MEL200 was as follows: CR 22 of 149 (15%), at least VGPR 55 of 149 (37%), at least PR 117 of 149 (78%), SD 27 of 149 (18%), and PD 1 of 149 (1%). Best response to MEL100 was as follows: CR 12 of 149 (8%), at least VGPR 32 of 149 (21%), at least PR 107 of 149 (72%), SD 34 of 149 (23%), and PD 5 (3%; Table 2).
Clinical outcomes
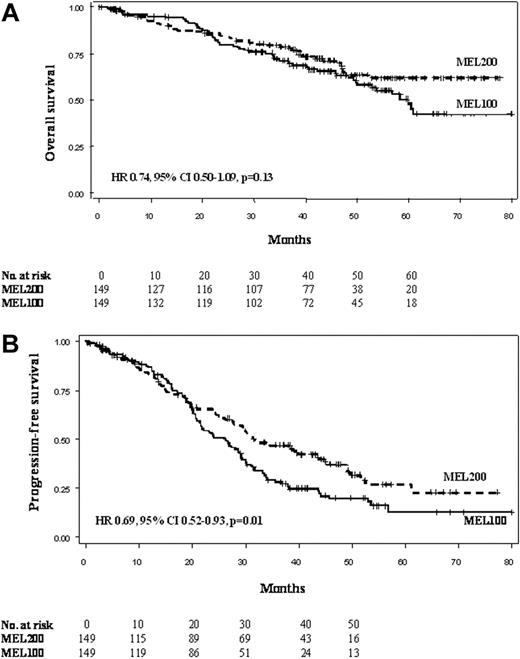
After a median follow-up of 44.6 (range, 0.5-79.9+) months from diagnosis in each group, median OS was not reached in the MEL200 group and was 60 months in the MEL100 group (HR = 0.74, 95% CI, 0.50-1.09, P = .13; Figure 2A). Projected OS at 5 years was 61.8% and 47.7% in the MEL200 and MEL100 groups, respectively. Median PFS was 31.4 (95% CI, 27.8-43.5) and 26.2 (95% CI, 21.5-29.1) months in MEL200 and MEL100, respectively (HR = 0.69, 95% CI, 0.52-0.93, P = .01; Figure 2B). PFS at 4 years was 37.1% and 19.6% in the MEL200 and MEL100 groups, respectively. Median TTP were 34.4 (95% CI, 29.8-44.7) and 27.0 (95% CI, 21.8-30.0) months in MEL200 and MEL100, respectively (HR = 0.69, 95% CI, 0.51-0.93, P = .01). Projected TTP at 4 years was 39.5% and 22.1% in the MEL200 and MEL100 groups, respectively. By applying a 12-month landmark analysis, the achievement of CR was not predictive of better OS (HR = 1.18, 95% CI, 0.65-2.14, P = .58) and PFS (HR = 0.96, 95% CI, 0.62-1.48, P = .85). Similarly, the achievement of at least VGPR did not influence OS (HR = 0.85, 95% CI, 0.50-1.44, P = .55) or PFS (HR = 0.77, 95% CI, 0.55-1.10, P = .15).
Clinical outcomes by intent-to-treat analysis of the 2 study cohorts. (A) Overall survival (OS). (B) Progression-free survival (PFS).
Clinical outcomes by intent-to-treat analysis of the 2 study cohorts. (A) Overall survival (OS). (B) Progression-free survival (PFS).
Age and response to DAV were prognostic factors for both OS and PFS. In patients older than 60 years, MEL200 did not prolong OS (HR = 0.83, 95% CI, 0.47-1.45) and PFS (HR = 0.84, 95% CI, 0.54-1.30); in patients younger than 60 years, MEL200 marginally improved OS (HR = 0.61, 95% CI, 0.34-1.08) and significantly enhanced PFS (HR = 0.60, 95% CI, 0.41-0.88; Figure 3). In patients who achieved at least PR to DAV, MEL200 improved neither OS (HR = 0.90, 95% CI, 0.46-1.75) nor PFS (HR = 0.83, 95% CI, 0.51-1.40); in patients who did not achieve at least PR to DAV, MEL200 prolonged both OS (HR = 0.65, 95% CI, 0.40-1.07) and PFS (HR = 0.63, 95% CI, 0.44-0.90).
OS and PFS. (A) OS in patients older than 60 years. (B) OS in patients younger than 60 years. (C) PFS in patients older than 60 years. (D) PFS in patients younger than 60 years.
OS and PFS. (A) OS in patients older than 60 years. (B) OS in patients younger than 60 years. (C) PFS in patients older than 60 years. (D) PFS in patients younger than 60 years.
Salvage treatments
Overall, 179 (60%) patients had progression or relapse: 80 (54%) in the MEL200 group and 99 (66%) in the MEL100 group, respectively. Second line treatment was given to 64 (43%) patients assigned to MEL200 and to 84 (56%) assigned to MEL100. Thirteen (9%) MEL200 patients and 16 (11%) MEL100 patients did not receive any salvage regimen, mainly because of death resulting from rapid disease progression. Chemotherapy-based salvage regimens were used in 10 (6%) MEL200 patients and 16 (11%) MEL100 patients. Thalidomide-based salvage regimens were used in 15 (10%) MEL200 patients and 34 (23%) MEL100 patients; bortezomib-based salvage regimens were used in 40 (27%) MEL200 patients and 27 (18%) MEL100 patients; lenalidomide-based regimens were used in 2 (1%) MEL200 patients and in 6 (4%) MEL100 patients.
Toxicities
Three patients died of treatment-related complications in both arms, accounting for a treatment-related mortality of 3.1% and 2.9% in the MEL200 and in the MEL100 group, respectively. In the MEL200 cohort, causes of death were stroke after induction, cardiac failure after the first cycle of cyclophosphamide, and pneumonia after the first MEL200; whereas in the MEL100 group, they were septic shock after induction, pneumonia after the first cycle of cyclophosphamide, and cerebral hemorrhage after the first MEL100.
Hematologic and nonhematologic toxicities are listed in Table 3. Platelet transfusion requirement was significantly higher in the MEL200 group (56% vs 38%, P = .002) as well as the use of intravenous broad-spectrum antibiotics (41% vs 29%, P = .03), gastrointestinal toxicities (11% vs 1%, P < .001), and mucositis (17% vs 3%, P < .001). Other nonhematologic grade 3 or 4 toxicities were evenly distributed. The overall occurrence of at least 1 nonhematologic grade 3 or 4 adverse event during treatment was significantly higher in the MEL200 group (45% vs 30%, P = .008).
Discussion
In this randomized study, 2 different doses of melphalan have been evaluated as the preparative regimen for autologous transplantation in newly diagnosed patients. MEL200 was more effective than MEL100: the median PFS was 31.4 versus 26.2 months (P = .01). This advantage was more pronounced in patients younger than 60 years.
The longer PFS and TTP, seen in the MEL200 group, did not translate into a survival advantage, although a plateau phase was observed after 50 months in patients who received MEL200. These data are consistent with previous observations, where the availability of several new effective agents, used as salvage therapies in both study groups, may offset the initial benefit of a more effective treatment at diagnosis.12 In the past, when melphalan-based regimens were the only effective treatments, the advantage induced at diagnosis translated into a longer OS. More recently, at least 3 other effective agents are available, and their use at relapse may highly reduce the clinical benefit induced at diagnosis. In our study, approximately 60% to 70% of relapsed patients received new drugs as first salvage treatments, and this may have affected OS. A longer follow-up is needed to better evaluate the effect of salvage therapy.
The superiority of autografting over conventional therapy for younger patients has been reported in prospective randomized trials. In the Intergroupe Francophone du Myelome-90 study, CR rates (22% vs 5%), 5-year PFS (28% vs 10%), and OS (52% vs 12%) were significantly better in the transplantation group.13 Similarly, in the Medical Research Council Myeloma VII Trial, the CR rate (44% vs 8%; P < .001), PFS (28 vs 20 months), and OS (54 vs 42 months) were superior in the transplantation group.2 The most appropriate preparative regimen for autologous transplantation is generally considered MEL200.14
The introduction of thalidomide, bortezomib, and lenalidomide in the late 1990s and early 2000s has dramatically changed the treatment options and the clinical outcome of both relapsed/refractory15 and newly diagnosed myeloma patients.16 Patients diagnosed after December 1996, when thalidomide was first introduced, have shown a 50% improvement in OS.15 The introduction of bortezomib as induction regimen before autologous transplantation has significantly improved response rate and PFS.17-20 According to these data, novel agents should be consistently used in the induction regimens before autologous transplantation. Which between MEL200 and MEL100 is the most appropriate preparative regimen for autologous transplantation in association with novel agents remains to be determined. MEL100 induces less adverse events and may be equally effective when new agents are included in the induction.
Alkylating agents, such as intravenous melphalan, still play an important role in the treatment of patients with myeloma. High-dose melphalan, 200 mg/m2, is the standard dose for the preparative regimen to autologous transplantation under the age of 60 to 65 years. In a large study, the outcome of patients older than 65 years, who received MEL200, was significantly inferior to those younger than 65 years.21 Most trials have primarily included patients with good organ functions. Although age per se should not be a contraindication for an autograft, comorbidities can be a limiting factor, even in younger patients. Our study is the first phase 3 trial conducted in untreated newly diagnosed myeloma patients younger than 65 years to compare 2 different doses of melphalan followed by autologous stem cell rescue. Our findings suggest that the greater benefit of MEL200 is in patients younger than 60 years and that MEL200 should be considered the standard conditioning regimen for autologous transplantation.
In elderly patients, conflicting results have been reported on the clinical efficacy of MEL100. An Intergroupe Francophone du Myelome study showed no differences in efficacy between MEL100 and the oral combination melphalan-prednisone in patients 65 to 75 years of age,22 whereas a Gruppo Italiano per le Malattie Ematologiche dell'Adulto study showed a superiority of MEL100 in patients 65 to 70 years of age.3 In a recent phase 2 study, including bortezomib in the induction, MEL100 as preparative regimen for autologous stem cell rescue, and lenalidomide as maintenance, clinical outcome was superior in patients 65 to 70 years of age compared with those 70 to 75 years of age.20 Although caution is imperative when evaluating nonrandomized trials, overall, these studies suggest that age 60 to 65 years and age 65 to 70 years should be considered the upper age limits for MEL200 and MEL100, respectively.
In our report, treatment-related mortality was approximately 3% in both arms, which is consistent with the mortality reported in other studies after a standard autograft.2,13,23 The occurrence of at least 1 grade 3 or 4 adverse event, either hematologic or nonhematologic, was significantly higher in the MEL200 group. The higher toxicity of MEL200 is also confirmed by the higher transfusion requirement and the higher use of intravenous antibiotics requiring inpatient hospital admittance. Overall, these findings may further support the use of MEL100 as an alternative option for patients younger than 65 years with comorbidities that may significantly increase the risks of toxicity after MEL200.
In conclusion, our study suggests that MEL200 is the conditioning regimen of choice in younger medically fit patients undergoing an autologous transplantation. However, MEL100, given the low toxicity profile, may be considered in younger patients with nonhematologic comorbidities.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the nurses and medical staff for caring for the patients and the study coordinators who collected the trial and follow-up data.
Authorship
Contribution: A.P. and M.B. designed the study, supervised its conduct and data analysis, and wrote the paper; B.B. contributed patients to the study and assisted in drafting and editing the manuscript; S.B. and F.C. participated in designing research/protocol, contributed patients to the study, verified data, and assisted in drafting the manuscript; A.P.F., A.M.L., M.G., R.R., F.P., C.C., T.C., A.L., G.M., A.N., P.P., A.G., V.C., M.R., L.A., V.D.S., P.M., M.T.P., and M.M. contributed patients to the study and reviewed manuscript; and I.B. performed the statistical analysis and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonio Palumbo, Divisione di Ematologia dell'Università di Torino, Azienda Ospedaliero-Universitaria San Giovanni Battista, Via Genova 3, 10126 Torino, Italy; e-mail: appalumbo@yahoo.com.