Abstract
Acute graft-versus-host disease (GVHD) remains the major obstacle to a more favorable therapeutic outcome of allogeneic hematopoietic stem cell transplantation (HSCT). GVHD is characterized by tissue damage in gut, liver, and skin, caused by donor T cells that are critical for antitumor and antimicrobial immunity after HSCT. One obstacle in combating GVHD used to be the lack of understanding the molecular mechanisms that are involved in the initiation phase of this syndrome. Recent research has demonstrated that interactions between microbial-associated molecules (pathogen-associated molecular patterns [PAMPs]) and innate immune receptors (pathogen recognition receptors [PRRs]), such as NOD-like receptors (NLRs) and Toll-like receptors (TLRs), control adaptive immune responses in inflammatory disorders. Polymorphisms of the genes encoding NOD2 and TLR4 are associated with a higher incidence of GVHD in HSC transplant recipients. Interestingly, NOD2 regulates GVHD through its inhibitory effect on antigen-presenting cell (APC) function. These insights identify important mechanisms regarding the induction of GVHD through the interplay of microbial molecules and innate immunity, thus opening a new area for future therapeutic approaches. This review covers current knowledge of the role of PAMPs and PRRs in the control of adaptive immune responses during inflammatory diseases, particularly GVHD.
Introduction
Acute graft-versus-host disease (GVHD) is a potentially lethal complication in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). It is characterized by damage of epithelial surfaces in target organs caused by alloactivated T cells recognizing host tissue antigens. The severity of GVHD is determined by many factors, including the level of immunogenic disparity,1 the T-cell dose,2 the conditioning regimen,3 and the degree of immunosuppression.4 A common feature of the primary GVHD target tissues is their exposure to microbes and microbial products through the epidermis, intestinal mucosa, and portal circulation. The significance of the intestinal microbial flora for the pathogenesis of GVHD was first discovered in experimental mouse models and confirmed in human trials in the 1970s.5,6 Based on these findings, it became common practice to perform intestinal decontamination using orally administered antibiotics in patients undergoing allogeneic HSCT. Despite a large body of data providing evidence that microbes or microbial products (pathogen-associated molecular patterns [PAMPs]) interact with innate receptors (pathogen recognition receptors [PRRs]), on hematopoietic cells, mucosal cells, and endothelial cells, it took until the mid 1990s before the first PRR was discovered: In 1996 the PRR “Toll,” was discovered and determined to be responsible for innate immune responses to Aspergillus fumigatus in Drosophila.7 Shortly after this publication, the human homologue of Toll was defined and named Toll-like receptor (TLR) and later on TLR4.8 From this point, research in the field of innate immunity has increased substantially and to date hundreds of receptors, proteins, and small molecules, which are involved in antimicrobial immunity as well as inflammatory disorders, have been discovered.
Mucosal surfaces and vascular endothelium, the potential sites of pathogen entry, are rich in resident innate immune cells, such as macrophages and dendritic cells (DCs).9 Signaling through PRRs regulates the activity of DCs, leading to phagocytosis, chemokine receptor expression, cytokine secretion, migration from peripheral tissue to draining lymph nodes, and antigen presentation.10 Several TLRs have been described to recognize different microbial molecules (PAMPs), including lipopolysaccharide (LPS, TLR4), bacterial lipoproteins (TLR2), flagellin (TLR5), RNA (TLR3+7), and cytosine-phosphorothioate-guanine (CpG) DNA (TLR9).11 TLR downstream signaling activates a complex signaling cascade, eventually leading to host resistance against pathogens by increased production of cytokines, chemokines, adhesion molecules, and antimicrobial peptides as well as to enhanced antigen presentation by antigen-presenting cells (APCs).12 However, PAMPs are recognized not only by TLRs, but also by a PRR family of intracellular NOD-like receptors (NLRs) present in various cell types.13,14 NLRs include proteins such as NALPs (NACHT-, LRR-, and PYD-containing proteins), NOD1 (nucleotide-binding oligomerization domain), and NOD2. NLRs are involved in diverse signaling pathways regulating the secretion of inflammatory cytokines, such as interleukin-1β (IL-1β) and IL-18, as well as the induction of cell death.
Recent advances in our understanding as to how the innate immune system senses microbial antigens and controls adaptive immune responses provided an insight in the molecular mechanisms, thus opening a new and exciting field for future therapies of inflammatory diseases. In this text, we review recently gained knowledge on the role of microbe-associated molecules as well as innate immune receptors and discuss these findings in the context of the pathophysiology of GVHD (Figures 1–2).
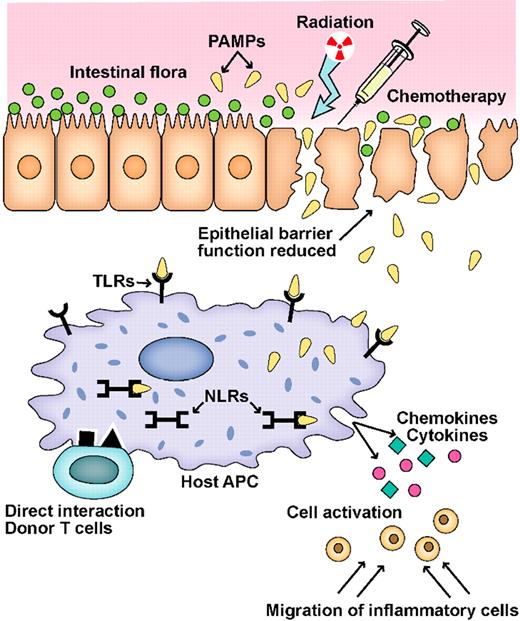
Schematic of the GVHD initiation phase. A key event in the initiation of inflammation during GVHD is the activation of pathogen recognition receptors (PRRs), such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs), by microbial products (PAMPs) produced by the intestinal flora.
Schematic of the GVHD initiation phase. A key event in the initiation of inflammation during GVHD is the activation of pathogen recognition receptors (PRRs), such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs), by microbial products (PAMPs) produced by the intestinal flora.
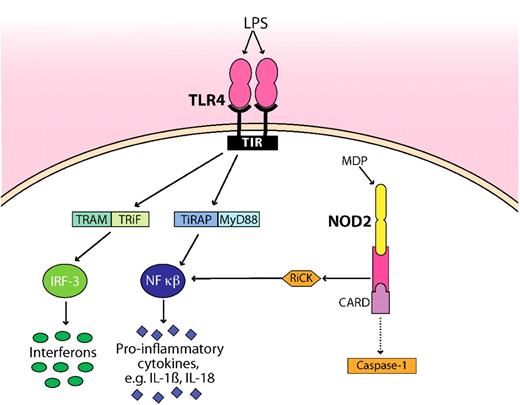
Schematic representation of how microbial products (LPS, MDP) are detected by TLR4 and NOD2. TLR4 and NOD2 are the best studied innate immune receptors present during GVHD. For clarity, the pathways have been simplified. NOD2 sense intracellular MDP, leading to recruitment of the adaptor protein RiCK as well as to activation of the caspase 1 inflammasome, which eventually results in cell death. Extracellular LPS is recognized by TLR4, which signals through its intracellular domain TIR. Subsequent steps involve the adaptor molecules MyD88, TiRAP, TRAM, and TRiF. The activation and translocation of nuclear factor κB (NF-κB) and IRF-3 result in the transcriptional up-regulation of proinflammatory genes. Whether the CARD domain interacts with caspase is controversial as indicated by a dotted line. TIR indicates Toll–IL-1 receptor, the cytoplasmic domain of TLR4; TRAM, Toll–IL-1R domain–containing adaptor inducing interferon-β–related adaptor molecule; TRiF, TIR domain–containing adapter-inducing interferon-β; MyD88, myeloid differentiation primary response gene; TiRAP, Toll–IL-1 receptor (TIR) domain–containing adaptor protein; and IRF-3, interferon regulatory factor 3.
Schematic representation of how microbial products (LPS, MDP) are detected by TLR4 and NOD2. TLR4 and NOD2 are the best studied innate immune receptors present during GVHD. For clarity, the pathways have been simplified. NOD2 sense intracellular MDP, leading to recruitment of the adaptor protein RiCK as well as to activation of the caspase 1 inflammasome, which eventually results in cell death. Extracellular LPS is recognized by TLR4, which signals through its intracellular domain TIR. Subsequent steps involve the adaptor molecules MyD88, TiRAP, TRAM, and TRiF. The activation and translocation of nuclear factor κB (NF-κB) and IRF-3 result in the transcriptional up-regulation of proinflammatory genes. Whether the CARD domain interacts with caspase is controversial as indicated by a dotted line. TIR indicates Toll–IL-1 receptor, the cytoplasmic domain of TLR4; TRAM, Toll–IL-1R domain–containing adaptor inducing interferon-β–related adaptor molecule; TRiF, TIR domain–containing adapter-inducing interferon-β; MyD88, myeloid differentiation primary response gene; TiRAP, Toll–IL-1 receptor (TIR) domain–containing adaptor protein; and IRF-3, interferon regulatory factor 3.
The intestinal microflora during GVHD
Early studies in allogeneic mouse radiation chimeras showed that the mortality due to the “secondary disease”—later called GVHD—is significantly reduced in germ-free mice or when decontamination of the gut flora was performed.15,16 In one of these studies, Jones et al transplanted bone marrow of DBA/2 mice into conventional or germ-free, lethally irradiated C3H/He mice.15 Four months after irradiation, 98% of the germ-free recipients were still alive—in contrast to the conventional mice, which had all died from wasting syndrome and diarrhea associated with intestinal GVHD. The authors proposed a model picturing equally severe intestinal lesions in conventional versus germ-free recipients, but a higher mortality as a result of secondary infections caused by intestinal microorganisms in the non–germ-free recipients. Soon afterward, this model of secondary infection was challenged by Van Bekkum et al, who infused bone marrow cells of C57BL/Rij donor mice into lethally irradiated CBA mice causing 95% mortality after 100 days. Strikingly, GVHD was virtually absent in the control groups on antibiotic prophylaxis or under germ-free conditions.16 When spleen cells were added (to induce a more acute form of GVHD), mortality of the recipient mice under germ-free conditions or with antibiotic prophylaxis was no longer completely prevented, but still significantly delayed, compared with recipient mice held under normal conditions without antibiotics. The same group successfully used fetal gut implants in germ-free mice after HSCT to show that the degree of histologic damage is positively correlated to the presence of intestinal microbes.17
These findings collectively gave reason to hypothesize that lymphocytes sensitized against microbial antigens cross-react with epithelial antigens in GVHD. This hypothesis became the most widely accepted model of microbial interactions in the pathogenesis of GVHD in the following decades and triggered several clinical studies in patients undergoing HSCT investigating “protected environment” and gastrointestinal decontamination in the 1980s. The majority of these trials showed efficacy of gastrointestinal decontamination and protected environment in the prevention of GVHD,5,6,18-20 and performance of gut decontamination became—and still is—standard practice in many transplant centers.21,22
A reduction of the bacterial translocation from the bowel lumen to the systemic circulation can be accomplished by administration not only of antibiotics, but also of oral probiotics or by use of antibodies against microorganisms. In a murine GVHD model, characterized by severe damage of the bowel mucosa and elevated serum LPS levels, the administration of the probiotic Lactobacillus rhamonosus GG resulted in reduced bacterial translocation to mesenteric lymph nodes, ameliorated systemic GVHD, and improved survival.23 Interestingly, the presence of antibody titers against a certain Escherichia coli strain (J5) is associated with a reduced incidence of GVHD in patients undergoing allo–bone marrow transplantation (BMT).24 On this basis, an polyclonal antibody against the same strain of E coli was produced and was successfully used to reduce the incidence of GVHD in a randomized clinical trial.25
TLRs present during GVHD
TLRs are expressed on hematopoietic cells, such as dendritic cells, T cells, and B cells, as well as on nonhematopoietic cells, such as endothelial cells, epithelial cells, and organ parenchyma cells.26 TLRs are involved in maintaining tolerance and eliminating pathogenic microorganisms, but they also play a role in amplifying autoimmune responses that ultimately cause inflammation.27,28 TLR signaling stimulated by PAMPs was found to regulate immune responses during inflammatory diseases with similarities to GVHD, such as systemic lupus erythematosus,29-31 arthritis,32 and inflammatory bowel disease.33-35 In experimental colitis, bacterial products aggravate acute inflammation via TLR2 and TLR4 signaling and direct the recruitment of inflammatory cells to intestinal sites.36 On the other hand, activation of specific TLRs can inhibit autoimmune diseases in certain mouse models: For example, the stimulation of TLR2 or TLR3 protects mice from experimental colitis.37,38 The repeated exposure to a TLR agonist can have anti-inflammatory effects by inducing hyporesponsiveness to subsequent TLR stimulation.39 Corr and coworkers demonstrated that repeated low-dose administration of a synthetic TLR7 agonist induced hyporesponsiveness or tolerance to TLR2, TLR7, and TLR9 activators and limited the course of neural inflammation in an experimental allergic encephalomyelitis model (Hayashi et al).40 TLR5 stimulation as well as TLR9 stimulation have mostly anti-inflammatory effects, as indicated by development of spontaneous intestinal inflammation in TLR5-deficient mice41 and the high susceptibility of TLR9-deficient mice to experimental colitis.42 Interestingly, TLR4 polymorphisms are associated with a higher risk of inflammatory bowel disease, suggesting that TLR4 stimulation is needed for intestinal homeostasis in humans.43,44
Convincing evidence of the significance of PAMP-TLR interactions in the pathogenesis of GVHD derives from experimental models of GVHD in rodents. Ferrara and coworkers demonstrated a crucial role of the LPS-TLR4 pathway for the pathophysiology of GVHD (Cooke et al).27 LPS is a structural component of Gram-negative bacteria and is a potent stimulator of TLR4. After allo-HSCT, the translocation of LPS and microorganisms from the bowel lumen through the damaged intestinal mucosa to the circulation can occur. LPS can trigger a broad range of inflammatory responses from different immune cells, such as macrophages, neutrophils, monocytes, and T cells. During GVHD, LPS stimulates the secretion of tumor necrosis factor-α by macrophages leading to increased GVHD mortality.45 Using a mouse BMT model, Ferrara and colleagues demonstrated that the transplantation of donor BM cells, which are resistant to LPS stimulation, results in less severe GVHD (Cooke et al).46 Next, they studied the therapeutic efficacy of LPS antagonism during GVHD by administration of a lipid-A analog from day 0 to day +6 after allo-BMT. They found that LPS antagonism resulted in reduced intestinal GVHD as well as reduced systemic GVHD, but did not alter T-cell activity to host antigens.27 In line with these findings are the observations that elevated LPS serum levels after allo-BMT positively correlate with histopathologic target organ damage during GVHD.46,47
Moreover, LPS was shown to play a role in transplant-related lung injuries after allogeneic HSCT: In a fully major histocompatibility complex (MHC)–mismatched allo-BMT model without systemic GVHD, the inhalation of LPS caused innate immune activation, accumulation of alloreactive T cells, and histologic damage. This effect was dependent on TLR4 signaling, and treatment with a TLR4 antagonist could protect against lung injury after allogeneic HSCT.48 The authors demonstrated that the absence of functional TLR4 in donor-derived hematopoietic cells abrogated the development of pulmonary damage, whereas the presence or absence of TLR4 on recipient structural lung tissue had no impact on lung injury after transplantation. Because the effects were a result of local activation of pulmonary innate immunity, as opposed to systemic innate immunity, the authors concluded that inhalation of LPS can promote the development of alloimmune lung injury after BMT independent from systemic GVHD.
Although the importance of the LPS-TLR4 pathway during GVHD is well documented in murine GVHD models, experiments with TLR4-deficient mice and MyD88-deficient mice show that TLR signaling is not absolutely required for GVHD.49,50 This might be because of the existence of alternative (non-TLR) activation pathways of APCs leading to alloactivation and proliferation of donor T cells in the absence of TLR signaling. The observation that defects in TLR signaling do not completely prevent GVHD is in line with the clinical data, which is described below. In humans, polymorphisms (Asp299Gly and Thr399Ile) affecting the extracellular domain of the TLR4 receptor are associated with a blunted response to inhaled LPS.51 Lorenz et al tested the impact of those 2 TLR4 polymorphisms on the outcome of 237 HLA-identical sibling allo-HSCTs.52 One or 2 polymorphisms were detected in approximately 10% of patients and donors. The authors found a trend toward a reduced incidence of grade II to IV acute GVHD when a TLR4 polymorphism was present (33% vs 47%) but failed to detect statistically significant results. They concluded that a much larger study population would be needed to confirm the role of LPS and TLR4 in the pathogenesis of GVHD in humans. Interestingly, in a subsequent study, Elmaagacli et al also found no correlation of the presence of TLR4 polymorphisms to the overall incidence of GVHD in a mixed cohort of 307 HSC transplants from HLA-identical siblings and matched unrelated donors.53 Surprisingly, they found that the severity GVHD was significantly increased in a univariate analysis if one TLR4 polymorphism (Thr399Ile) was present in both donor and recipient of an allo-HSC transplant compared with wild-type donor/recipient pairs (42% vs 15% severe GVHD). However, the TLR4 polymorphism failed to influence the occurrence of severe GVHD in the multivariate analysis, indicating that its role might not be very pronounced, which is in line with the data of Lorenz et al. The differences of a strong effect of TLR4 signaling in mouse models and a weaker effect in the clinical studies might be caused in part by the routine performance of bacterial gut decontamination in clinical allo-HSCT. Elmaagacli and coworkers used metronidazol and ciprofloxacin, which reduces the concentration of anaerobic bacteria very effectively by several log steps and is associated with a reduced incidence of acute GVHD (Beelen et al).20 It is reasonable to argue that reducing the concentration of intestinal anaerobic bacteria may also reduce the concentration of microbial products, including bacterial cell wall compounds such as LPS, leading to a reduced stimulation of the TLR4 pathway. Another explanation for the different effects of TLR4 stimulation in humans and in mouse models could be a difference in the balance of proinflammatory effects versus tissue-regenerative/anti-inflammatory effects upon TLR4 activation between humans and mice. Taken together, the clinical data on the connection between TLR4 and GVHD do not draw a clear picture yet. Therefore, futures studies addressing the role of TLR4 polymorphisms in allo-HSCT are needed, taking into account the type of bacterial gut decontamination used.
Sykes and coworkers were able to demonstrate that localized tissue inflammation caused by TLR ligands controls the recruitment of alloreactive T cells to GVHD target organs (Chakraverty et al).54 They transferred B6 T cells either to freshly irradiated BALB/c recipients or to B6-BALB/c mixed chimeras (a model for donor lymphocyte infusions). Both models showed a massive GVH reaction, characterized by T-cell expansion and activation, with similar up-regulation of skin- as well as gut-homing molecules, leading to eradication of host hematopoietic tissue. However, exclusively the irradiated BALB/c recipients (not the B6-BALB/c mixed chimeras) developed intestinal and skin GVHD with profound tissue injury caused by alloreactive T cells. Strikingly, innate immune activation was able to overcome the tolerance of B6-BALB/c mixed chimeras to adoptive transfer of B6 T cells: The systemic application of a TLR7 agonist (R-848) induced recruitment of alloreactive T cells and tissue damage in gut, liver, skin, and lung. These observations indicate that tissue inflammation, induced by signaling through PRRs, controls the development of GVHD at the local level. Blazar and colleagues showed that the TLR7/8 agonist (3M-011) can have differential effects on GVHD depending on the timing of administration. When 3M-011 was given after allo-BMT, the GVHD-related mortality rate was increased from 0% to 50% in a major mismatched model (Taylor et al).55 In a subsequent work the same group demonstrated, however, that pretreatment (prior to allo-BMT) with the same TLR7/8 agonist significantly delayed GVHD lethality and reduced GVHD target organ injury.56 Interestingly, pretreatment with 3M-011 resulted in increased levels of the immunosuppressive intracellular enzyme indoleamine 2,3-dioxygenase, which plays a crucial role in GVHD regulation.56,57 Collectively these data suggest that detection of pathogens (viral DNA and RNA) by TLR7 can lead to localized and systemic inflammation as well as to inhibition of inflammation during GVHD.
In a murine GVHD model, Balsari and colleagues demonstrated reduced systemic GVHD leading to improved survival in TLR9−/− allo-BM transplant recipients (Calcaterra et al).50 A possible mechanism was provided by the authors: The stimulatory activity of spleen APCs from irradiated TLR9−/− mice showed a reduced percentage of cells expressing costimulatory molecules and a significantly lower allostimulatory ability leading to less proliferation of allogeneic donor T cells. Experiments performed in BM chimeric mice showed that TLR9 in the nonhematopoietic system influenced GVHD, whereas the presence or absence of TLR9 on hematopoietic cells had no effect on GVHD. The authors argued that nonhematopoietic cells, such as intestinal epithelial cells expressing high levels of TLR9, might directly participate in the process of antigen presentation during GVHD. However, it is well known that TLRs are highly expressed on dendritic cells (DCs) and that antigen presentation by host DCs is central to the pathophysiology of GVHD.58,59 Cytosine-phosphorothioate-guanine oligodeoxynucleotides (CpG ODNs) mimic bacterial and viral DNA and stimulate TLR9, leading to innate immune activation. Blazar and colleagues found that ligation of TLR9 with CpG ODNs at the time of allo-BMT enhanced alloreactive T-cell responses, resulting in increased lethal GVHD (Taylor et al).55 The CpG ODN–mediated effects were dependent on TLR9 signaling in host APCs, which underlines the importance of TLR signaling for host APC function during GVHD. Elmaagacli and colleagues analyzed the impact of 2 polymorphisms of the TLR9 gene (T1486C and T1237C), which are associated with a lower TLR9 expression, on the clinical outcome in 413 donors and allo-HSC transplant recipients.53 The TLR9 polymorphisms were not associated with incidence or severity of GVHD. However, patients with the TLR9 1486 polymorphism had a significantly improved survival because of reduction of treatment-related mortality and relapse rate. The authors hypothesized that overwhelming TLR9-mediated immune responses, such as systemic inflammatory response syndrome and sepsis, might have occurred less often in allo-HSC transplant recipients with TLR9 polymorphisms, in comparison with wild-type allo-HSC transplant recipients. This hypothesis is based on experimental results demonstrating that systemic inflammatory response syndrome and sepsis can be prevented by inhibition of TLR9.60
NLRs present during GVHD
NOD2 is the best studied NLR present during GVHD. NOD2 (CARD15) detects muramyl dipeptide (MDP), a molecule that is produced during the synthesis and degradation of peptidoglycan, which is a cell wall component of most bacteria. Single nucleotide polymorphisms (SNPs) near or within the NOD2 LRR region (G908R, L1007insC, and R702W) are present in approximately 15% of the population and constitute genetic risk factors for the development of Crohn disease.61,62 In addition, polymorphisms in the NACHT region of NOD2 are linked to other inflammatory diseases, such as Blau syndrome63 and early onset sarcoidosis.64
We performed studies in murine allo-BMT models to determine the role of NOD2 during GVHD.65 We found that NOD2 deficiency of the allo-BM transplant donor (either donor T cells or BM) has no significant impact on the development of GVHD and does not regulate alloactivation of donor T cells. Our data suggest that NOD2 has no cell-intrinsic role in the regulation of T-cell activity during GVHD despite the increasing evidence that innate immune receptors can modulate T-cell function and activation during inflammation.66-68 In NOD2−/− allo-BM transplant recipients, we observed increased GVHD in both MHC-mismatched and MHC-matched models. Using chimeric mice, we demonstrated that NOD2 deficiency in the hematopoietic system, but not in the nonhematopoietic system, aggravates both experimental GVHD as well as experimental trinitrobenzene sulfonate (TNBS) colitis. We studied DCs of NOD2−/− allo-BM transplant recipients and found increased activation and function leading to augmented proliferation as well as activation of allogeneic donor T cells, which resulted in target organ damage. These data support the hypothesis that NOD2 can negatively regulate the activity and function of host DCs, resulting in increased alloactivation and proliferation of donor T cells in NOD2−/− allo-BM transplant recipients. Our results suggest that loss of intestinal epithelial cell function causing impaired antibacterial resistance, which has been proposed as a mechanism for increased inflammation in NOD2−/− mice,69 is not responsible for the increased GVHD and colitis. Our results are in agreement with the findings of Strober and colleagues (Strober et al,70 Watanabe et al,71-73 and Yang et al74 ), who found increased susceptibility to different types of experimental colitis in NOD2−/− mice that was due to enhanced ability of NOD2−/− APCs to trigger inflammatory T-cell responses.
In clinical allo-HSCT, multiple studies have been performed on the relation of NOD2 polymorphisms and the incidence of GVHD. Several studies show a connection between NOD2 SNPs and increased GVHD incidence as well as GVHD severity. Our group (Holler et al) was the first to investigate the impact of NOD2 SNPs (SNPs 8 [Arg702Trp], 12 [Gly908Arg], and 13 [Leu1007fsinsC]) on clinical outcome after allo-HSCT. We screened donors and recipients of an allogeneic HSC transplant in several European transplant centers in search of SNPs in the LRR region of the NOD2 gene and compared the results to clinical outcome. In a first single-center analysis and also in a subsequent multicenter study, we found an association between a higher incidence of GVHD and NOD2 SNPs of the donor or recipient.75,76 These associations were particularly strong in recipients of an HLA-identical sibling allograft, whereas only the donor L1007insC SNP predicted increased nonrelapse mortality in recipients of an HLA-matched unrelated donor allograft. This difference could reflect either a higher baseline mortality after unrelated donor SCT or the higher frequency of both donor and recipient SNPs in relatives.77 Because the lung has also an extensive exposure to bacterial ligands, we tested the hypothesis that a defective inflammatory response might trigger alloreactivity. In a large analysis of more than 400 patients, the cumulative incidence of bronchiolitis obliterans syndrome was 23% in patients with NOD2 SNPs but only 1% in recipients without NOD2 SNPs.78 Interestingly, Donnelly and coworkers confirmed the connection between NOD2 SNPs and GVHD in a smaller study on T cell–depleted allo-HSCT using the same SNPs (8, 12, and 13; van der Velden et al).79 They found that there was significantly more GVHD when NOD2 SNPs were present in the donor or in the recipient. The effect was strongest in allo-HSC transplant recipients with NOD2 SNPs receiving a transplant from a donor with NOD2 SNPs. The authors hypothesized that defective antimicrobial activity due to NOD2 dysfunction in hematopoietic cells and epithelial cells leads to immune dysregulation. In a large single-center analysis, Elmaagacli et al also found increased incidence of GVHD in allo-HSC transplant recipients with NOD2 SNPs receiving grafts from donors with NOD2 SNPs (8, 12, and 13).53 Surprisingly, GVHD was reduced in wild-type recipients of grafts from donors with NOD2 SNPs. The authors hypothesized that the NOD2 SNPs on the donor side could negatively influence alloreactivity of donor T cells, leading to reduced GVHD.
In contrast to the studies described previously, other transplant centers could not find a significant impact of NOD2 SNPs on the incidence of GVHD. Granell et al reported that the NOD2 SNPs 8, 12, and 13 were not associated with acute or chronic GVHD, but still increased mortality because of pulmonary complications, in a small cohort of 85 patients receiving CD34-selected HLA-identical sibling transplants.80 Sairafi et al also found no significant impact of these NOD2 SNPs on incidence of acute GVHD in a series of 198 patients who underwent transplantation in Stockholm.81 In a study by Marsh and coworkers, in a similarly sized patient cohort the incidence of acute GVHD was low and there was no significant difference due to the presence of NOD2 SNPs (8, 12, and 13), but this trial focused on unrelated donor transplants recipients undergoing alemtuzumab prophylaxis (Mayor et al).82 Steinbach and colleagues also found no significant impact of these 3 NOD2 SNPs on the incidence of GVHD in a cohort of 231 children who underwent allo-HSCT (Gruhn et al).83
The conflicting results between the different clinical studies on the role of NOD2 polymorphisms during GVHD are most likely explained by differences between the study cohorts including the NOD2 SNP frequency, overall incidence of GVHD, T-cell depletion, type of conditioning regimen, intestinal microbial decontamination, donor source, and environmental factors. In conclusion, it appears that polymorphisms in the NOD2 gene locus of the recipient can negatively influence survival after allogeneic HSCT. However, transplantation-specific strategies such as T-cell depletion or gut decontamination may alter the activation of dendritic cells or epithelial cells and the role of NOD2 in these processes. The effect of NOD2 SNPs of the allo-HSC transplant donor on the incidence of GVHD is controversial. Experimental data suggest that there is no major effect of NOD2 on the allo-HSC transplant donor side on the regulation of GVHD.65
In addition, functional data on the impact of NOD2/CARD15 SNPs in humans are urgently needed: As shown for inflammatory bowel disease, peripheral blood mononuclear cells from recipients and donors with NOD2/CARD15 SNPs have a significantly decreased capacity to release IL-8 after stimulation with the ligand MDP.75,77,78,84,85 More recently, we observed a diminished recruitment of both CD4 cells and neutrophils in intestinal biopsies from patients suffering from gastrointestinal GVHD, indicating that NOD2/CARD15 may play a role in recruitment of these cells via chemokines released by APCs.86 Based on these data, GVHD can be seen as an imbalance in protective CD4 cells that is further enhanced in the presence of NOD2/VCARD15 SNPs.
Table 1 summarizes studies investigating innate immune receptors during GVHD.
Studies on innate immune receptors during GVHD
| PRR/species . | Observation . | Reference no. . |
|---|---|---|
| TLR4 | ||
| Human | TLR4 polymorphisms on patient and donor side cause more severe GVHD | 53 |
| Human | TLR4 polymorphisms do not cause more severe GVHD | 52 |
| Mouse | LPS antagonism results in less lethal GVHD | 27 |
| Mouse | LPS antagonism protects against lung injury after allo-BMT | 48 |
| TLR7 | ||
| Mouse | TLR7 agonism controls trafficking of T cells to GVHD target organs | 54 |
| TLR7/TLR8 | ||
| Mouse | TLR7/8 agonism after allo-BMT increases GVHD | 55 |
| Mouse | TLR7/8 agonism before allo-BMT ameliorates GVHD | 56 |
| TLR9 | ||
| Mouse | TLR9 agonism increases GVHD | 55 |
| Mouse | Lethal GVHD is reduced in TLR9−/− allo-BM transplant recipients | 50 |
| NOD2 | ||
| Human | NOD2 polymorphisms on patient and donor side cause more severe GVHD | 75 |
| Human | NOD2 polymorphisms on patient and donor side cause more severe GVHD | 76 |
| Human | NOD2 polymorphisms on patient and donor side cause more severe GVHD in recipients of a T cell–depleted HSC transplant | 79 |
| Human | NOD2 polymorphisms on patient and donor side cause more severe GVHD; Incidence of GVHD is reduced when NOD2 polymorphisms are on the donor side | 53 |
| Human | Increased pulmonary complications and death in patients with NOD2 polymorphisms after allo-HSCT. No significant difference in GVHD | 80 |
| Human | GVHD is not significantly increased in patients with NOD2 polymorphisms | 81 |
| Human | GVHD is not significantly increased in patients with NOD2 polymorphisms; Leukemia relapse rate is increased after allo-HSCT in patients with NOD2 polymorphisms | 82 |
| Human | GVHD is not significantly increased in children with NOD2 polymorphisms | 83 |
| Human | NOD2 polymorphisms in allo-HSC transplant recipients with GVHD are associated with diminished recruitment of CD4+ T cells and neutrophils in intestinal biopsies | 86 |
| Mouse | Lethal GVHD is increased in NOD2−/− allo-BM transplant recipients; NOD2 negatively regulates antigen-presenting cell (APC) function during GVHD | 65 |
| PRR/species . | Observation . | Reference no. . |
|---|---|---|
| TLR4 | ||
| Human | TLR4 polymorphisms on patient and donor side cause more severe GVHD | 53 |
| Human | TLR4 polymorphisms do not cause more severe GVHD | 52 |
| Mouse | LPS antagonism results in less lethal GVHD | 27 |
| Mouse | LPS antagonism protects against lung injury after allo-BMT | 48 |
| TLR7 | ||
| Mouse | TLR7 agonism controls trafficking of T cells to GVHD target organs | 54 |
| TLR7/TLR8 | ||
| Mouse | TLR7/8 agonism after allo-BMT increases GVHD | 55 |
| Mouse | TLR7/8 agonism before allo-BMT ameliorates GVHD | 56 |
| TLR9 | ||
| Mouse | TLR9 agonism increases GVHD | 55 |
| Mouse | Lethal GVHD is reduced in TLR9−/− allo-BM transplant recipients | 50 |
| NOD2 | ||
| Human | NOD2 polymorphisms on patient and donor side cause more severe GVHD | 75 |
| Human | NOD2 polymorphisms on patient and donor side cause more severe GVHD | 76 |
| Human | NOD2 polymorphisms on patient and donor side cause more severe GVHD in recipients of a T cell–depleted HSC transplant | 79 |
| Human | NOD2 polymorphisms on patient and donor side cause more severe GVHD; Incidence of GVHD is reduced when NOD2 polymorphisms are on the donor side | 53 |
| Human | Increased pulmonary complications and death in patients with NOD2 polymorphisms after allo-HSCT. No significant difference in GVHD | 80 |
| Human | GVHD is not significantly increased in patients with NOD2 polymorphisms | 81 |
| Human | GVHD is not significantly increased in patients with NOD2 polymorphisms; Leukemia relapse rate is increased after allo-HSCT in patients with NOD2 polymorphisms | 82 |
| Human | GVHD is not significantly increased in children with NOD2 polymorphisms | 83 |
| Human | NOD2 polymorphisms in allo-HSC transplant recipients with GVHD are associated with diminished recruitment of CD4+ T cells and neutrophils in intestinal biopsies | 86 |
| Mouse | Lethal GVHD is increased in NOD2−/− allo-BM transplant recipients; NOD2 negatively regulates antigen-presenting cell (APC) function during GVHD | 65 |
Conclusions and future directions
Recent research has illuminated details as to how stimulation of innate immune receptors by microbial-associated molecules significantly contributes to the inflammatory processes that consequently lead to recruitment of alloactivated T cells as well as tissue damage in GVHD target organs. The inflammatory cascade that characterizes GVHD is initiated by PAMPs that activate PRR signaling in resident innate immune cells, epithelial cells, and endothelial cells. The stimulation of PRRs lead to transcription of inflammatory genes and processing of proinflammatory cytokines, resulting in local tissue inflammation, migration of leukocytes, presentation of host antigens, and eventually antihost reactivity of donor T cells.
To date, most therapeutic and prophylactic efforts in GVHD, such as T-cell depletion or inhibition of T-cell activation/proliferation, intervene with the T cell–dependent effector phase. The main drawback of this approach is the inhibition of graft-versus-tumor activity and reduction of antimicrobial immunity mediated by donor T cells, which are major determinants of the overall outcome in HSCT. Finding therapeutic strategies exclusively targeting unwanted GVH reactions toward epithelial surfaces and sparing the beneficial reactivity toward malignant host cells and microbial pathogens could be the key to improve survival in patients undergoing HSCT. One such strategy is the prophylactic or therapeutic intervention in innate immunity aiming at the activation of innate immune cells by PAMPs; an additional strategy would aim for local modulation of epithelial and/or dendritic cells. Hopefully, improved understanding of the cellular players, receptors, and signaling pathways of innate immunity in GVHD will provide the basis for development of new and effective therapeutic tools in the near future.
Acknowledgments
The authors thank Sabine Czylwik and Ulrike Heider for proofreading the paper.
Authorship
Contribution: O.P., E.H., and M.R.M.v.d.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcel van den Brink, Departments of Immunology and Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY 10065; e-mail: vandenbm@mskcc.org.