Abstract
We report a rapid and highly efficient approach to generate mice in which the hematopoietic system is derived from embryonic stem (ES) cells. We show that ES cells injected into blastocysts from the c-kit–deficient W41/W41 mouse strain have a clear advantage over the W41/W41 blastocyst-derived inner cell mass cells in founding the hematopoietic system. Fetal liver hematopoietic stem cells from W41/W41 blastocyst complementation embryos can be transplanted to establish large cohorts of bone marrow chimeras with hematopoiesis of practically pure ES-cell origin. Using ES cells with site-directed modifications, we show how this system can be used to drive inducible transgene expression in hematopoietic cells in a robust and reliable manner both in vitro and in vivo. The approach avoids the cost and time constraints associated with the creation of standard transgenic mouse strains while taking advantage of the sophisticated site-directed manipulations that are possible in ES cells.
Introduction
To date, the most common approaches to study hematopoieisis and hematopoietic stem cells (HSCs) in genetically engineered systems have been the creation of transgenic mouse strains or the use of viral vectors to deliver genes directly into the hematopoietic cells. For many purposes, the generation of transgenic mice is limited by time and cost aspects, whereas the use of viral vectors often is coupled with constraints, including limited control of expression, transduction difficulties, and insertional mutagenesis.1-3 We have sought to establish an alternative system that would take advantage of the versatile and site-directed genetic manipulations that are possible in embryonic stem (ES) cells. Genetically modified ES cells can be injected into blastocysts, and hematopoietic cells can be rapidly derived from the fetal liver of chimeric embryos. The fetal liver in day 14 to 15 mouse embryos is the main site of hematopoiesis and contains large numbers of transplantable long-term repopulating stem cells.4,5 The use of this approach is, however, limited by the highly variable levels of chimerism obtained from standard blastocyst complementation procedures. Blastocyst complementations using tetraploid blastocysts generate offspring of 100% ES-cell origin, but this requires special embryo manipulation techniques that are not available to most laboratories and many ES-cell lines fail to generate offspring by this procedure.6-8 Here, we reasoned that implanting ES cells into blastocysts from a mouse strain with impaired HSC function could give the ES cells an advantage over the blastocyst inner cell mass cells in founding the hematopoietic system and generate embryos with hematopoiesis of preferential ES-cell origin. A similar approach has previously been demonstrated for the generation of mice with purely ES cell–derived B and T lymphocytes by injecting ES cells into RAG-deficient blastocysts.9
Methods
ES cells
ES cells with constitutive green fluorescent protein (GFP) expression were generated by transduction of KH2 ES cells10 with the pLKO.1-GFP lentivirus and subsequent cloning of GFP+ cells. ES cells with inducible GFP expression were generated by ligating a GFP cassette between the MluI and EcoRI sites of the pBS31 vector, which was then used to target KH2 ES cells as described.10 For blastocyst complementations, ES cells were injected into W41/W41 or wild-type (C57/B6 or NFR/N) embryonic day (E) 3.5 blastocysts.
Antibodies
For identification of lineage-negative cells from fetal liver, antibodies against B220, CD5, Gr1, and Ter119 were used; for bone marrow cells, CD4 and Mac-1 were also included. In addition, antibodies against c-Kit, Sca-1, CD45.1, and CD45.2 were used as indicated. All antibodies were from BioLegend.
Cell culture
Fetal liver cells were depleted of Ter119+ cells using Dynabeads (Invitrogen) and cultured in serum-free medium (StemSpanSFEM; StemCell Technologies) supplemented with cytokines recombinant murine stem cell factor, recombinant human thrombopoietin, recombinant human interleukin-6, and recombinant human FMS-like tyrosine kinase-3 at 50 ng/mL and recombinant human interleukin-3 at 20 ng/mL (all cytokines from PeproTech). GFP expression was induced with doxycycline (Sigma-Aldrich) at indicated concentrations.
Transplantation assays
A total of 2 million unfractionated fetal liver cells were transplanted into lethally irradiated (900 Gy) B6/SJL recipient mice. Doxycycline was given in the drinking water supplemented with 10 mg/mL sucrose. Control animals were only given sucrose.
Results and discussion
To test whether ES cells injected into blastocysts from a strain with impaired HSC function would have an advantage in founding the hematopoietic system, we used the W41/W41 mouse strain. These mice have a point mutation in the gene for the c-kit receptor, which negatively affects both the numbers and function of HSCs.11-15 Importantly, homozygous mutant mice are viable and fertile and can therefore easily be used as donors for blastocysts without any screening for genotype. Although it has been shown that intact c-kit signaling is not required for the generation and expansion of HSCs during early development,13,15,16 we hypothesized that, in the competitive setting of a developing chimeric embryo, the W41/W41 HSCs would exhibit reduced fitness compared with the normal ES cell–derived HSCs.
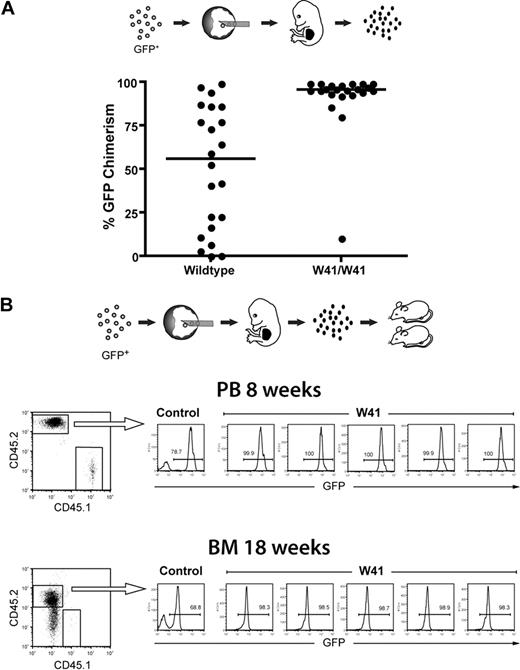
GFP-labeled ES cells were injected into wild-type or W41/W41 blastocysts, and fetal livers of E14.5 embryos were isolated and analyzed by fluorescence-activated cell sorter (FACS) to assess the percentage of cells that were of ES-cell origin. As shown in Figure 1A, the lineage-negative fetal liver cells from W41/W41 blastocysts had a very high percentage of ES cell–derived cells (> 90% in the majority of cases). By contrast, fetal livers from wild-type blastocysts had a widespread distribution of GFP chimerism. These results clearly demonstrate that the HSC defect in W41/W41 blastocysts gives ES cells a selective advantage in founding the hematopoietic system during early development and reveal a previously undefined role for c-kit signaling in regulation of the earliest stages of embryonic hematopoiesis.
High-level fetal liver chimerism in embryos generated from W41/W41 blastocyst complementations. (A) GFP-expressing ES cells were injected into wild-type or W41/W41 blastocysts, and fetal livers were isolated from E14.5 embryos. The percentage of GFP+ cells in each liver was measured by FACS analysis within the Lin− subpopulation. Distribution of GFP chimerism in the fetal liver of embryos from W41/W41 or wild-type blastocysts. (B) GFP+ ES cells were injected into W41/W41 or wild-type blastocysts. E14.5 embryos were harvested, and 2 million chimeric fetal liver cells (CD45.2) were transplanted into CD45.1 recipient mice. FACS profiles for 5 mice transplanted with pooled cells from 4 randomly picked W41/W41 chimeric embryos. A control mouse transplanted with cells from a high-level wild-type chimera is shown as reference. Percentage of GFP+ donor cells in peripheral blood (PB) and bone marrow (BM) after 8 and 18 weeks, respectively.
High-level fetal liver chimerism in embryos generated from W41/W41 blastocyst complementations. (A) GFP-expressing ES cells were injected into wild-type or W41/W41 blastocysts, and fetal livers were isolated from E14.5 embryos. The percentage of GFP+ cells in each liver was measured by FACS analysis within the Lin− subpopulation. Distribution of GFP chimerism in the fetal liver of embryos from W41/W41 or wild-type blastocysts. (B) GFP+ ES cells were injected into W41/W41 or wild-type blastocysts. E14.5 embryos were harvested, and 2 million chimeric fetal liver cells (CD45.2) were transplanted into CD45.1 recipient mice. FACS profiles for 5 mice transplanted with pooled cells from 4 randomly picked W41/W41 chimeric embryos. A control mouse transplanted with cells from a high-level wild-type chimera is shown as reference. Percentage of GFP+ donor cells in peripheral blood (PB) and bone marrow (BM) after 8 and 18 weeks, respectively.
We then transplanted lethally irradiated recipients with fetal liver cells from the W41/W41 blastocyst complementations. Analysis of peripheral blood after 8 weeks and bone marrow after 18 weeks showed that essentially all donor cells expressed GFP and were of ES-cell origin with no signs of W41/W41 blastocyst-derived cells (Figure 1B). This is in agreement with previous work showing that W41/W41 FL HSCs have a competitive disadvantage in transplantation assays.13 The complete ES-cell origin of donor cells was further confirmed by PCR on bone marrow colonies where 100% (80 of 80) of colonies analyzed were of ES-cell origin (data not shown). Taken together, the combined W41/W41 blastocyst complementation and fetal liver transplantation approach described here makes it possible to generate large cohorts of mice with hematopoiesis of practically pure ES-cell origin in just a few weeks. The approach is further very flexible and cost-effective because fetal liver cells can be freezer-stored while maintaining a high repopulation ability, and we have found that a single chimeric fetal liver contains sufficient stem cells for robust engraftment of at least 20 recipients (L.J., unpublished observations, May 2007).
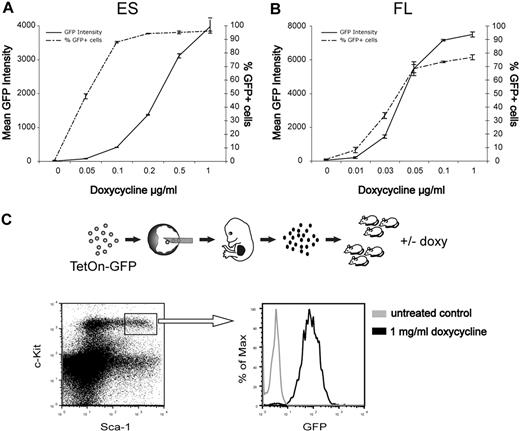
We next tested the potential of these findings for genetic studies in the hematopoietic system. We used a pre-engineered ES cell line (KH2) in which tetracycline-inducible transgenes are inserted by FLT/FRT recombination into the collagen 1A locus to ensure predictable and robust expression levels.10,17 KH2 cells were targeted to generate ES cells with tetracycline-inducible GFP expression. When cultured under different concentrations of doxycycline, these cells showed a dose-dependent induction of GFP expression (Figure 2A). The ES cells were then injected into W41/W41 blastocysts to produce fetal liver stage embryos. Cultured fetal liver cells showed similar doxycycline-inducible and dose-dependent GFP expression as the original ES cells (Figure 2B). Finally, we transplanted lethally irradiated mice with fetal liver cells and after reconstitution administered doxycycline in the drinking water during 1 week to 1 cohort of mice and left the others untreated. GFP expression was efficiently induced in the lineage −c-kit+Sca1+ compartment of induced mice, whereas no GFP could be detected in noninduced control mice (Figure 2C), demonstrating that the gene induction can be tightly controlled in primitive hematopoietic cells in vivo. Inducible control of gene expression is an important feature, especially when evaluating effects on self-renewal of HSCs or when assessing malignant phenotypes.18
Tightly regulated doxycycline-inducible GFP expression in ES cells and hematopoietic cells. (A) ES cells were cultured with indicated concentrations of doxycycline and analyzed by FACS for GFP fluorescence after 3 days. Data are mean ± SD of triplicate cultures from a representative experiment. (B) Fetal liver cells were depleted of red cells and cultured in serum-free medium supplemented with cytokines and the indicated concentrations of doxycycline. GFP fluorescence was measured by FACS after 3 days. Data are mean ± SD of duplicate cultures from a representative experiment. (C) Lethally irradiated mice were transplanted with GFP-inducible fetal liver cells. Four months after transplantation, half of the mice were given doxycycline in the drinking water for 1 week and the mice were then killed for bone marrow analysis. Representative FACS profiles depicting GFP expression within the lineage −c-kit+Sca1+ compartment.
Tightly regulated doxycycline-inducible GFP expression in ES cells and hematopoietic cells. (A) ES cells were cultured with indicated concentrations of doxycycline and analyzed by FACS for GFP fluorescence after 3 days. Data are mean ± SD of triplicate cultures from a representative experiment. (B) Fetal liver cells were depleted of red cells and cultured in serum-free medium supplemented with cytokines and the indicated concentrations of doxycycline. GFP fluorescence was measured by FACS after 3 days. Data are mean ± SD of duplicate cultures from a representative experiment. (C) Lethally irradiated mice were transplanted with GFP-inducible fetal liver cells. Four months after transplantation, half of the mice were given doxycycline in the drinking water for 1 week and the mice were then killed for bone marrow analysis. Representative FACS profiles depicting GFP expression within the lineage −c-kit+Sca1+ compartment.
In conclusion, we have established an inexpensive and efficient strategy for the rapid creation of mice with purely ES cell–derived hematopoiesis. Combined with site-directed modifications for inducible expression in ES cells, this is a versatile and accurate method for genetic studies of the mouse hematopoietic system.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Konrad Hochedlinger and Rudolph Jaenisch for the KH2 ES-cell line and Mary-Ann Sällström and Ragnar Mattson at the transgenic core facility, Lund University, for expert technical assistance with blastocyst complementations.
This work was supported by grants from the Swedish Research Council (Stockholm, Sweden), the Swedish Cancer Foundation (Stockholm, Sweden), and the Swedish Childhood Cancer Foundation (Stockholm, Sweden) to J.L.
Authorship
Contribution: J.L. conceptualized and designed the study together with L.J.; L.J. performed all experiments; and L.J. and J.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonas Larsson, Molecular Medicine and Gene Therapy, BMC A12, 22184, Lund, Sweden; e-mail jonas.larsson@med.lu.se.