Abstract
Extracellular matrix metalloproteinase inducer (EMMPRIN/CD147) is thought to promote tumor angiogenesis mostly through its protease-inducing function and more recently by its ability to increase tumor cell expression of vascular endothelial growth factor (VEGF). In this study, we present evidence that EMMPRIN can promote angiogenesis by a direct effect on endothelial cells through a paracrine regulation of the VEGF/VEGF-receptor (VEGFR) system. Using human microvascular endothelial cell line–1 endothelial cells, we show that EMMPRIN selectively increased the soluble VEGF isoforms (121 and 165), but not the matrix-bound VEGF 189 form. In addition, EMMPRIN up-regulated the expression of VEGFR-2 without an effect on VEGFR-1. This increase in VEGFR-2 was responsible for the observed EMMPRIN stimulation of the migratory and tube formation capacity of endothelial cells. EMMPRIN′s effects, which were matrix metalloproteinase and urokinase-type plasminogen activator independent, were mediated primarily through hypoxia-inducible factor-2α expression, also up-regulated by EMMPRIN. VEGFR-2 increase was also observed in vivo in a mouse model of xenograph tumors overexpressing EMMPRIN. These results suggest that in addition to increasing protease production, EMMPRIN may contribute to the formation of a reactive stroma also through the up-regulation of hypoxia-inducible factor-2α, VEGFR-2, and the soluble forms of VEGF in endothelial cells, thus directly regulating the angiogenic process.
Introduction
Angiogenesis is essential for the primary and metastatic growth of tumors, and has become a target for anticancer therapy, although the mechanisms by which the tumor regulates angiogenesis are not fully known. The ability of tumors to recruit endothelial cells and stimulate their proliferation, migration, or survival is thought to be central in tumor-induced angiogenesis. Much attention has been focused on the vascular endothelial growth factor (VEGF) family of growth factors and the receptor tyrosine kinases that mediate their angiogenic effects.1,2 The implication of VEGF as a mediator of tumor angiogenesis is supported by multiple evidence. Up-regulation of VEGF has been observed in many human tumors, and VEGF expression is closely correlated with tumor progression and less favorable prognosis.3-6 Furthermore, monoclonal antibodies to VEGF and other anti-VEGF treatments have been shown to inhibit growth of several tumor cell lines in nude mice.7,8 VEGF expression can be induced by numerous environmental factors. Several growth factors, oncogenic proteins, or transcription factors were shown to up-regulate VEGF mRNA expression. VEGF gene expression is also induced by exposure to low oxygen tension.
The biologic significance of VEGF depends on the content and ratio of the different isoforms of this growth factor and on the expression of their receptors. VEGF is expressed through alternative splicing as 6 different isoforms (VEGF121, VEGF145, VEGF165, VEGF183, VEGF189, and VEGF206) having, respectively, 121, 145, 165, 183, 189, and 206 amino acids that have been described.9 VEGF121, VEGF165, and VEGF189 are the predominant isoforms secreted by a wide range of normal and transformed cells.10 Whereas VEGF121 diffuses relatively freely in tissues, VEGF189 has high affinity for heparin and is almost completely retained in the extracellular matrix (ECM); VEGF165 exists in both a soluble and an ECM-bound form.11 Thus, the ECM represents an important source of VEGF that can be released in a diffusible and bioactive form by proteolysis.
VEGF acts through 2 high-affinity receptor tyrosine kinases, VEGF receptor (VEGFR)-1/flt-1 and VEGFR-2/kinase domain receptor (KDR)/flk-1; both are expressed on normal vascular endothelial cells.12 VEGF signals mainly through VEGFR-2, which upon ligand binding becomes tyrosine phosphorylated and activates multiple signaling networks that lead to increase proliferation, sprouting, migration, and tube formation of endothelial cells.13-15 VEGFR-2 is expressed at elevated levels by endothelial cells engaged in angiogenesis. VEGFR-1 is expressed not only in endothelial cells, but also in macrophage-lineage cells, and was shown to promote tumor growth and inflammation at least partly in a macrophage-dependent manner.16 The role of VEGFR-1 in neoangiogenesis is much less clear, as it binds VEGF with approximately 10 times the affinity of VEGFR-2 binding, but its signal-transducing properties are extremely weak.17 As was already shown in embryogenesis, VEGFR-1 may function as a negative regulator of angiogenesis, by binding and trapping VEGF and preventing its binding to VEGFR-2.18
It is generally held that in tumor angiogenesis, VEGF action is attributable to a paracrine mechanism by tumor cells, which produce VEGF, but cannot respond to it directly because they do not have cell surface VEGFRs. In contrast, endothelial cells engaged in angiogenesis express numerous VEGFRs, but they produce only low levels of endogenous VEGF.19 However, a recent study has shown that this low expression of endogenous autocrine-acting VEGF is nevertheless crucial for endothelial cell survival and is needed to regulate vascular homeostasis by signaling through intracellular VEGFR-2.20 It seems, therefore, that even though VEGF can originate from a variety of cells and be present in sufficient amount in the tumor environment, its expression by the endothelial cells themselves may present an essential role in driving the angiogenesis process.
To form new blood vessels, activated endothelial cells must degrade the basement membrane of the original vessel and remodel the ECM around neovasculature sites, and proteases, such as the matrix metalloproteinase (MMPs) and the urokinase-type plasminogen activator (uPA), have been largely implicated in this process. CD147/extracellular MMP inducer (EMMPRIN), a membrane glycoprotein greatly enriched on the surface of tumor cells, was shown to stimulate neighboring stromal cells, such as fibroblasts and endothelial cells, to increase their synthesis of several MMPs.21-23 EMMPRIN was also shown to increase expression of the plasminogen activation system, including uPA and its receptor, both in tumor and endothelial cells, further increasing its proteolytic potential in the stroma.24 Elevated EMMPRIN levels were detected in numerous malignant tumors and have been correlated with tumor progression in experimental and clinical conditions.25,26 In some cases, it was also associated with poor prognosis.25,27-29 Experimentally increasing EMMPRIN by cDNA transfection into human breast cancer cells greatly enhanced tumor growth in nude mice.30
The role of EMMPRIN in tumor progression has been attributed mostly to its protease-inducing function in both tumor cells and the surrounding stromal cells. However, Tang et al31 have recently reported that the up-regulation of EMMPRIN in MDA-MB231 tumor cells can also increase VEGF expression in these cells, evoking a potential role of EMMPRIN in tumor angiogenesis. This prompted us to explore whether EMMPRIN can promote angiogenesis through a direct effect on endothelial cells in a paracrine manner. Therefore, the effect of EMMPRIN on the expression of VEGF as well as that of its receptors in endothelial cells was examined.
Methods
Cell culture
Human microvascular endothelial cell line (HMEC-1) derived from dermal microvasculature (T. Lawley, Emory University, Atlanta, GA) was maintained in MCDB-131 medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS), 2 mL glutamine (Invitrogen), 10 ng/mL endothelial growth factor (Upstate Biotechnology/Millipore), and 1 μg/mL hydrocortisone (Sigma-Aldrich). Primary human umbilical vein endothelial cells (HUVECs) obtained from PromoCell (Promo Cell) were cultured in endothelial cell growth medium (Promo Cell) following the instructions. HUVECs at early passages (passages 2-4) were used in all of the experiments. Chinese hamster ovary (CHO) cells (ATCC) were cultured in Dulbecco modified Eagle medium/F12 (Invitrogen) supplemented with 10% FBS and 2 mL glutamine. CHO cells were transfected with a plasmid containing EMMPRIN full-lengh cDNA, as previously described,24 and stably transfected cells were designated as CHO-Emp cells.
In the different experiments, HMEC-1 cells were incubated with 20 μg/mL membrane extract from CHO-Emp or CHO control cells, designated Emp and Ctl, respectively. To determine how many CHO-Emp, or, by comparison, tumor cells (mammary and melanoma MDA-MB231 and WM278 obtained from ATCC and Wistar Institute, respectively) are needed to deliver 20 μg of EMMPRIN-enriched membranes, membrane fractions were immunoblotted for EMMPRIN alongside extracts obtained from increasing number of the above cells. The results show that 20 μg of EMMPRIN-enriched membranes corresponds to between 25 000 (CHO-Emp and WM278) and 50 000 (MDA-MB231) cells. Coculture experiments were performed by incubating endothelial cells (HMEC-1 or HUVEC) with either EMMPRIN- or mock-transfected CHO cells at a 1:1 ratio for 24 hours.
In some experiments, cells were incubated with either EMMPRIN blocking antibody (20 μg/mL, UM-8D6; Ancell), uPA inhibitor amiloride (25nM; Sigma-Aldrich), MMP inhibitor marimastat (20μM; British Biotechnology), anti-uPA blocking antibody (50 μg/mL; American Diagnostica), recombinant tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 (20 μg/mL), or the iron chelator deferoxamine (Sigma-Aldrich) to mimic hypoxia (100μM).
Membrane preparation
Cell membranes were isolated, as previously described.32 Briefly, CHO cells were scrapped in serum-free medium containing 1/100 protease inhibitor mixture Set V (AEBSF [4-(2-aminoethyl)benzene sulfonyl hydrocholoride], aprotinin, E-64, leupeptin, and 1mM EDTA [ethylenediaminetetraacetic acid]), sonicated on ice, and centrifuged at 1000g for 10 minutes at 4°C to remove unbroken cells. After removal of the organelles by 19 000g centrifugation for 30 minutes, the membranes were pelleted at 100 000g for 1 hour, washed, and resuspended in serum-free Dulbecco modified Eagle medium. The bioactivity of EMMPRIN-containing membranes was verified by its activity in stimulating uPA expression in HMEC-1 cells.24
Enzyme-linked immunosorbent assay measurement of VEGF
HMEC-1 cells were seeded in 12-well plates (105 cells/well). After 24 hours, the cells were incubated with serum-free medium for 6 hours and then treated for 1 hour with 20 μg/mL anti-EMMPRIN blocking antibody (Covalab) or with an immunoglobulin G (IgG) control antibody before the addition of Emp for 24- and 48-hour incubation. The concentration of VEGF protein in the conditioned media (CM) of treated HMEC-1 cells was determined using human VEGF enzyme-linked immunosorbent assay (ELISA) quantikine kit (R&D Systems). This kit recognizes the different VEGF isoforms, and the detection limit was 5 pg/mL. Results were normalized to cell number in the corresponding well at the time of sample collection.
RNA extraction, reverse transcription, and real-time quantitative polymerase chain reaction
Total RNA was isolated using TRIzol (Invitrogen). cDNA was synthesized using random hexamers and Moloney murine leukemia virus (Invitrogen), according to the manufacturer's protocol. VEGF isoforms (121, 165, and 189), VEGFRs (R1 and R2), hypoxia-inducible factor (HIF)-1α, and HIF-2α mRNA expression levels were measured by real-time quantitative polymerase chain reaction (qRT-PCR) using Perfect MasterMix-Probe (AnyGenes) on LightCycler 2.0 (Roche), according to the manufacturer's protocol. The expression levels of interest transcripts were normalized to the housekeeping TATA-box binding protein (TBP) gene transcripts.
Western blotting analysis
Cells were lysed in TBS-Nonidet P-40 solution comprising 50mM Tris buffer, pH 7.5, 150mM NaCl, 1% Nonidet P-40, 2mM EDTA (ethylenediaminetetraacetic acid), and protease inhibitor mixture Set V. Cell lysates (30 μg) and the concentrated CM (10 μg) were analyzed by being subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with anti-VEGF (Tebu-bio), anti–VEGFR-2 (R&D Systems), anti–HIF-1α (BD Biosciences), or anti–HIF-2α antibodies (Interchim). The proteins were visualized with enhanced chemiluminescence reagent, and their relative expression was determined by densitometry using ImageJ software program and normalized relative to the total protein concentration of cell lysates or to β-actin.
Small interfering RNA transfection
VEGF, VEGFR-1, VEGFR-2, HIF-1α, HIF-2α, and EMMPRIN small interfering RNA (siRNA) oligos (Ambion/Applied Biosystems) or scrambled siRNA oligos (25 nmol/L) were transfected into HMEC-1 cells using the BLOCK-iT transfection kit and Lipofectamine-2000 (Invitrogen), according to the manufacturer's protocol. Cells were then incubated for 24 hours before qRT-PCR and Western blotting, or trypsinized for endothelial capillary-like structure formation or migration assays.
Endothelial capillary-like structure formation
Human fibrinogen (Biogenic) suspended in phosphate-buffered saline was added to human thrombin (Calbiochem). Plates coated with this suspension (500 μL/well) were placed at 37°C for 1 hour. The fibrin gel was equilibrated overnight with MCDB-131 medium supplemented with 2% FBS. HMEC-1 previously transfected with siRNA directed against VEGF, VEGFR-2, or control siRNA were seeded (2.5 × 105 cells/well) on top of these gels in 1 mL of serum-free medium, and 20 μg/mL Emp was then added. A mix of human recombinant VEGF (25 ng/mL) and basic fibroblast growth factor (bFGF; 25 ng/mL; R&D Systems) was used as a positive control, and serum-free medium was used as a negative control. After 24 hours, the formation of capillary-like structures was photographed, and the measure of the capillary tube length was carried out using the ImageJ software program.
In vitro migration assay
In vitro migration was assessed using a modified Boyden chamber assay.33 HMEC-1 previously transfected with siRNA directed against VEGF, VEGFR-1, VEGFR-2, or control siRNA were seeded (105cells/well) on the upper chamber of the insert, and 20 μg/mL Emp was then added. Human recombinant VEGF (200 ng/mL; R&D Systems) was used as a positive control. After 48 hours of incubation, cells were fixed, stained with Diff Quik (Dade Behring), and counted under a microscope.
Animal experiments
All in vivo experiments were carried out with local ethical committee approval of University Paris 12 and according to United Kingdom Co-ordinating Committee on Cancer Research guidelines. Nu/nu mice (Janvier) were purchased at 4 weeks of age. Transformed mammary tumor cells NS2T2A24 were transfected with EMMPRIN cDNA, as described above for CHO cells. Suspensions of NS2T2A-Emp or NS2T2A-mock cells (4 × 106 in 100 μL of phosphate-buffered saline) were subcutaneously injected into the left side of 6 nude mice per group.24 Tumors were resected 5 weeks after injection and stored in liquid nitrogen before RNA extraction and immunohistochemistry.
Immunohistochemistry
Immunohistochemical analyses of tissue sections of tumors obtained as above were carried out using antibody directed against VEGFR-2 (R&D Systems). Sections were incubated overnight with the primary antibody and then incubated with the biotinylated secondary antibody. Peroxidase reactivity was visualized using 3-amino-9-ethylcarbazole (DakoCytomation).
Statistical analysis
Data are presented as the mean values plus or minus SD. Mann-Whitney test was used to evaluate differences between groups. P value less than .05 was accepted as significant.
Results
EMMPRIN up-regulates VEGF121, VEGF165, and VEGFR-2, but not VEGF189 and VEGFR-1 expression in endothelial cells
As EMMPRIN is an intrinsic membrane protein and primarily functions by direct cell-cell contact mechanism, we used EMMPRIN-enriched membranes to study EMMPRIN′s effect on endothelial cells. To determine whether EMMPRIN could induce VEGF production in a paracrine manner, we analyzed by ELISA the level of VEGF in the conditioned media of HMEC-1 cells treated with 20 μg/mL membranes issue from EMMPRIN-transfected CHO cells (designated Emp) and compared with membranes prepared in parallel from mock-transfected CHO cells. Western blot calibration using purified EMMPRIN has shown that 20 μg of Emp membrane proteins contained 0.5 μg of EMMPRIN, whereas none could be detected in the mock control membranes. By similar calibration alongside cell extracts from CHO-Emp and 2 different tumor cell lines, 20 μg of Emp was estimated to be derived from between 25 000 (CHO-Emp and WM278) and 50 000 (MDA-MB231) cells.
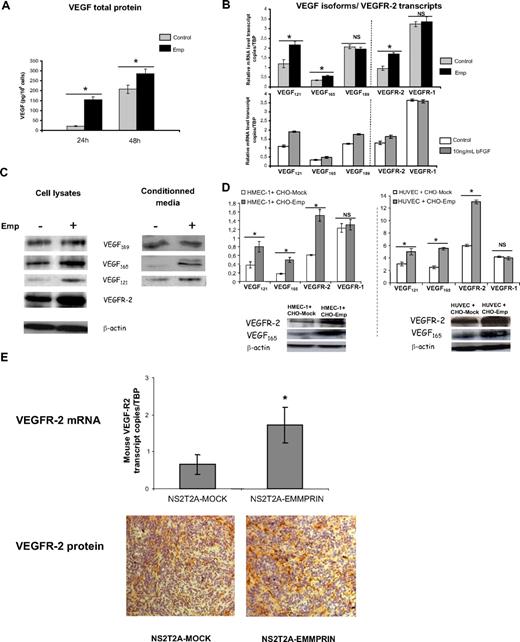
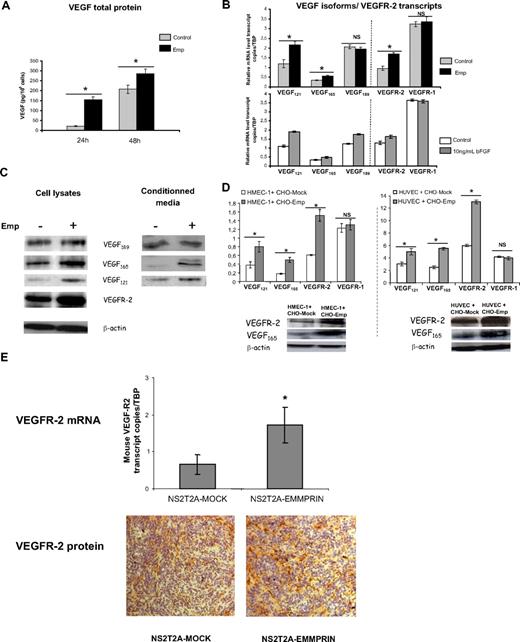
As shown in Figure 1A, treatment with 20 μg/mL Emp resulted in a marked increase in VEGF secretion, from 21 pg/106 cells in control cells to 154 pg/106 cells in Emp-treated cells after 24 hours and from 207 to 287 pg/106 cells after 48-hour incubation.
EMMPRIN up-regulates VEGF121, VEGF165, and VEGFR-2, but not VEGF189 and VEGFR-1 expression in endothelial cells. HMEC-1 cells were incubated with 20 μg/mL Emp in serum-free medium. (A) After 24 and 48 hours, CM were collected and soluble VEGF production was measured by ELISA. Results were normalized to the cell number and expressed as pg/106 cells. Columns indicate means of 3 independent experiments; and bars, SD. *P < .05. (B) Total RNA was extracted from HMEC-1 cells treated with Emp or with 10 ng/mL bFGF for 4 hours. mRNA levels for VEGF121, VEGF165, VEGF189, VEGFR-2, and VEGFR-1 were quantified using qRT-PCR. Columns indicate means of relative expression to TBP housekeeping gene of at least 3 independent experiments; and bars, SD. *P < .05. (C) Cell lysates were immunoblotted for VEGF isoforms and VEGFR-2 (β-actin was used as loading control). Concentrated CM were immunoblotted with anti-VEGF antibody. Band densities were expressed relative to total protein concentration of the corresponding cell lysates. Representative blots of 3 independent experiments. (D) HMEC-1 and HUVECs cocultured with EMMPRIN- or mock-transfected CHO cells. Total RNA was extracted from cocultured cells. mRNA levels for VEGF121, VEGF165, VEGFR-2, and VEGFR-1 were quantified using qRT-PCR. Columns indicate means of relative expression to TBP housekeeping gene of at least 3 independent experiments; and bars, SD. *P < .05. Cocultured cell lysates were immunoblotted for VEGF isoforms and VEGFR-2 (β-actin was used as loading control). Representative blots of 3 independent experiments. (E) EMMPRIN regulates mouse VEGFR-2 in xenograph tumors in vivo. Tumors grown in nude mice obtained from EMMPRIN- or mock-transfected mammary tumor sections (NS2T2A) were analyzed for mouse VEGFR-2 expression by both qRT-PCR and immunohistochemistry. Mouse VEGFR-2 transcripts were quantified using qRT-PCR. Columns indicate means of relative expression to mouse TBP housekeeping gene of at least 3 independent experiments. Bars indicate SD. *P < .05. Mouse flk-1/VEGFR-2 protein was analyzed by immunohistochemistry.
EMMPRIN up-regulates VEGF121, VEGF165, and VEGFR-2, but not VEGF189 and VEGFR-1 expression in endothelial cells. HMEC-1 cells were incubated with 20 μg/mL Emp in serum-free medium. (A) After 24 and 48 hours, CM were collected and soluble VEGF production was measured by ELISA. Results were normalized to the cell number and expressed as pg/106 cells. Columns indicate means of 3 independent experiments; and bars, SD. *P < .05. (B) Total RNA was extracted from HMEC-1 cells treated with Emp or with 10 ng/mL bFGF for 4 hours. mRNA levels for VEGF121, VEGF165, VEGF189, VEGFR-2, and VEGFR-1 were quantified using qRT-PCR. Columns indicate means of relative expression to TBP housekeeping gene of at least 3 independent experiments; and bars, SD. *P < .05. (C) Cell lysates were immunoblotted for VEGF isoforms and VEGFR-2 (β-actin was used as loading control). Concentrated CM were immunoblotted with anti-VEGF antibody. Band densities were expressed relative to total protein concentration of the corresponding cell lysates. Representative blots of 3 independent experiments. (D) HMEC-1 and HUVECs cocultured with EMMPRIN- or mock-transfected CHO cells. Total RNA was extracted from cocultured cells. mRNA levels for VEGF121, VEGF165, VEGFR-2, and VEGFR-1 were quantified using qRT-PCR. Columns indicate means of relative expression to TBP housekeeping gene of at least 3 independent experiments; and bars, SD. *P < .05. Cocultured cell lysates were immunoblotted for VEGF isoforms and VEGFR-2 (β-actin was used as loading control). Representative blots of 3 independent experiments. (E) EMMPRIN regulates mouse VEGFR-2 in xenograph tumors in vivo. Tumors grown in nude mice obtained from EMMPRIN- or mock-transfected mammary tumor sections (NS2T2A) were analyzed for mouse VEGFR-2 expression by both qRT-PCR and immunohistochemistry. Mouse VEGFR-2 transcripts were quantified using qRT-PCR. Columns indicate means of relative expression to mouse TBP housekeeping gene of at least 3 independent experiments. Bars indicate SD. *P < .05. Mouse flk-1/VEGFR-2 protein was analyzed by immunohistochemistry.
To determine whether this increased VEGF in the conditioned medium is due to a transcriptional up-regulation of the specific isoforms of VEGF, we conducted qRT-PCR assay using isoform-specific VEGF primers and probes. Figure 1B shows that only the soluble VEGF isoforms were significantly induced by EMMPRIN (1.8- and 1.6-fold for VEGF121 and VEGF165, respectively). Analysis of VEGFRs mRNA revealed that EMMPRIN also up-regulates VEGFR-2 transcript (∼ 1.7-fold) without an effect on VEGFR-1 (Figure 1B). To examine the specificity of this pattern of regulation by EMMPRIN, the results were compared with those obtained after treatment of HMEC-1 with bFGF, a known potent angiogenic factor. Unlike EMMPRIN, bFGF increased the transcription of all VEGF isoforms, including VEGF189 (1.7-, 1.5-, and 1.35-fold for VEGF121, VEGF165, and VEGF189, respectively), although its effect on the VEGFRs was similar to that of EMMPRIN (VEGFR-2, 1.25-fold).
This up-regulation was observed also at the protein level, as shown by the Western blot analysis. VEGF121 and VEGF165 protein levels were increased by approximately 45% and 80%, respectively, in the conditioned media, and by 55% and 60% in the cell lysates (Figure 1C) after treatment with Emp. VEGFR-2 protein level was increased by 65%.
We next performed coculture experiments using EMMPRIN- or mock-transfected CHO cells with HMEC-1 cells to confirm that the same effect could be observed using intact cells. Figure 1D shows that both VEGF and VEGFR-2 mRNA are up-regulated in HMEC-1 cells when cultured with CHO-Emp cells, as was found with the membrane fractions. Similar results were obtained with primary umbilical endothelial cells (HUVECs, Figure 1D), showing that this effect is not specific for the HMEC-1 cell line. Western blot analysis confirmed this regulation at the protein level in both cell type cocultures (Figure 1D).
In vivo analysis of VEGFR-2 expression, by both qRT-PCR and immunohistochemistry in sections obtained from tumors grown in nude mice (as we previously described24 ), suggests that a similar regulation of VEGFR-2 by EMMPRIN occurs in vivo (Figure 1E). Mouse VEGFR-2 transcripts were more expressed (∼ 2.4-fold) in the tumors obtained from EMMPRIN-transfected mammary tumor cells (NS2T2A) compared with mock-transfected cells. This was associated with a more intense staining of VEGFR-2 protein shown by immunostaining.
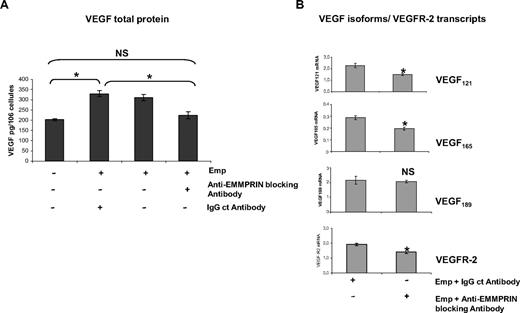
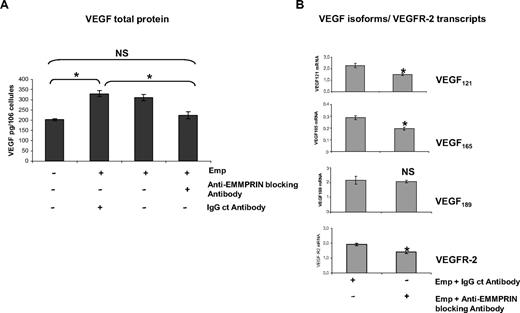
To evaluate the specificity of EMMPRIN′s effects, we then examined the effect of blocking anti-EMMPRIN antibody added with the EMMPRIN-containing membranes. As shown in Figure 2A, HMEC-1 cells treated for 48 hours with 20 μg/mL blocking anti-EMMPRIN antibody resulted in a significant decrease in VEGF level in the culture supernatants, whereas IgG control antibody had almost no effect. This down-regulation was also observed at the transcript level (Figure 2B), where the incubation with the anti-EMMPRIN antibody showed a maximal decrease at 2-hour incubation for VEGF121, VEGF165, and VEGFR-2 (∼ 25%, 35%, and 32%, respectively) with no effect on VEGF189.
Anti-EMMPRIN antibody inhibits VEGF isoforms and VEGFR-2 expression induced by EMMPRIN. (A) HMEC-1 cells were incubated with anti-EMMPRIN blocking antibody or with an IgG control antibody in serum-free medium before the addition of Emp for 48 hours, and VEGF in the CM was quantified by ELISA. Results were normalized to the cell number and expressed as pg/106 cells. Columns indicate means of 3 independent experiments; and bars, SD. *P < .05. (B) HMEC-1 cells were incubated for 2 hours with 20 μg/mL anti-EMMPRIN blocking antibody or with an IgG control antibody in serum-free medium. VEGF121, VEGF165,VEGF189, and VEGFR-2 transcripts were quantified by qRT-PCR. Columns indicate means of relative expression to TBP housekeeping gene of at least 3 independent experiments; and bars, SD. *P < .05.
Anti-EMMPRIN antibody inhibits VEGF isoforms and VEGFR-2 expression induced by EMMPRIN. (A) HMEC-1 cells were incubated with anti-EMMPRIN blocking antibody or with an IgG control antibody in serum-free medium before the addition of Emp for 48 hours, and VEGF in the CM was quantified by ELISA. Results were normalized to the cell number and expressed as pg/106 cells. Columns indicate means of 3 independent experiments; and bars, SD. *P < .05. (B) HMEC-1 cells were incubated for 2 hours with 20 μg/mL anti-EMMPRIN blocking antibody or with an IgG control antibody in serum-free medium. VEGF121, VEGF165,VEGF189, and VEGFR-2 transcripts were quantified by qRT-PCR. Columns indicate means of relative expression to TBP housekeeping gene of at least 3 independent experiments; and bars, SD. *P < .05.
EMMPRIN induces endothelial cell tube formation through VEGF/VEGFR-2
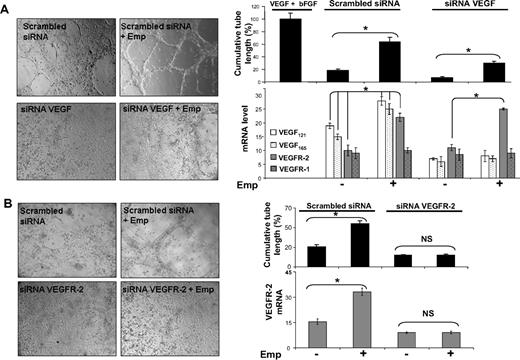
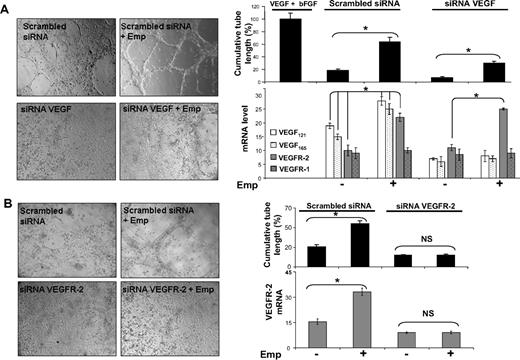
To determine the biologic implications of the regulation of VEGF by EMMPRIN, we studied tube formation by HMEC-1 cells grown on a fibrin gel (Figure 3). EMMPRIN was able to significantly induce tube formation of HMEC-1 cells (3.6-fold compared with control), reaching 60% of the maximal value obtained with the mix of human recombinants VEGF and bFGF used as a positive control. VEGF siRNA transfection of HMEC-1 cells significantly reduced the VEGF expression both at the protein and mRNA levels (60% reduction of RNA levels) as well as their capillary-like formation compared with scrambled control siRNA transfection. Treatment of the VEGF-siRNA–transfected cells with EMMPRIN partially restored tube formation. This increased tube formation by EMMPRIN, which did not involve a parallel increase in VEGF levels, may be explained by the increased VEGFR-2 transcription by EMMPRIN (Figure 3A), which could then be activated by the remaining uninhibited VEGF protein.
EMMPRIN induces capillary-like structure formation by endothelial cells via VEGF and VEGFR-2. HMEC-1 transfected with VEGF, VEGFR-2 siRNA, or scrambled siRNA were seeded on top of a fibrin gel and treated with Emp for 24 hours in serum-free medium. (A) Capillary-like structure formation by HMEC-1 cells transfected with siRNA-targeting common sequence of VEGF isoforms. A mix of VEGF and bFGF and serum-free medium was used as positive and negative controls, respectively. VEGF121, VEGF165,VEGF189, VEGFR-1, and VEGFR-2 transcript quantification by qRT-PCR. (B) Capillary-like structure formation by HMEC-1 cells transfected with VEGFR-2 siRNA. VEGFR-2 transcript quantification by qRT-PCR. Results are presented as the mean of 3 independent experiments and expressed as a percentage of the cumulative tube length obtained for the positive control set at 100%. Columns indicate means of relative expression to TBP housekeeping gene of 3 independent experiments; and bars, SD. *P < .05.
EMMPRIN induces capillary-like structure formation by endothelial cells via VEGF and VEGFR-2. HMEC-1 transfected with VEGF, VEGFR-2 siRNA, or scrambled siRNA were seeded on top of a fibrin gel and treated with Emp for 24 hours in serum-free medium. (A) Capillary-like structure formation by HMEC-1 cells transfected with siRNA-targeting common sequence of VEGF isoforms. A mix of VEGF and bFGF and serum-free medium was used as positive and negative controls, respectively. VEGF121, VEGF165,VEGF189, VEGFR-1, and VEGFR-2 transcript quantification by qRT-PCR. (B) Capillary-like structure formation by HMEC-1 cells transfected with VEGFR-2 siRNA. VEGFR-2 transcript quantification by qRT-PCR. Results are presented as the mean of 3 independent experiments and expressed as a percentage of the cumulative tube length obtained for the positive control set at 100%. Columns indicate means of relative expression to TBP housekeeping gene of 3 independent experiments; and bars, SD. *P < .05.
On the contrary, the inhibition of tube formation observed with VEGFR-2 siRNA-transfected cells could not be restored by the addition of EMMPRIN (Figure 3B) in spite of the presence of VEGFR-1 and the induction of VEGF by EMMPRIN. In addition, the measured level of VEGFR-2 transcript mirrored that of tube formation (Figure 3B), further suggesting that the induction of this biologic function by EMMPRIN passes primarily through VEGFR-2.
EMMPRIN induces endothelial cell migration through VEGFR-2
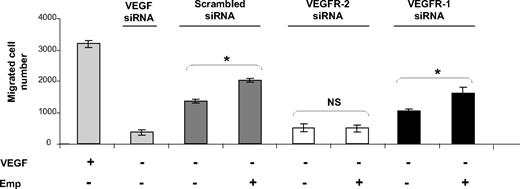
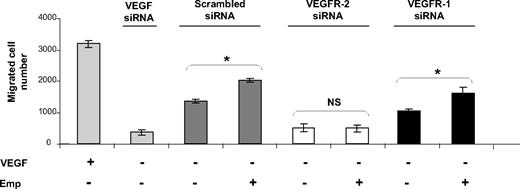
VEGF has been shown to regulate endothelial cell migration, an essential step in the angiogenesis process, and both VEGFR-1 and VEGFR-2 have been implicated. To determine whether EMMPRIN can regulate endothelial cell migration through the up-regulation of VEGFR-2, the expression of both VEGFR-1 and VEGFR-2 was inhibited by siRNA transfection and cell migration was followed by a modified Boyden chamber. Migration induced by VEGF (∼ 2.3-fold increase) or inhibited by VEGF siRNA (70% inhibition) was used as control (Figure 4).
EMMPRIN induces migration in HMEC-1 endothelial cells via VEGFR-2. HMEC-1 cells transfected with VEGF, VEGFR-1, VEGFR-2 siRNA, or scrambled siRNA were seeded in a 12-well insert of Boyden chambers and then treated or not with Emp for 24 hours. VEGF (200 ng/mL) was used as a positive control. After 48 hours of incubation, cells were fixed, stained with Diff-Quik, and counted under a microscope. Columns indicate means of 3 independent experiments carried out in triplicate; and bars, SD. *P < .05.
EMMPRIN induces migration in HMEC-1 endothelial cells via VEGFR-2. HMEC-1 cells transfected with VEGF, VEGFR-1, VEGFR-2 siRNA, or scrambled siRNA were seeded in a 12-well insert of Boyden chambers and then treated or not with Emp for 24 hours. VEGF (200 ng/mL) was used as a positive control. After 48 hours of incubation, cells were fixed, stained with Diff-Quik, and counted under a microscope. Columns indicate means of 3 independent experiments carried out in triplicate; and bars, SD. *P < .05.
Transfection of HMEC-1 cells with VEGFR-2 siRNA inhibited cell migration to a much greater extent (63%) than with VEGFR-1 siRNA (24%). EMMPRIN enhanced cell migration (25% in comparison with control cells) and was able, in addition, to restore cell migration in VEGFR-1, but not VEGFR-2 siRNA-inhibited cells. This may be explained by the fact that EMMPRIN, by up-regulating both VEGF and VEGFR-2, can compensate for the inhibited VEGFR-1, but because it does not regulate VEGFR-1, the addition of EMMPRIN to the cells treated by VEGFR-2 siRNA had no significant effect. This was confirmed by the RNA measurements showing that EMMPRIN increased both VEGF and VEGFR-2 transcripts in the VEGFR-1–inhibited cells, but only the VEGF transcript in VEGFR-2 siRNA-transfected cells (data not shown).
EMMPRIN up-regulates VEGF and VEGFR-2 in a MMP- and uPA-independent manner
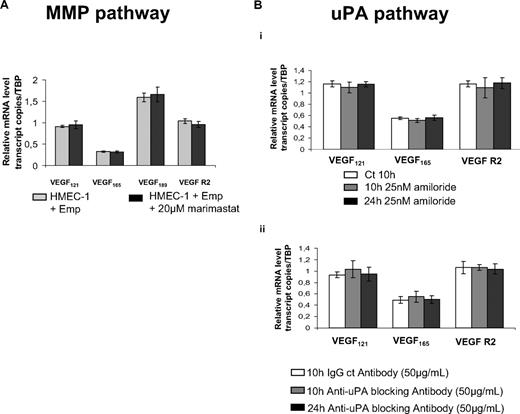
As EMMPRIN is best known as a MMP inducer, and MMPs, such as membrane type 1 (MT1)–MMP,34 were shown to transcriptionally regulate VEGF, we sought to determine whether the induction of VEGF by EMMPRIN may involve a MMP-dependent mechanism. To this end, we examined the effect of marimastat, large spectrum inhibitor of MMPs, or recombinant physiologic MMP inhibitors (TIMP-1 and TIMP-2) on VEGF and VEGFR-2 mRNA expression in HMEC-1 cells treated by EMMPRIN.
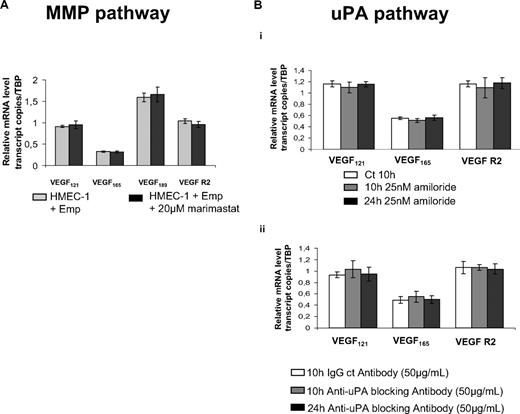
As shown in Figure 5A, the levels of either VEGF isoforms or VEGFR-2 expression induced by EMMRPIN were not affected by marimastat (20μM), suggesting a MMP-independent mechanism. The implication of MT1-MMP, known to be inhibited only by TIMP-2 and not by TIMP-1, was also excluded by demonstrating that EMMPRIN-mediated VEGF induction was similarly unaffected by either TIMPs (data not shown).
EMMPRIN up-regulates VEGF and VEGFR-2 in a MMP- or uPA-independent manner. (A) HMEC-1 cells were incubated for 12 hours with 20μM marimastat in serum-free medium before treatment with Emp for 2 hours. VEGF121, VEGF165, VEGF189, and VEGFR-2 transcripts were quantified by qRT-PCR. (B) HMEC-1 cells were incubated with 25nM amiloride (i) or 50 μg/mL anti-uPA blocking antibody (ii) for 10 hours and 24 hours in serum-free medium. VEGF121, VEGF165,VEGF189, and VEGFR-2 transcripts were quantified by qRT-PCR. Columns indicate means of relative expression to TBP housekeeping gene of at least 3 independent experiments; and bars, SD.
EMMPRIN up-regulates VEGF and VEGFR-2 in a MMP- or uPA-independent manner. (A) HMEC-1 cells were incubated for 12 hours with 20μM marimastat in serum-free medium before treatment with Emp for 2 hours. VEGF121, VEGF165, VEGF189, and VEGFR-2 transcripts were quantified by qRT-PCR. (B) HMEC-1 cells were incubated with 25nM amiloride (i) or 50 μg/mL anti-uPA blocking antibody (ii) for 10 hours and 24 hours in serum-free medium. VEGF121, VEGF165,VEGF189, and VEGFR-2 transcripts were quantified by qRT-PCR. Columns indicate means of relative expression to TBP housekeeping gene of at least 3 independent experiments; and bars, SD.
We next examined the possible implication of uPA, which we have previously shown to also be induced by EMMPRIN in both epithelial and endothelial cells.24 The treatment of the cells with 25nM amiloride, a synthetic uPA inhibitor (Figure 5Bi) or 50 μg/mL anti-uPA blocking antibody (Figure 5Bii) for 10 hours and 24 hours in serum-free medium did not affect EMMPRIN-induced VEGF or VEGFR-2 mRNA expression.
EMMPRIN up-regulates VEGF and VEGFR-2 through stimulation of HIF
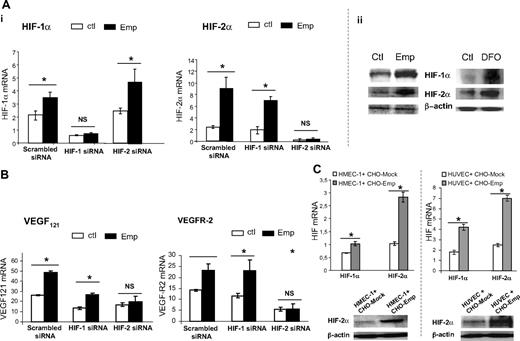
As HIF-1α and HIF-2α are considered as key regulators of VEGF and VEGF-R gene expression, we sought to determine whether the increased expression of VEGF and VEGFR-2 after EMMPRIN treatment may result from a stimulation of these transcription factors. The data in Figure 6 show that EMMPRIN up-regulated HIF-1α and HIF-2α both at the RNA and protein level. This increase, already noted after 30 minutes at the RNA and 1 hour at the protein level, preceded that of VEGF and VEGFR-2 (data not shown). The increase in HIF-1α and HIF-2α was confirmed with both HMEC-1s and HUVECs when cultured in a coculture system with intact CHO-Emp cells (Figure 6C).
EMMPRIN up-regulates VEGF and VEGFR-2 through HIF stimulation. (A) EMMPRIN up-regulates HIF-1α and HIF-2α under normoxic conditions. (i) HMEC-1 cells were transfected with HIF-1α, HIF-2α siRNA, or scrambled siRNA before treatment with 20 μg/mL Emp for 1 hour. HIF-1α and HIF-2α transcripts were quantified by qRT-PCR. (ii) The cells were incubated with Emp or desferrioxamine (DFO). The HIF-1α and HIF-2α levels were detected by Western blotting. (B) EMMPRIN up-regulates VEGFR-2 and soluble VEGF isoforms via HIF-2α. HMEC-1 cells were transfected with HIF-1α, HIF-2α siRNA, or scrambled siRNA before treatment with Emp. VEGFR-2 and VEGF121 transcripts were quantified by qRT-PCR. Columns indicate means of relative expression to TBP housekeeping gene of at least 3 independent experiments; and bars, SD. *P < .05. (C) HMEC-1 and HUVECs cocultured with EMMPRIN- or mock-transfected CHO cells. Total RNA was extracted from cocultured cells. mRNA levels for HIF-1α and HIF-2α were quantified using qRT-PCR. Columns indicate means of relative expression to TBP housekeeping gene of at least 3 independent experiments; and bars, SD. *P < .05. Cocultured cell lysates were immunoblotted for HIF-2α (β-actin was used as loading control). Representative blots of 3 independent experiments.
EMMPRIN up-regulates VEGF and VEGFR-2 through HIF stimulation. (A) EMMPRIN up-regulates HIF-1α and HIF-2α under normoxic conditions. (i) HMEC-1 cells were transfected with HIF-1α, HIF-2α siRNA, or scrambled siRNA before treatment with 20 μg/mL Emp for 1 hour. HIF-1α and HIF-2α transcripts were quantified by qRT-PCR. (ii) The cells were incubated with Emp or desferrioxamine (DFO). The HIF-1α and HIF-2α levels were detected by Western blotting. (B) EMMPRIN up-regulates VEGFR-2 and soluble VEGF isoforms via HIF-2α. HMEC-1 cells were transfected with HIF-1α, HIF-2α siRNA, or scrambled siRNA before treatment with Emp. VEGFR-2 and VEGF121 transcripts were quantified by qRT-PCR. Columns indicate means of relative expression to TBP housekeeping gene of at least 3 independent experiments; and bars, SD. *P < .05. (C) HMEC-1 and HUVECs cocultured with EMMPRIN- or mock-transfected CHO cells. Total RNA was extracted from cocultured cells. mRNA levels for HIF-1α and HIF-2α were quantified using qRT-PCR. Columns indicate means of relative expression to TBP housekeeping gene of at least 3 independent experiments; and bars, SD. *P < .05. Cocultured cell lysates were immunoblotted for HIF-2α (β-actin was used as loading control). Representative blots of 3 independent experiments.
We next examined the effect of HIF silencing, by using both HIF-1α and HIF-2α siRNA, on EMMPRIN regulation of VEGF system. Specific inhibition of either HIF-1α or HIF-2α was achieved, as shown by HIF-1α and HIF-2α RNA analysis (Figure 6A). When silencing HIF-1α, EMMPRIN only increased HIF-2α with no effect on HIF-1α; similarly, silencing HIF-2α did not affect EMMPRIN induction of HIF-1α. Figure 6B shows that VEGFR-2 up-regulation by EMMPRIN was significantly inhibited by siRNA of HIF-2α, but not of HIF-1α. However, the induction of VEGF121 (and VEGF165; data not shown) appears to involve both HIF-1α and HIF-2α, although HIF-2α silencing had a much greater inhibitory effect than that of HIF-1α. These results suggest that HIF-2α plays a central role in EMMPRIN regulation of VEGFR-2 as well as VEGF.
Discussion
EMMPRIN is highly expressed on the surface of tumor cells and stimulates surrounding fibroblasts and endothelial cells to produce MMPs and uPA in a paracrine fashion.24,25,35 Certain MMPs can release bound VEGF, thus involving EMMPRIN in the regulation of VEGF bioavailability.36 UPA was also shown to cleave the matrix-bound VEGF189, releasing a diffusible fragment having the ability to activate VEGFR-2 and induce proliferation.37 However, EMMPRIN was also shown to stimulate the transcriptional expression of VEGF in tumor cells via the phosphatidylinositol 3-kinase–Akt signaling pathway.31,38 In the present study, we further show that EMMPRIN can have a direct effect on endothelial cells, specifically up-regulating VEGFR-2 and its soluble ligands 121 and 165, and as a consequence, increasing both migration and tube formation. Neither the expression of VEGFR-1 nor that of the larger matrix-bound VEGF isoforms was affected by EMMPRIN. This may have an important biologic significance, as although VEGF can bind to both receptors, VEGFR-2 appears to play a more important role in initiating signal transduction pathways within endothelial cells due to its greater kinase activity and, thus, in promoting endothelial cell tube formation.9 These results further expand on the function of EMMPRIN in tumorogenesis and metastasis, as in addition to its known effect on tumor invasion through the induction of proteinases in the surrounding tissue, EMMPRIN can enhance tumor angiogenesis by a paracrine effect on endothelial cells' expression of VEGFR-2 and may account, at least in part, for the elevated VEGFR-2 expression observed in tumor vasculature.39 As VEGF can be expressed by a variety of cells, the effect of EMMPRIN on VEGF production in tumor cells31 and in endothelial cells, as shown in this study, would be expected to increase the pool of VEGF in the tumoral tissue. It is interesting in this respect that endogenous endothelial VEGF was shown, albeit under nonpathologic conditions, to serve a particular role in cell survival that cannot be provided by VEGF secreted from adjacent cell types.20 It remains to be seen whether this endothelial autocrine production induced by EMMPRIN may also have a more specific function in tumor angiogenesis, such as increasing viability and stability of the newly formed blood vessels.
EMMPRIN has already been suggested to play a role in angiogenesis through the induction of MMPs and, hence, tissue remodeling, endothelial cell migration, and invasion. MT1-MMP, a transmembrane metalloproteinase whose role as an activator of pro-MMP-2 has been extensively studied, was also shown to promote angiogenesis independently of MMP-2 by enhancing VEGF production in tumor cells.40 This effect of MT1-MMP, which was shown to involve the Src tyrosine kinase signaling pathway, was nevertheless dependent on the catalytic domain of the molecule.34 As EMMPRIN was shown to increase expression of MT1-MMP,41 we investigated the possibility that its effect on VEGF production may be mediated through this proteinase. Our results show, however, that neither marimastat, a broad spectrum MMP inhibitor, nor TIMP-1 and TIMP-2, which differentially inhibit MT1-MMP, nor MT1-MMP siRNA (data not shown) had any effect on the synthesis of VEGF induced by EMMPRIN. Furthermore, the inhibition of uPA, the serine protease also induced by EMMPRIN24 and which was likewise shown to up-regulate VEGF, was without an effect, further confirming that VEGF regulation by EMMPRIN is independent of either the MMP or the uPA systems.
It was previously suggested that the MMP-inducing function of EMMPRIN within tumor cells involves homophilic interactions, with EMMPRIN acting as a counterreceptor for itself.42 Such interactions may exist in our system, as the inhibition of endogenous EMMPRIN expression in endothelial cells with siRNA inhibited cell migration as well as the mRNA levels of VEGR-2 and HIF-2α, although it did not completely abrogate EMMPRIN's effects (see supplemental File 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). This may be due to the noninhibited fraction of EMMPRIN that still remains after the siRNA treatment, although the possibility that EMMPRIN can also exert its effects on endothelial cell migration through other nonhomophilic cell-cell interactions may not be excluded.
EMMPRIN regulated differently the various VEGF isoforms; those are known to be generated by different processes. These may involve the alternative 5′ splicing, giving rise to either VEGF189 or VEGF206, the alternative 3′ splicing to form either VEGF165 or the antiangiogenic peptide VEGF165b,43 and the cassette exons forming VEGF165 or VEGF121.15 Little is known at present on the mechanism of the regulation of VEGF splicing, although 2 factors, the Sam68-like mammalian protein 244 and coactivator of activator protein 1 and estrogen receptor alpha (CAPERα),45 have both been shown to be able to alter splicing of the VEGF gene. It is hard to speculate at this point on the mechanism by which EMMPRIN modifies the ratio of the different VEGF isoforms, but it is interesting to note that CAPERα inhibition resulted in an increase in VEGF121/189 ratio similar to EMMPRIN′s effect.
Our results also show that the regulation of VEGF and VEGFR-2 by EMMRPIN is mediated through the regulation of HIFα, transcription factors that have a central role in angiogenesis.46 Even though these factors are known to be highly regulated by cellular oxygen levels, other elements of the inflammatory and tumor microenvironment were shown to influence their accumulation and activity under normal oxygen concentration. Whereas both HIF-1α and HIF-2α were up-regulated by EMMPRIN, our results show that HIF-2α has a more prominent role in EMMPRIN regulation of the VEGF system and more particularly of VEGFR-2, as the inhibition of HIF-2α, but not that of HIF-1α, almost completely blocked EMMPRIN′s induction of VEGFR-2. This is in agreement with the previously reported specific regulation of VEGFR-2 by HIF-2α, but not by HIF-1α.47
By inducing HIF-2α under normal oxygen levels, EMMRPIN may contribute to the development of tumor aggressiveness by inducing the program for a hypoxic phenotype also at near physiologic oxygen tensions, thus promoting angiogenesis, through VEGF and VEGFR-2 production, in nonhypoxic conditions. This view is supported by the experimental evidence from different cellular models showing that HIF-2α is more stable than HIF-1α at normoxia and is transcriptionally active at such conditions.48 It is interesting that HIF-2α was also shown to regulate other gene targets such as uPA receptor49 and MT1-MMP,50 which are both increased by EMMPRIN. It remains to be seen, however, whether the increase in HIF-2α represents a central pathway for the increase of these EMMPRIN target genes.
Our results presented in this study strengthen the emerging view that the role of EMMPRIN in tumor progression is not strictly restrained to its widely demonstrated role in MMP induction and matrix turnover, but also through VEGF/VEGFR-2–mediated control of the angiogenesis process by a paracrine effect on the endothelial cells. Such findings have direct impact in cancer therapy by providing a possibility, through the inhibition of EMMPRIN, to inhibit simultaneously VEGF signaling and tissue remodeling associated with tumoral angiogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr E. Huet for stimulating discussions.
This work was supported by the Fondation Charles Oberling (C.Q.), the Société Française de Dermatologie, and the Fondation de L'Avenir (study ET8-489).
National Institutes of Health
Authorship
Contribution: F.B., C.Q. and E.E.G. performed research and analyzed data; S.K. performed research and analyzed data; B.N. performed research, provided analytic tools, and analyzed data; M.-P.P. performed research; G.M. performed microscopic imaging; F.C. designed the research; C.D. analyzed data and conducted research discussions; C.L. analyzed data and conducted research discussions; and S. Menashi and S. Mourah designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Samia Mourah, Laboratoire de Pharmacologie and Inserm Unité 940, Hôpital Saint-Louis, 27, rue Juliette Dodu, 75010 Paris, France; e-mail: samia.mourah@sls.aphp.fr.
References
Author notes
*F.B. and C.Q. contributed equally to this work.