Abstract
Platelet-type von Willebrand disease (PT-VWD) is a bleeding disorder of the platelet glycoprotein Ib-IX/von Willebrand factor (VWF) axis caused by mutations in the glycoprotein Ib-IX receptor that lead to an increased affinity with VWF. In this report, platelets from a mouse expressing a mutation associated with PT-VWD have been visualized using state-of-the art image collection and processing. Confocal analysis revealed that VWF bound to the surface of single platelets and bridging micro-aggregates of platelets. Surface-bound VWF appears as a large, linear structure on the surface of 50% of the PT-VWD platelets. In vivo thrombus formation after chemical injury to the carotid artery revealed a severe impairment to occlusion as a consequence of the PT-VWD mutation. In vitro stimulation of PT-VWD platelets with adenosine diphosphate or thrombin demonstrates a significant block in their ability to bind fibrinogen. The impairment of in vivo thrombus formation and in vitro fibrinogen binding are more significant than might be expected from the observed platelet binding to VWF polymers over a small portion of the plasma membrane. Visualization of the receptor/ligand interaction and characterization of a severe antithrombotic phenotype provide a new understanding on the molecular basis of bleeding associated with the PT-VWD phenotype.
Introduction
As an initiating step in hemostasis, surface-bound von Willebrand factor (VWF) tethers platelets to a damaged vascular surface via the platelet membrane receptor, glycoprotein (GP) Ib-IX.1 Before the hemostatic or thrombotic response, soluble von Willebrand factor and platelets coexist without a detectable interaction. Indeed, the regulation of binding between VWF and GP Ib-IX is one of key steps controlling hemostasis and thrombosis.2 Much of our understanding of these processes has been aided by human bleeding disorders representing “experiments of nature” that identify the key proteins. To this end, platelet-type von Willebrand disease (PT-VWD) is a hereditary bleeding disorder caused by point mutations within the extracellular α-subunit domain of the GP Ib-IX receptor.3-6 A documented result of PT-VWD mutations is an increased affinity between mutant GP Ib-IX and soluble VWF.7 A mechanistically similar situation exists with type 2B VWD where single mutations in VWF have an increased affinity for a normal platelet GP Ib-IX receptor.8 Whether the mutation exists in the receptor, PT-VWD, or the ligand, type 2B VWD, the net result in both cases is a bleeding disorder.
The bleeding phenotype in PT-VWD and type 2B VWD has been explained as a consequence of circulating platelet micro-aggregates composed of bridged platelets and VWF.7,9 The aggregates are removed from the circulation resulting in a thrombocytopenia, albeit borderline in PT-VWD, and this can partially explain bleeding.3 In addition, there is an absence of the largest circulating plasma VWF multimeric species in both PT-VWD and type 2B VWD.3,10 The largest VWF multimer species are the most hemostatically efficient, and removal from the circulation would further add to the impairment of normal hemostasis.11 However, the removal of the largest multimers by binding to platelets is somewhat counterintuitive because these multimers would presumably be bound to platelets and might be expected to preload a platelet for a hemostatic or thrombotic event. Nevertheless, PT-VWD and type 2B VWD remain 2 of the more interesting bleeding phenotypes providing mechanistic clues on the regulation of hemostasis and thrombosis.
We have recently reported the development of a mouse expressing a mutant human subunit associated with PT-VWD.12 The mouse expresses a human α-subunit of GP Ib-IX in which Gly233 is replaced by Val233 (G233V), the first reported genetic basis for human PT-VWD.13 The mice display several diagnostic attributes of human PT-VWD, including the ability of platelets to agglutinate in the presence of low doses of ristocetin and a bleeding phenotype as judged by a simple assessment of hemostasis, the tail bleeding time.12 Similar to human PT-VWD, the mice do not display significant thrombocytopenia, suggesting that the defect observed in the tail bleeding time is not solely explained by a reduced platelet count. In the present manuscript, we have used state-of-the-art imaging analysis to visualize mouse VWF bound to the surface of platelets. We observed a small number of linear VWF species bound to the surface of individual platelets. The phenotypic consequence of the G233V mutation is documented in vivo with a complete abrogation of thrombosis after ferric chloride damage to the carotid artery. Finally, we demonstrate that the mutant GP Ib-IX receptor results in an inhibition of normal platelet function using common platelet agonists, such as adenosine diphosphate (ADP) and thrombin. Visualization of the receptor/ligand interaction and characterization of a severe antithrombotic phenotype provide a new understanding on the molecular basis of the human PT-VWD phenotype.
Methods
Animal models and husbandry
A mouse model lacking the gene encoding mouse GP Ibα has been previously described.14 These mice lack a surface-expressed GP Ib-IX complex and exhibit a severe bleeding phenotype. Rescue of the GP Ib-IX–deficient phenotype by platelet-specific expression of a human GP Ibα transgene was also achieved.14 A congenic strain of these animals has been generated in the C57BL/6J background after extensive backcrossing (> 10 generations).15 We refer to animals from this colony as hTgWT. More recently, we have used a similar strategy to express a mutant human GP Ib-IX complex in the mouse GP Ib-IX null background in which residue Gly233 of human GP Ibα has been replaced by Val.12 We refer to animals from this colony as hTgG233V. The G233V mutation corresponds to one of a limited number of point mutations that have been identified as the molecular basis of human PT-VWD.7,13 However, human PT-VWD is inherited in an autosomal dominant manner in which a normal GP Ibα allele is expressed in the presence of the mutant GP Ibα allele. The mouse model used in this study is somewhat different as only the mutant allele product is present on the surface of hTgG233V platelets. hTgG233V animals express a similar level of human GP Ibα antigen on their platelet surface as their control counterpart, hTgWT.12 In a simple model of mouse hemostasis, the tail bleeding time assay, hTgG233V animals display a bleeding phenotype in contrast to hTgWT animals, which are indistinguishable from normal or wild-type mice.12,14 hTgG233V animals have a slightly reduced platelet count (reduced by ∼ 20%) compared with control hTgWT animals.12 hTgG233V animals used in the current study have been backcrossed for 6 generations with C57BL/6J animals. All mouse procedures were performed after approval from the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee.
Immunostaining of megakaryocytes and platelets
For megakaryocytes, femur and tibia were collected, and bone marrow was obtained and centrifuged in a Percoll gradient as described.16 Megakaryocytes were then fixed, attached to the coverslip, and were ready for immunostaining.
For platelets, whole blood was drawn in citrate and immediately fixed with 2% formaldehyde in phosphate-buffered saline (PBS, 137mM NaCl, 2.7mM KCl, 10mM Na2HCO3, 0.42mM Na2HPO4, 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 2mM MgCl2, 0.1% dextrose, pH 7.4) at room temperature. Platelets were washed by centrifugation, finally resuspended in Tyrode buffer, pH 7.4, containing 1mM CaCl2 and 0.1% bovine serum albumin, and allowed to adhere to coverslips for 90 minutes (37°C). The immunostaining procedure was followed basically as described elsewhere.17 Platelets were treated with ammonium chloride to eliminate remaining fixative and then permeabilized with PBS containing 0.1% saponin and 0.2% gelatin. Rat anti–mouse CD41 (BD Biosciences PharMingen) was used to label the platelet surface. Rabbit polyclonal anti–human VWF (Code A0082, Dako Glostrup), rat anti–mouse fibrinogen (BD Biosciences PharMingen), and rat anti–mouse P-selectin (Santa Cruz Biotechnology) were also used. Secondary antibodies used were fluorescein isothiocyanate-conjugated goat anti–rat IgG and Cy3-conjugated goat anti–rabbit IgG, both from Invitrogen. After incubation with antibodies, samples were washed in saponin-gelatin-PBS and mounted in Mowiol (Calbiochem). Primary and secondary antibodies were used at 1/200 and 1/1000 dilution, respectively.
Image acquisition
Spinning-disk confocal images were taken with a Zeiss Axiovert 200 M microscope fitted with a BD Biosciences CARVII Confocal imager. The objective was 100× with a numerical aperture of 1.4 (oil objective). The software used was iVision 4.0.5 (BioVision). Further image processing consisted of deconvolution of the raw confocal image stacks using Huygens Essential v2.10 (Scientific Volume Imaging). Maximum-intensity projections of the deconvolved stacks were obtained as previously described using iVision software.17 Three-dimensional projections and surface-rendered projections of the deconvolved images were also generated using Huygens Essential. The images shown represent examples obtained from different platelet preparations.
Ferric chloride-induced carotid artery occlusion
The carotid artery was separated from other tissues in isofluorane (2.5%) anesthetized mice. A 4 × 10-mm strip of Whatman No. 1 filter paper saturated in 10% FeCl3 solution was placed on the carotid artery for 3 minutes, followed by rinsing 3 times with physiologic saline. Blood flow was monitored using a laser Doppler system (Trimflo Fiber Optic Probe) connected to a BPM Blood Perfusion Monitor (Vasemedics Inc). The monitoring of carotid artery blood flow was initiated at the time of FeCl3 treatment. Blood flow was monitored for 40 minutes, and time to first occlusion was determined. Complete occlusion was considered when flow dropped to less than 0.2 blood flow units per minute.
Studies of platelet activation by flow cytometry
Whole blood was drawn, diluted in PBS with a 2mM CaCl2 final concentration, and platelet activation was stimulated with 10μM ADP or 1 U/mL thrombin (Chrono-Log Corp). Oregon green-fibrinogen (Invitrogen) was added, and samples were further incubated at room temperature with no stirring. FL-1 was read in a FACScan (BD Biosciences). Control samples included no agonists in the presence or absence of ethyleneglycoltetraacetic acid. Flow cytometry experiments have been repeated 3 times, and the results from each experiment were consistent with those presented in Figure 6.
Results
Visualization of VWF bound to murine PT-VWD platelets
Mice expressing normal human GP Ibα on the surface of their platelets (hTgWT) and assembling into a chimeric mouse/human GP Ib-IX complex have been previously described.14 Similar mice expressing a human subunit with a PT-VWD mutation (G233V) have also been described (hTgG233V).12 Both models express similar levels of human GP Ibα as measured by flow cytometry, and both lack endogenous mouse GP Ibα alleles. Whole blood was collected from a retro-orbital sinus and immediately fixed with paraformaldehyde. After the preparation of washed platelets, a platelet suspension was allowed to adhere to glass coverslips. Immunofluorescence staining of the platelets was performed using a rat anti–mouse CD41 monoclonal antibody (integrin αIIb subunit) followed by a detecting antibody, anti–rat Alexa488 secondary antibody. A rabbit anti-VWF polyclonal antibody was used and detected using an anti–rabbit IgG Cy3 secondary antibody.
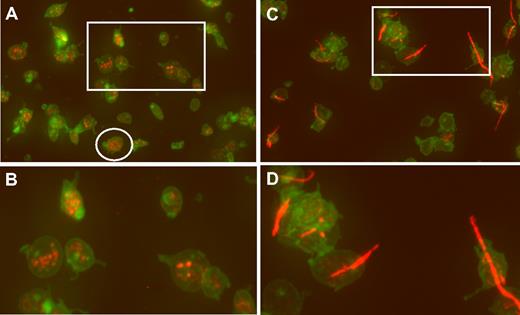
An example of wide-field microscopy is shown in Figure 1A for platelets that were allowed to adhere to glass. Wide-field images of hTgWT platelets are normal appearing with some evidence of filodopodia (slight activation) and platelets with visible VWF-positive α-granules (Figure 1B). In contrast, platelets from hTgG233V animals have a large amount of adhering linear VWF bridging minor platelet clumps together (Figure 1C-D). Additional wide-field images are available for viewing (supplemental Figures 1-7, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Confocal analysis of CD41 and VWF localization. (A) Fixed platelets from hTgWT animals were allowed to adhere to glass coverslips and subjected to immunofluorescent staining. Maximum intensity projections of a deconvolved image stack were obtained using confocal microscopy. The raw confocal stack images were deconvolved with iterations limited to 10 using Huygens Essential Software. The presented image is a color merge of VWF (red) and CD41 (green) in hTgWT platelets. VWF staining was obtained with a rabbit polyclonal anti–human VWF antibody and an anti–rabbit CY3 secondary antibody. The hTgWT platelet surface was labeled using a rat anti–mouse CD41 antibody and an anti–rat Alexa488 secondary antibody. The circled platelet was further analyzed and is presented as a surface rendered image in Figure 2. (B) A higher magnification of the boxed region is shown for clarification. VWF labeling is restricted to platelet granules. (C) As described for panel A, wide-field microscopy of platelets from mice expressing a G233V GP Ibα subunit (hTgG233V) is shown. (D) A higher magnification of an isolated region is shown for clarification.
Confocal analysis of CD41 and VWF localization. (A) Fixed platelets from hTgWT animals were allowed to adhere to glass coverslips and subjected to immunofluorescent staining. Maximum intensity projections of a deconvolved image stack were obtained using confocal microscopy. The raw confocal stack images were deconvolved with iterations limited to 10 using Huygens Essential Software. The presented image is a color merge of VWF (red) and CD41 (green) in hTgWT platelets. VWF staining was obtained with a rabbit polyclonal anti–human VWF antibody and an anti–rabbit CY3 secondary antibody. The hTgWT platelet surface was labeled using a rat anti–mouse CD41 antibody and an anti–rat Alexa488 secondary antibody. The circled platelet was further analyzed and is presented as a surface rendered image in Figure 2. (B) A higher magnification of the boxed region is shown for clarification. VWF labeling is restricted to platelet granules. (C) As described for panel A, wide-field microscopy of platelets from mice expressing a G233V GP Ibα subunit (hTgG233V) is shown. (D) A higher magnification of an isolated region is shown for clarification.
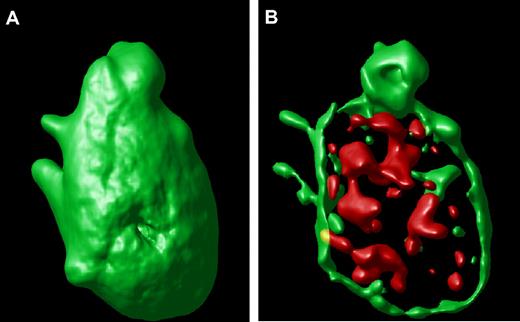
An example of a surface-rendered projection from the stacked image of a single hTgWT platelet is shown (Figure 2A). This result provides an example of the ability to image the platelet surface in a three-dimensional format along with an ability to visualize compartmentalized VWF inside the control platelet (Figure 2B). This is the typical staining pattern from platelets expressing the wild-type or control human GP Ibα transgene.
Surface-rendered platelet projection. (A) A representative hTgWT platelet is shown after image analysis reveals the surface-rendered fluorescence from anti-CD41 antibodies. The stack confocal image of this platelet is visible in Figure 1A within the circle. (B) If the CD41-dependent fluorescence intensity is decreased, the internal VWF+ α-granules are readily visible filling the platelet interior.
Surface-rendered platelet projection. (A) A representative hTgWT platelet is shown after image analysis reveals the surface-rendered fluorescence from anti-CD41 antibodies. The stack confocal image of this platelet is visible in Figure 1A within the circle. (B) If the CD41-dependent fluorescence intensity is decreased, the internal VWF+ α-granules are readily visible filling the platelet interior.
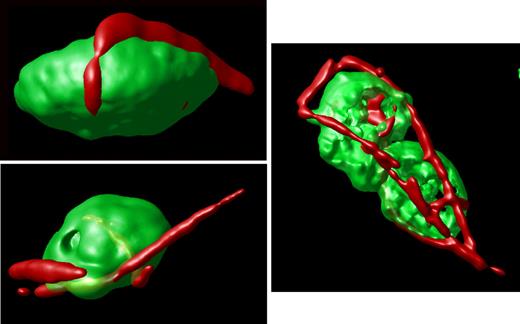
In contrast, an analysis of stacked images from hTgG233V platelets revealed a striking appearance of VWF staining on the surface of platelets (Figure 1C). Bound VWF was commonly observed in a relatively linear form, as opposed to the globular structure that is proposed for circulating unbound plasma VWF.18 Approximately 50% of the isolated platelets contained some linear VWF visible on the surface of the platelet (Figure 1C). The actual incidence in vivo may be higher. Moreover, bridging of platelets to produce micro-aggregates held together by a single fluorescent VWF species was also observed (Figures 1,3). The linear arrangement of VWF staining suggests that this represents a single VWF multimer. A range of VWF lengths were observed, with some approximating a length longer than the width of a single platelet. Additional hTgG233V platelet images are provided as supplemental files (supplemental Figures 8-10). Representative Z-section images are also presented for hTgWT and hTgG233V platelets (supplemental Figure 11). Subsequent staining for granules containing fibrinogen and P-selectin revealed no significant differences between hTgWT and hTgG233V platelets (supplemental Figures 12-13).
Surface-rendered projections. Representative platelets from an hTgG233V animal are shown. Surface-rendered fluorescence for CD41 antigen (green) and VWF fluorescence (red) reveals the linear surface adhesion of VWF on the platelet surface from animals expressing a PT-VWD mutation. The example shown to the right highlights the linear nature of the bound VWF and the ability of VWF to bridge platelets and support micro-aggregate formation. Additional images are available online (see the supplemental Figures).
Surface-rendered projections. Representative platelets from an hTgG233V animal are shown. Surface-rendered fluorescence for CD41 antigen (green) and VWF fluorescence (red) reveals the linear surface adhesion of VWF on the platelet surface from animals expressing a PT-VWD mutation. The example shown to the right highlights the linear nature of the bound VWF and the ability of VWF to bridge platelets and support micro-aggregate formation. Additional images are available online (see the supplemental Figures).
Megakaryocytes in the mouse model of PT-VWD
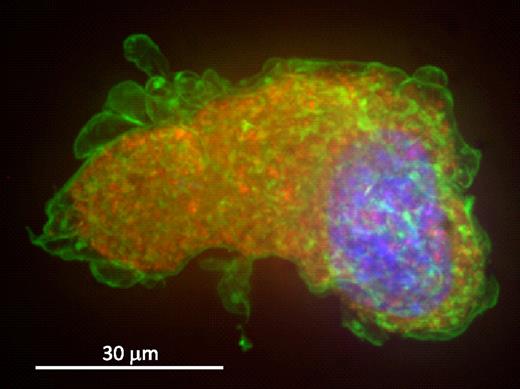
It has been suggested that the increased affinity between GP Ib-IX and VWF could result in an intracellular association during synthesis in the megakaryocyte, leading to surface localization of VWF. Thus, we analyzed hTgG233V bone marrow megakaryocytes stained with a surface marker (CD41) and VWF to examine the distribution of VWF in the platelet progenitor cell. Figure 4 shows a representative hTgG233V megakaryocyte labeled for nuclear material, CD41 and VWF. The image is the maximum-intensity projection of the deconvolved image stack. A typical granular location of VWF is present throughout the cytoplasm with no sign of surface VWF staining. CD41 mostly labels the membrane of megakaryocytes; and, unlike platelets from the mutant mice, no visible VWF is present on the surface of the megakaryocyte. No differences were observed for CD41 and VWF distribution comparing hTgG233V and hTgWT megakaryocytes. We conclude that synthesis, maturation, and localization of VWF in hTgG233V megakaryocytes are unremarkable. We also conclude that the surface-bound VWF observed on platelets from hTgG233V animals occurs after the generation of platelets from the megakaryocyte.
Confocal-stacked image of a bone marrow megakaryocyte. hTgG233V megakaryocytes were analyzed and show a typical granular distribution of VWF in the cytoplasm. Percoll-gradient megakaryocytes were fixed and labeled for VWF, CD41, and 4,6-diamidino-2-phenylindole. Maximum-intensity projection of the deconvolved image stack was obtained using a spinning-disk confocal microscope. Deconvolution was performed to get a sharper picture. A composite of VWF in red, CD41 in green, and nuclear staining in blue is shown. VWF is visualized as part of the megakaryocyte granule content, as opposed to CD41, which is most visible on the surface of the platelet precursor. No surface-bound VWF was observed.
Confocal-stacked image of a bone marrow megakaryocyte. hTgG233V megakaryocytes were analyzed and show a typical granular distribution of VWF in the cytoplasm. Percoll-gradient megakaryocytes were fixed and labeled for VWF, CD41, and 4,6-diamidino-2-phenylindole. Maximum-intensity projection of the deconvolved image stack was obtained using a spinning-disk confocal microscope. Deconvolution was performed to get a sharper picture. A composite of VWF in red, CD41 in green, and nuclear staining in blue is shown. VWF is visualized as part of the megakaryocyte granule content, as opposed to CD41, which is most visible on the surface of the platelet precursor. No surface-bound VWF was observed.
In vivo impairment of occlusive thrombi in hTgG233V mice
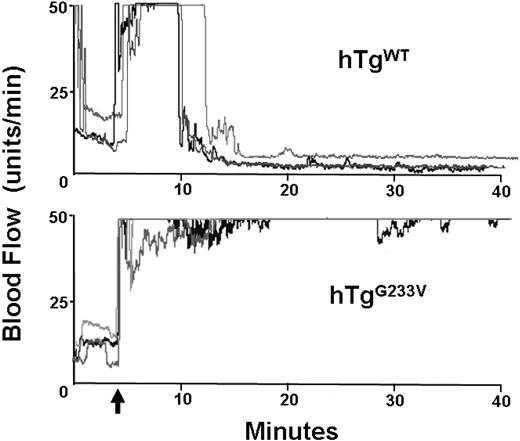
Impaired hemostasis in patients with PT-VWD and their tendency for bleeding have been documented for many years. In addition, induction of arterial thrombosis in mouse models has helped to identify the relevant elements that play a significant role in hemostasis and thrombosis in vivo.19 GP Ibα knockout mice have a significant defect in the occlusion of the carotid artery after vascular damage induced by ferric chloride.20 Consequently, we analyzed the ability of hTgG233V mice to develop an occlusive thrombus after chemical injury. In control hTgWT mice, the flow dropped within approximately 5 minutes after the removal of ferric chloride, and the carotid remained occluded through completion of the experiment (40 minutes). Figure 5 (top panel) illustrates 3 representative tracings of blood flow, but similar results were observed with all hTgWT animals tested (n > 10). In contrast, hTgG233V animals were unable to occlude the carotid artery (Figure 5 bottom panel). There was little evidence of blood flow reduction, and maximal blood flow continued throughout the entire 40 minutes of monitoring using hTgG233V animals. The failure of an occlusive thrombus to form was observed for every hTgG233V mouse analyzed (n = 10). These results confirm a significant defect in thrombosis as a consequence of the G233V mutation.
In vivo thrombosis. Thrombosis in a carotid artery occlusion model is presented. After damage via FeCl3 to an exposed carotid artery, blood flow in an anesthetized animal was followed for approximately 40 minutes. hTgWT animals display a rapid and stable reduction in blood flow initiated approximately 5 minutes after chemical damage (top graph). Three representative tracings are shown but are indicative of results obtained from 10 different animals. In contrast, hTgG233V mice were unable to occlude the carotid artery as evidenced by only small changes in the blood flow during the 40-minute experiment (bottom graph). Again, only 3 representative tracings are shown, but similar results were obtained with all hTgG233V animals tested (n = 10). The patent carotid artery of hTgG233V animals is in stark contrast to the occlusive thrombi that form with hTgWT animals.
In vivo thrombosis. Thrombosis in a carotid artery occlusion model is presented. After damage via FeCl3 to an exposed carotid artery, blood flow in an anesthetized animal was followed for approximately 40 minutes. hTgWT animals display a rapid and stable reduction in blood flow initiated approximately 5 minutes after chemical damage (top graph). Three representative tracings are shown but are indicative of results obtained from 10 different animals. In contrast, hTgG233V mice were unable to occlude the carotid artery as evidenced by only small changes in the blood flow during the 40-minute experiment (bottom graph). Again, only 3 representative tracings are shown, but similar results were obtained with all hTgG233V animals tested (n = 10). The patent carotid artery of hTgG233V animals is in stark contrast to the occlusive thrombi that form with hTgWT animals.
Fibrinogen binding to hTgG233V platelets
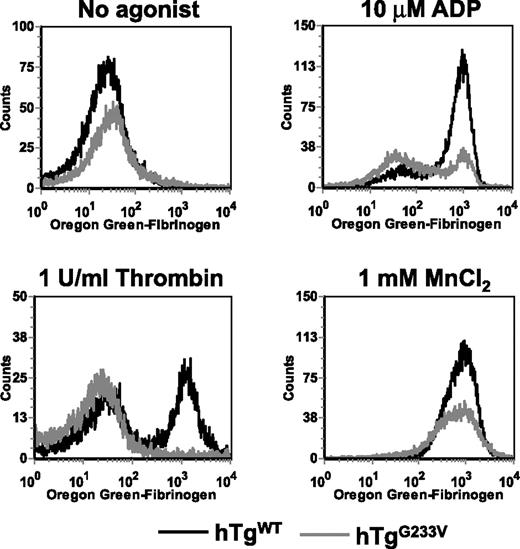
The inability of hTgG233V animals to occlude the carotid artery after injury (Figure 5) suggested a major impairment of platelet function. Thus, the question remains as to how such a significant impairment of platelet function occurs when only a limited number of GP Ib-IX molecules appear to be occupied by VWF and a seemingly large amount of the fibrinogen receptor (CD41, integrin αIIbβ3) remains unoccupied. Therefore, we measured the fibrinogen-binding capacity of platelets after in vitro activation with agonists, such as thrombin and ADP, both presumably bypassing a GP Ib-IX–dependent pathway. Whole blood was exposed to agonists and incubated with Oregon green-fibrinogen before analysis by flow cytometry. Fibrinogen binding increased dramatically in hTgWT platelets in the presence of 10μM ADP or 1 U/mL thrombin (Figure 6). In contrast, hTgG233V platelets were unable to bind fibrinogen when stimulated with either agonist (Figure 6). These results demonstrate that platelet stimulation is blocked in hTgG233V animals using common platelet agonists. We assume that the absence of linear VWF, as seen on approximately half of the hTgG233V platelets, suggests the inability of hTgG233V platelets to become activated via ADP or thrombin is not a direct consequence of binding between VWF and GP Ib-IX. Moreover, the light microscopic images suggest a lot of unoccupied αΙΙbβ3 on the platelet surface (green fluorescence in Figures 1,Figure 2–3). The accessibility of antiαIIb antibodies to bind the surface of G233V platelets, as measured by flow cytometry, was also similar among normal mouse and hTgWT and hTgG233V platelets (supplemental Figure 14). To test the hypothesis that the extracellular G233V mutation has created a more general defect in inside-out signaling, we investigated fibrinogen binding via an outside-in pathway using divalent cations. Here, the G233V platelets were able to bind fibrinogen, albeit at reduced levels, compared with hTgG233V platelets (Figure 6), further illustrating the accessibility of fibrinogen to the platelet surface.
In vitro fibrinogen binding. Whole blood was drawn into citrate from a retro-orbital sinus of anesthetized animals. Whole blood was stimulated with 10μM ADP, 1 U/mL thrombin, or 1mM MnCl2 and then incubated with Oregon green-fibrinogen for 20 minutes at room temperature. FL1 was read using a FACScan (BD Biosciences). Results are shown for blood from hTgWT animals (black line) and hTgG233V animals (gray line) without agonist or in the presence of ADP, thrombin, or MnCl2. hTgG233V platelets are severely impaired in their ability to respond to either ADP or thrombin.
In vitro fibrinogen binding. Whole blood was drawn into citrate from a retro-orbital sinus of anesthetized animals. Whole blood was stimulated with 10μM ADP, 1 U/mL thrombin, or 1mM MnCl2 and then incubated with Oregon green-fibrinogen for 20 minutes at room temperature. FL1 was read using a FACScan (BD Biosciences). Results are shown for blood from hTgWT animals (black line) and hTgG233V animals (gray line) without agonist or in the presence of ADP, thrombin, or MnCl2. hTgG233V platelets are severely impaired in their ability to respond to either ADP or thrombin.
Discussion
PT-VWD is a rare bleeding disorder inherited in an autosomal dominant manner.4 One hallmark feature is the ability of platelets to agglutinate in the presence of low doses of ristocetin, necessitating the need to do plasma versus platelet mixing studies to determine whether the defect is platelet-dependent (PT-VWD) or plasma-dependent (type 2B VWD).21 Recombinant expression of PT-VWD mutations in heterologous cells has confirmed that the mutations result in an in vitro higher affinity between receptor and ligand.7,22,23 We provide imaging data verifying that the increased affinity does yield a platelet/VWF interaction in platelet preparations from mice expressing a G233V variant GP Ibα subunit. However, the in vivo niche where such an interaction first occurs, either megakaryocytes or platelets, has yet to be established in PT-VWD. It has been previously reported that VWF exists on the surface of cultured megakaryocytes in a 2B-VWD patient.24 Unlike the findings with this 2B-VWD patient, we observed a VWF distribution in megakaryocytes of hTgG233V animals that is granular and intracellular with no detectable surface VWF antigen. This still does not address whether the VWF adhering to the surface of hTgG233V platelets is a consequence of platelet release or plasma-derived. Staining for fibrinogen and P-selectin revealed a regular granule distribution in the cytoplasm with a similar number of granules comparing hTgWT and hTgG233V platelets. We suggest that the VWF imaged on the surface of platelets is plasma-derived and most probably represents the product of endothelial cell synthesis.
The conformation of circulating VWF is influenced by shear forces.18 At shear rates ranging from 10 to 1000 seconds, multimers exhibit a globular conformation with a 2-μm diameter, whereas higher shear stress (> 1000 seconds), such as that found in smaller arteries, results in an elongation of VWF multimers, estimated to reach 15 μm in length.18 It has also been proposed that the transition between a globular and linear conformation is reversible.18 In our analysis by confocal microscopy, we have observed a single linear VWF binding between 2 or more platelets. Estimating the mean platelet diameter as 2 to 3 μm, we can extrapolate that, according to our images, the size of a single VWF multimer could range between 0.5 μm and 15 μm long, as has been previously suggested.18 Longer multimers containing a higher number of platelets could exist in vivo, but they would probably be rapidly cleaved by the metalloprotease, ADAMTS13.25
Under normal conditions, the stretched conformation of VWF would be expected to have more binding sites exposed to solvent than globular VWF. Our images show mostly linear VWF multimers adhering to platelets, although we cannot exclude that platelets bind coiled multimers and, once bound, a conformational change results in the adoption of a linear form. Previous predictions suggest 12 000 to 25 000 GP Ib-IX receptors on the platelet surface.26,27 As such, we are observing only a small fraction of GP Ib-IX receptors occupied on the platelet surface at any one time.
Our data suggest that 50% of the hTgG233V platelet population is free of surface-attached VWF. However, we cannot exclude the possibility of a higher in vivo incidence resulting from insufficient affinity to maintain binding during platelet isolation and before subsequent staining. We have attempted to limit this possibility by immediately fixing the blood on withdrawal. Does the binding of VWF to the platelet lead to the significant impairment of hemostasis and thrombosis we have observed in hTgG233V mice? hTgG233V mice have a patent open carotid artery after vascular damage with no signs of blood flow reduction through the end of the assay (Figure 5). Two common agonists, ADP and thrombin, were unable to generate significant binding of fibrinogen using hTgG233V platelets (Figure 6). A steric hindrance by VWF on fibrinogen binding is possible, but the images suggest that the majority of the platelet surface is not occupied by VWF and only half the platelets have adherent VWF. Further evidence that fibrinogen has access to the G233V platelet surface is the ability of divalent cations to support fibrinogen binding (Figure 6) and the binding of antiαIIb antibodies (supplemental Figure 14). Thus, the data suggest that the major impairment in hemostasis and thrombosis is independent of the bound VWF, reflecting other platelet changes caused by a point mutation in the GP Ibα subunit. We suggest that the inability of ADP and thrombin to initiate fibrinogen binding most probably reflects a global change in platelet activation signals regardless of whether VWF is bound to GP Ib-IX or not. The PT-VWD extracellular mutation could propagate a structural change to the GP Ibα tail where several key platelet-signaling molecules are bound.28
Human PT-VWD differs from the mouse studied in this work because of the presence of both normal and mutant GP Ibα subunits, an autosomal dominant mode of inheritance. To simplify interpretations, we have restricted our analysis to mice expressing either a normal human GP Ibα subunit, hTgWT, or the mutant human GP Ibα subunit, hTgG233V. Thus, the phenotypic consequences of the G233V subunit in this model are probably more severe than would be expected in the human situation or with a mouse carrying both transgenes. The transgenic expression approach has yielded 2 models with similar levels of GP Ibα surface expression,10 but a single animal expressing both subunits might not completely reflect the human situation because we cannot control the relative gene expression of each GP Ibα subunit and the ability to assemble with the other subunits of the complex, GP Ibβ and GP IX, a prerequisite for surface expression. Nevertheless, for purposes of defining the consequences of a PT-VWD mutation, the presented studies should provide a mechanistic framework to further evaluate the bleeding associated with human PT-VWD.
Overall, the current murine model has provided us insights into the pathophysiology of PT-VWD. The study illustrates how perturbation of GP Ibα structure can have a significant impact on normal platelet physiology. Imaging analysis has provided new questions, and some answers, into the molecular regulation of hemostasis and thrombosis. For the future, the hTgG233V mouse model will be useful to investigate the intrinsic defects within PT-VWD platelets. Although human PT-VWD is rare and difficult to study, the hTgG233V mouse is a readily available animal model for studies of PT-VWD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by funds from the National Heart, Lung, and Blood Institute (HL50545; J.W.). J.A.G. is supported by a Sara Borrell postdoctoral contract.
National Institutes of Health
Authorship
Contribution: J.A.G. performed the majority of the research, analyzed data, and wrote the manuscript; M.K. aided in analysis of images and flow cytometry; S.R. genotyped and maintained all of the mouse colonies used in the work; J.L. and T.K.G. performed the FeCl3 experiments with the aid of J.A.G. and J.W.; B.S. guided the image analysis; and J.W. directed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
J.A.G.'s current affiliation is University of Murcia, Murcia, Spain.
Correspondence: Jerry Ware, Department of Physiology and Biophysics, Slot 505, University of Arkansas for Medical Sciences, 4301 W Markham St, Little Rock, AR 72205; e-mail: jware@uams.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal