Abstract
Abstract 4248
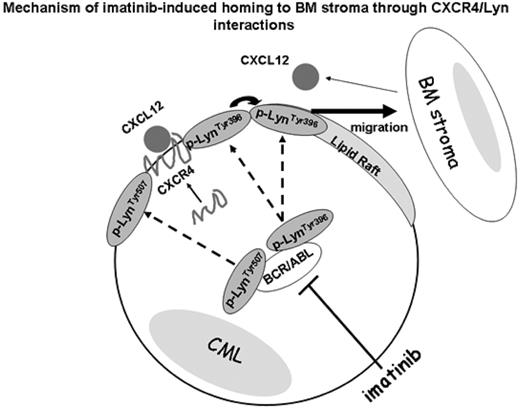
In patients with chronic-phase chronic myeloid leukemia (CML), imatinib resistance is of increasing importance. We have recently reported that the constitutively activated Bcr-Abl tyrosine kinase in CML suppresses CXCL12/CXCR4-mediated migration of CML cells to the bone marrow (BM) stroma. This finding can explain the characteristic leukocytosis in CML. In turn, tyrosine kinase inhibitor imatinib inhibits Bcr-Abl, enhances migration of CML cells towards CXCL12-producing BM stromal cells which in turn promotes cell quiescence and development of the microenvironment-mediated, non-pharmacological drug resistance (Jin, Mol Cancer Ther 2008;7:48). In this study, we further investigated the molecular mechanisms of imatinib-induced CML cell migration and adherence to the bone marrow-derived stromal cells (MSC). Src-related kinase Lyn regulates survival and responsiveness of CML cells to inhibition of BCR-ABL kinase and is known to interact with CXCL12/CXCR4 signaling. Lyn frequently localizes in lipid raft fractions, which act as signal transduction platforms for a variety of intracellular processes. Therefore, we investigated the effects of imatinib on the localization of activated Lyn in the lipid raft structures of KBM-5 CML cells under co-culture conditions with CXCL12-secreting MSC or recombinant CXCL12. Confocal microscopy and discontinuous sucrose density gradient fractionation demonstrated that CXCR4 and phosphorylated CXCR4 localized in the higher-density detergent-soluble non-raft cell surface regions in KBM5 cells in the presence and absence of imatinib, with or without MSC, which suggests that CXCR4 does not directly associate with lipid rafts. In contrast, Lyn was present both in the low-density raft and in the high-density non-raft fractions, which contained CXCR4. We have further demonstrated co-localization of CXCR4 with Lyn, and their direct interaction was confirmed by co-immunoprecipitation. Notably, the active form of phosphorylated p-LynTyr396 clustered in lipid rafts, while inactive p-LynTyr507 in non-raft fractions. In suspension KBM-5 cultures imatinib depleted both, p-LynTyr396 and p-LynTyr507. In contrast, under MSC co-culture conditions imatinib repressed p-LynTyr507, but failed to deplete p-LynTyr396 in lipid rafts, and p-LynTyr396 further accumulated in non-raft fractions, likely associating with CXCR4. Knock-down of Lyn by siRNA, Src inhibitor treatment or lipid raft destruction by methyl-b cyclodextrin (MbCD) abrogated imatinib-induced KBM5 migration to MSCs and CXCL12, indicating the critical role of p-LynTyr396 in cell migration. Since the a4b1 integrin VLA-4 represents a cooperative molecular pathway guiding BM homing in addition to CXCL12/CXCR4, we next investigated the localization and expression of VLA-4 in KBM5 cells. Imatinib decreased VLA-4 protein expression both in lipid raft and non-raft fractions without affecting VLA-4 gene expression levels as determined by quantitative RT-PCR. Interestingly, VLA-4 reduction by imatinib or lipid raft destruction by MbCD did not affect the ability to adhere to fibronectin.
In conclusion, these findings demonstrate that under conditions mimicking BM microenvironment imatinib restores CXCL12-dependent migration through interactions between CXCR4 and active p-Lyn Tyr396 in non-raft microdomains of CML cells and that p-Lyn Tyr396 localized in lipid rafts is contributing to the CML cell migration. We propose that while CXCR4 is segregated from lipid raft fractions, imatinib through Bcr-Abl kinase inhibition induces the compartmental changes of multivalent Lyn complex between lipid raft and non-raft fractions, restoring the interactions between Lyn and CXCR4 and stimulating cell migration.
Our findings indicate that leukemic BM microenvironment may be involved in imatinib resistance in a subset of CML patients through activation of Lyn kinase, consistent with reported higher clinical activity of Bcr-Abl/Src inhibitor dasatinib in patients with imatinib-resistant CML. We propose that BM stroma cells produce abundant CXCL12 and attract migrating cells through adhesive interactions with the extracellular matrix, which may in turn facilitate lodging into BM niches of imatinib-exposed CML cells and promote non-pharmacological resistance to this agent.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.