Abstract
Adult T-cell leukemia (ATL) is caused by human T-cell lymphotropic virus I (HTLV-1) and is an aggressive malignancy of CD4, CD25-expressing leukemia, and lymphoma cells. There is no accepted curative therapy for ATL. Depsipeptide, a histone deacetylase inhibitor, has demonstrated major antitumor effects in leukemias and lymphomas. In this study, we investigated the therapeutic efficacy of depsipeptide alone and in combination with daclizumab (humanized anti-Tac) in a murine model of human ATL. The Met-1 ATL model was established by intraperitoneal injection of ex vivo leukemic cells into nonobese diabetic/severe combined immunodeficiency mice. Either depsipeptide, given at 0.5 mg/kg every other day for 2 weeks, or daclizumab, given at 100 μg weekly for 4 weeks, inhibited tumor growth as monitored by serum levels of soluble IL-2R-α (sIL-2R-α) and soluble β2-microglobulin (β2μ) (P < .001), and prolonged survival of the leukemia-bearing mice (P < .001) compared with the control group. Combination of depsipeptide with daclizumab enhanced the antitumor effect, as shown by both sIL-2R-α and β2μ levels and survival of the leukemia-bearing mice, compared with those in the depsipeptide or daclizumab alone groups (P < .001). The significantly improved therapeutic efficacy by combining depsipeptide with daclizumab supports a clinical trial of this combination in the treatment of ATL.
Introduction
Adult T-cell leukemia (ATL) is an aggressive malignancy of mature activated CD4+ T cells associated with human T-cell lymphotropic virus type 1 (HTLV-1) infection.1-3 The leukemic cells are characterized by the expression of interleukin-2 receptor α (IL-2R-α, CD25) on their cell surfaces.4-6 At present, there is no accepted curative therapy for ATL, and the patients progress to death with a median survival duration of 9 months in acute ATL and 24 months in chronic ATL.7 A preclinical in vivo murine model of ATL was developed by introducing leukemic cells (MET-1) from a patient with ATL into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice.8 New therapeutic approaches have been tested in this model before initiating human clinical trials.9,10 Daclizumab (Zenapax; Hoffman La Roche, Basel Switzerland), a monoclonal antibody targeted at the IL-2R-α (CD25), showed excellent therapeutic efficacy in this murine model.8 In clinical trials, it has also yielded some partial or rare complete remissions in patients with ATL, suggesting that this new IL-2R-α–targeting monoclonal antibody therapy is promising in the treatment of patients with ATL. Recently, a high response rate after azidothymidine/interferon-α (AZT/IFN-α) treatment of ATL patients has been reported in several human trials.11-13 The tumor suppressor p53 appears to be a predictive marker for AZT response, as patients responded to AZT therapy only when p53 was wild-type in sequence; inversely, disease relapse or absence of response was associated with mutation and inactive p53.14 A paradigm has emerged that the combination of a monoclonal antibody with chemotherapeutic reagents that function via different mechanisms of action may be greater than additive in their cytotoxic action, leading to malignant cell death. Thus, it will be of great value to find chemotherapy reagents that could enhance the antitumor efficacy of daclizumab.
Histone deacetylase (HDAC) inhibitors are a new class of antitumor agents that are currently undergoing intensive preclinical and clinical testing. HDAC inhibitors are potent inducers of apoptosis and growth inhibition with a variety of transformed cells in vitro and in vivo, including malignancies originating from lymphoid cells.15-17 Depsipeptide (FR901228, FK228), isolated from Chronobacterium violaceum, is a member of the cyclic peptide class of HDAC inhibitors. Depsipeptide has shown cytotoxic effects on several malignant lymphoid cell lines,18 including HTLV-1–infected T-cell lines.19 Depsipeptide is currently in clinical trials for evaluation of its anticancer efficacy.20 Recent results using FR901228 in patients with cutaneous and peripheral T-cell lymphoma suggest significant activity in these diseases.15 Furthermore, the study shows that the IL-2R-α expression level on the patients' malignant cells after depsipeptide treatment is increased. Based on these findings, it has been speculated that the combination of depsipeptide with IL-2R-α targeting therapy may enhance the antitumor efficacy of depsipeptide.15,21 As discussed in this introduction, daclizumab is an IL-2R-α–targeting agent. Thus, the combination of depsipeptide with daclizumab in the treatment of T-cell malignancy could enhance the antitumor efficacy, not only through 2 different modes of killing, but also through enhancing the expression of the target of daclizumab.
In this study, we investigated the therapeutic efficacy of depsipeptide alone and its combination with daclizumab in a murine model of ATL. Both depsipeptide and daclizumab inhibited tumor growth as monitored by soluble IL-2R-α and soluble β2μ levels (P < .001), and prolonged the survival of the leukemia-bearing mice significantly (P < .001). Combination of these 2 agents demonstrated much greater therapeutic efficacy in the MET-1 model of ATL. The median survival of leukemia-bearing mice was 180 days in the combination group, compared with 113 days in the depsipeptide alone group, 98 days in the daclizumab group, and 57 days in the phosphate-buffered saline (PBS) group. Our data would support the use of depsipeptide, preferably combined with daclizumab, in the treatment of patients with ATL.
Methods
Drug and antibody
Depsipeptide was obtained from Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute (Bethesda, MD). Daclizumab, which recognizes IL-2R-α, was acquired from Hoffman-La Roche.22
Proliferation assay
HTLV-1 positive T-cell lines, HUT102, CaGT, MT-2, MT-1, and MJ, were maintained in RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 100 U/mL of penicillin, and 100 μg/mL of streptomycin in an atmosphere containing 5% CO2. Aliquots of 5 × 103 cells were seeded in 96-well culture plates and incubated with medium alone or with serial dilutions of depsipeptide (0.125, 0.5, 2, and 5 ng/mL). The cells were pulsed after 72 hours of culture for 6 hours with 1 μCi (0.037 MBq) of [3H]thymidine (GE Healthcare, Little Chalfont, United Kingdom). Then, the cells were harvested with a 96-well harvester (Tomtec, Hamden, CT) and counted in a β counter (PerkinElmer Wallac, Turku, Finland). The assay was performed in triplicate and repeated 3 times.
Annexin V staining and apoptosis analysis
Quantification of apoptosis was performed by immunostaining cells with annexin V, which specifically detects phospholipid phosphatidylserine redistributed from the inner to the outer leaflet during apoptosis.23,24 Cells cultured with 2 ng/mL of depsipeptide or media for 24 and 48 hours were labeled with annexin V fluorescein isothiocyanate and subsequently analyzed by flow cytometry (fluorescein-activated cell sorting). The dead cells were labeled with propidium iodide.
Caspase-3 and caspase-9 activity assay
The caspase-3 and caspase-9 activities were measured using caspase-3 and caspase-9 colorimetric assays from R&D Systems (Minneapolis, MN). The activity was expressed as fold increase in depsipeptide-treated cells over that of nontreated cells. The background values were subtracted from the experimental results before calculating the fold induction.
Western blot analysis
Treated cells were solubilized at 4°C in ristocetin-induced platelet aggregation lysis buffer (50 mM Tris-Cl, pH 7.4, 0.5% sodium deoxycholate, 1% Nonidet P-40, 150 mM of NaCl, 66 μg/mL aprotinin, 100 μg/mL phenylmethylsulfonyl fluoride, and 1 mM sodium orthovanadate). Cell lysates (50 μg) were resolved by electrophoresis on sodium dodecyl sulfate–polyacrylamide (4%-12%) gels and transferred to polyvinylidine difluoride membranes. After blocking of the membranes in 5% skim milk and 0.05% Tween 20 in Tris-buffered saline, the blots were incubated with the mouse monoclonal antibody to p21, cyclin D1, Bcl-2, α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA), or the rabbit polyclonal antibody to cyclin A (Santa Cruz Biotechnology), acetyl-histone H3, histone H3 (Cell Signaling Technology, Danvers, MA), or Bcl-XL (Transduction Laboratories, Lexington, KY). The anti-Tax antibody was kindly provided by Dr Steven Jacobson (National Institute of Neurological Disorders and Stroke, National Institutes of Health). After several washes, the protein bands recognized by the antibodies were visualized with an enhanced chemiluminescence Western blotting detection system (GE Healthcare).
Mouse model of ATL
The ATL cell population, MET-1, was established from the peripheral blood of a patient with acute ATL, and the cells were maintained by serial transfer in NOD/SCID mice (The Jackson Laboratories, Bar Harbor, ME). MET-1 cells have a distinct phenotype elucidated by fluorescein-activated cell sorting analysis: CD3dim, CD4+/−, CD7−, CD20−, and CD25+. The leukemia model was established by intraperitoneal injection of 1.5 × 107 MET-1 cells into NOD/SCID mice as described previously.8 The therapy experiments were performed on these mice when their serum-soluble IL-2R-α (sIL-2R-α) levels were more than 1000 pg/mL, which occurs approximately 10 to 14 days after tumor inoculation. All animal experiments were performed in accordance with National Institutes of Health Animal Care and Use Committee guidelines.
Definition of the maximum tolerated dose
Before the initiation of the therapeutic studies, the maximum tolerated dose of depsipeptide was determined in NOD/SCID mice. Doses of 0.125, 0.25, 0.5, 1.0, and 2.0 mg depsipeptide per kilogram of body weight were administered intraperitoneally daily for 2 weeks. All mice in the 2.0 mg/kg group died at day 7 and 80% of the mice in the 1.0 mg/kg group died at day 14. The mice in the 0.5, 0.25, and 0.125 mg/kg group were still alive 6 months after depsipeptide injection. Therefore, a dose of 0.5 mg/kg every other day for 14 days was chosen to use in the therapeutic trials; this dose is consistent with what other researchers have used in mice.19,25
Therapy study
Therapeutic studies were performed in MET-1 leukemia-bearing mice with serum surrogate tumor marker sIL-2R-α values of 1000 to 10 000 pg/mL in the small tumor burden trial and 10 000 to 25 000 pg/mL in the large tumor burden trial. There were 5 groups in the therapeutic trials. Group 1, the depsipeptide group, received intraperitoneal injections of 0.5 mg depsipeptide/kg body weight every other day for 2 weeks. Group 2, the immunotherapy (daclizumab) group, was given intravenous injections of 100 μg daclizumab on days 0, 7, 14, and 21. Group 3, the combination therapy group, received a combined therapy of depsipeptide and daclizumab (dosing schedule as in group 1 plus group 2). Group 4 received 200 μL of PBS weekly for 4 weeks and served as a control. Group 5, with no tumor and no therapy, served as a control for the natural death of NOD/SCID mouse. There were 13 mice per group in the small tumor burden therapeutic trial (sIL-2R-α, 1000-10 000 pg/mL), and there were 8 mice per group in the large tumor burden trial (sIL-2R-α, 10 000-25 000 pg/mL). The groups were randomly assigned and had comparable average levels of the surrogate tumor marker, sIL-2R-α, at the beginning of the experiments.
Monitoring of tumor growth
Measurements of the serum concentrations of the soluble IL-2R-α or soluble β2-microglobulin (β2μ) were performed using enzyme-linked immunosorbent assay (R&D Systems). The enzyme-linked immunosorbent assays were performed following the manufacturer's recommendation.
Statistical analysis
The serum levels of human sIL-2R-α and β2μ were analyzed at different time points for the different treatment groups using the Student t test for unpaired data. Statistical significance of differences in survival of mice in different groups was determined by the log-rank test using the StatView program (Abacus Concepts, Berkeley, CA).
Results
Depsipeptide inhibited the proliferation and induced apoptosis in HTLV-1–positive T-cell lines
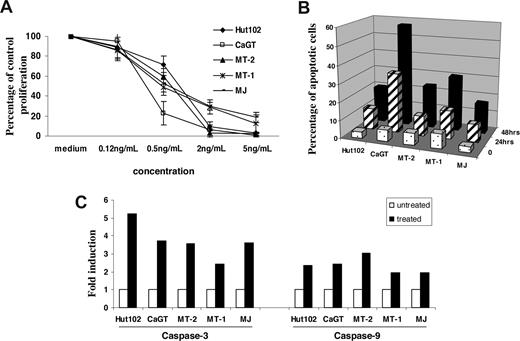
We first examined the effects of depsipeptide on proliferation and apoptosis of HTLV-1–infected T-cell lines. The cell lines (Hut102, MT-2, CaGT, MT-1, and MJ) were treated with various concentrations (0.12-5 ng/mL) of depsipeptide for 72 hours. Depsipeptide inhibited the proliferation in a dose-dependent manner in all 5 cell lines tested (Figure 1A). Staining of depsipeptide-treated cells with annexin V fluorescein isothiocyanate showed that a significant proportion of the cells had undergone apoptosis 24 and 48 hours after depsipeptide treatment (2 ng/mL; Figure 1B). Both caspase-9 and caspase-3 activities were induced in the HTLV-1–infected cell lines 24 hours after depsipeptide treatment (5 ng/mL; Figure 1C). It has been reported by Nguyen et al that depsipeptide induced apoptosis of lung or esophageal cancer cells by activating the mitochondria-dependent death-signaling pathway.26 Our data suggested a similar action of depsipeptide in HTLV-1–infected cell lines.
Depsipetide treatment inhibited the cell proliferation and induced apoptosis in HTLV-I–infected cell lines. (A) The cells were treated with various concentrations of depsipeptide (0, 0.12, 0.5, 2, or 5 ng/mL), and the cell proliferation was measured 72 hours later by 3H thymidine incorporation. The data are shown as percentage of untreated control, represent the mean plus or minus SD of triplicates, and are representative of 3 independent experiments. (B) Induction of apoptosis by depsipeptide. The cells were treated with 2 ng/mL of depsipeptide for 24 hours or 48 hours, and the apoptotic cells were measured by annexin V staining. Data represent the mean percentage of apoptotic cells from 3 independent experiments. (C) Induction of caspase-3 and caspase-9 activities by depsipeptide. The data are representative of 3 independent experiments.
Depsipetide treatment inhibited the cell proliferation and induced apoptosis in HTLV-I–infected cell lines. (A) The cells were treated with various concentrations of depsipeptide (0, 0.12, 0.5, 2, or 5 ng/mL), and the cell proliferation was measured 72 hours later by 3H thymidine incorporation. The data are shown as percentage of untreated control, represent the mean plus or minus SD of triplicates, and are representative of 3 independent experiments. (B) Induction of apoptosis by depsipeptide. The cells were treated with 2 ng/mL of depsipeptide for 24 hours or 48 hours, and the apoptotic cells were measured by annexin V staining. Data represent the mean percentage of apoptotic cells from 3 independent experiments. (C) Induction of caspase-3 and caspase-9 activities by depsipeptide. The data are representative of 3 independent experiments.
Depsipeptide induced accumulation of histone acetylation and affected the expression of intracellular regulators of cell cycle and apoptosis
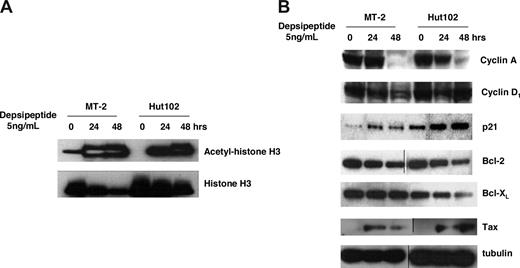
To clarify the molecular mechanisms by which depsipeptide induces inhibition of cell growth and apoptosis in HTLV-1–infected T-cell lines, we examined the histone acetylation level (histone H3) and some regulators of the cell cycle (cyclin A, cyclin D1, p21) and apoptosis (Bcl-2, Bcl-XL) by Western blot analysis. As expected, the acetylation level of histone H3 was dramatically increased in depsipeptide-treated (5 ng/mL) HTLV-1–positive Hut102 and MT-2 cells both at 24 and 48 hours after treatment (Figure 2A). The accumulation of histone acetylation levels could profoundly affect the transcription of genes involved in proliferation, cell cycle, and apoptosis.27 As shown in Figure 2B, the expression levels of the CDK inhibitor p21 were up-regulated, and the expressions of cyclin A were down-regulated in depsipeptide-treated (5 ng/mL) Hut102 and MT-2 cells, whereas the expression of cyclin D1 was not altered by depsipeptide. Furthermore, the expressions of antiapoptotic proteins Bcl-2 and Bcl-XL were decreased in depsipeptide-treated Hut102 and MT-2 cells, which is consistent with the observation that depsipeptide induced apoptosis in these cell lines. Interestingly, depsipeptide treatment induced the viral gene Tax expression in Hut102 and MT-2 cells. It has been reported that HDAC1 negatively regulates viral gene expression and the HDAC inhibitor trichostatin A induced viral gene Tax expression in the HTLV-1–infected cell line C81.28,29 Depsipeptide inhibits both class I and class II HDAC, including HDAC130 ; our data suggested a similar action of depsipeptide in HTLV-1–infected cell lines.
Depsipeptide treatment induced accumulation of histone acetylation and altered the expression of cyclin A, p21, Bcl-2, and Bcl-XL in HTLV-I–infected cell lines. (A) Accumulation of histone H3 acetylation after depsipetide treatment. MT-2 and Hut102 cells were treated with 5 ng/mL of depsipeptide for 24 hours and 48 hours. Total cell lysate (50 μg/lane) was separated on sodium dodecyl sulfate-polyacrylamide gels and transferred to the membrane. Acetyl-histone H3 or histone H3 levels were detected by Western blot with specific antibodies. (B) Effect of depsipeptide on the expression of cyclin A, cyclin D, p21, Bcl-2, Bcl-XL, and viral protein Tax. Cell lines were treated with 5 ng/mL of depsipeptide for 24 hours and 48 hours, and then total cellular protein was extracted and Western blot analysis was performed.
Depsipeptide treatment induced accumulation of histone acetylation and altered the expression of cyclin A, p21, Bcl-2, and Bcl-XL in HTLV-I–infected cell lines. (A) Accumulation of histone H3 acetylation after depsipetide treatment. MT-2 and Hut102 cells were treated with 5 ng/mL of depsipeptide for 24 hours and 48 hours. Total cell lysate (50 μg/lane) was separated on sodium dodecyl sulfate-polyacrylamide gels and transferred to the membrane. Acetyl-histone H3 or histone H3 levels were detected by Western blot with specific antibodies. (B) Effect of depsipeptide on the expression of cyclin A, cyclin D, p21, Bcl-2, Bcl-XL, and viral protein Tax. Cell lines were treated with 5 ng/mL of depsipeptide for 24 hours and 48 hours, and then total cellular protein was extracted and Western blot analysis was performed.
Definition of the maximum tolerated dose
Different doses of depsipeptide (0.125, 0.25, 0.5, 1.0, and 2.0 mg/kg body weight 5 days/week for 2 weeks) were injected intraperitoneally in the NOD/SCID mice to determine the maximum tolerated dose. The mice injected with 2.0 mg/kg died (5 of 5) during the injection course, and 80% of the 1.0 mg/kg group mice died right after the injection (day 14), indicating high toxicity at those doses. The groups of 0.5, 0.25, and 0.125 mg/kg survived for more than 6 months. Considering the tolerance of depsipeptide in tumor-bearing NOD/SCID mice might be lower than that in non–tumor-bearing mice, we choose to administer 0.5 mg/kg every other day for 2 weeks in the therapy study. It has been reported in the literature that intraperitoneal administration of 0.5 mg/kg of depsipeptide 3 times a week is sufficient to inhibit HDAC in mice.31,32
Effective treatment of ATL with depsispeptide alone and in combination with daclizumab in the Met-1 model
The small tumor burden therapeutic trial in MET-1 model.
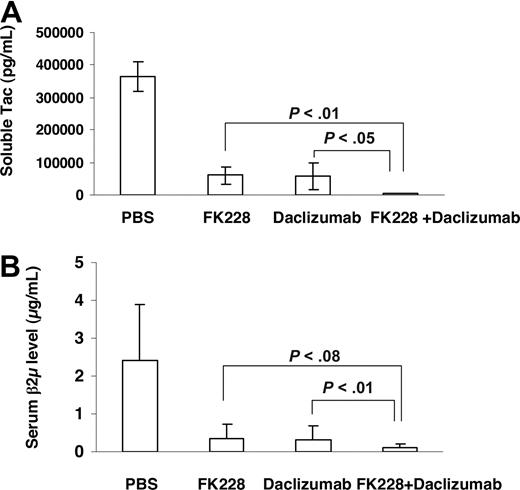
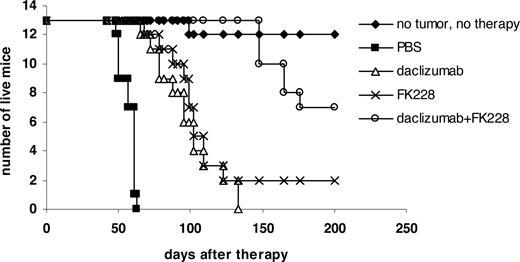
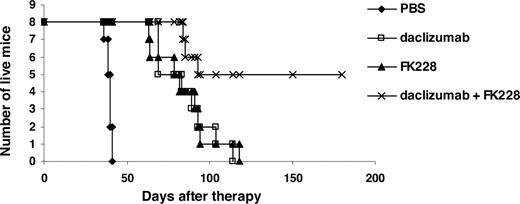
In a therapeutic trial in the MET-1 model of human ATL, a 2-week course of treatment with depsipeptide (0.5 mg/kg every other day), a 4-week course of treatment with daclizumab (100 μg/mouse, weekly), and the combination of depsipeptide with daclizumab demonstrated therapeutic efficacy by both their effects on the serum levels of human soluble Tac (sIL-2R-α) and soluble β2μ (Figure 3) and on the survival of leukemia-bearing mice (sIL-2R-α, 1000-10 000 pg/mL; Figure 4). Compared with the serum concentration of sIL-2Ra and β2μ in the PBS control group of mice at 8 weeks after therapy, there was a significant reduction of sIL-2Ra as well as β2μ levels in treated animals in the depsipeptide group (P < .001), the daclizumab group (P < .001), and the combination of depsipeptide with daclizumab group (P < .001). Furthermore, there were significant reductions in human sIL-2Ra and reductions in β2μ levels that did not achieve significance in the combination therapy group of mice (depispeptide plus daclizumab) compared with that of the daclizumab alone group (sIL-2Ra, P < .05; β2μ, P < .1) or the depsipeptide alone group (sIL-2Ra, P < .01; β2μ, P < .08). The human sIL-2R-α and β2μ levels were undetectable in 9 of 13 mice that received the combination of depsipeptide and daclizumab treatment when measured at 8 weeks after therapy, suggesting that those mice were virtually tumor-free. However, the leukemia progressed in those mice gradually, as demonstrated by the increased human sIL-2R-α and β2μ levels when we terminated the experiment at day 200 after therapy (data not shown). The PBS control group mice died between day 48 and day 63. The control mice had extensive infiltrations of leukemic cells into a variety of organs, including the lungs, liver, and spleen.8 In contrast, the depsipeptide treatment alone (P < .001), daclizumab treatment alone (P < .001), and the combination of depsipeptide and daclizumab treatment (P < .001) had significantly prolonged survival of the leukemia-bearing mice (Figure 4). Furthermore, the mice in the treatment groups had much less infiltration in the lungs, liver, and spleen but had tumor outgrowth subcutaneously when examined at the endpoint. This may be the result of incomplete saturation of the IL-2 receptor by daclizumab in the skin compared with blood or lymph nodes where the receptor is easily saturated as observed with patients with ATL (T.A.W., unpublished data, September 2005). All the mice in the PBS group died before day 63 after therapy. However, at 120 days after therapy, 2 of 13 mice in the depsipeptide treatment alone and daclizumab treatment alone groups and 13 of 13 mice in the combination therapy group were still alive. The combination of depsipeptide and daclizumab treatment significantly prolonged the survival of leukemia-bearing mice compared with depsipeptide treatment alone (P < .001) or daclizumab treatment alone (P < .001). Seven of 13 mice survived more than 200 days in the combination group (Figure 4).
The growth of MET-1 ATL cells in NOD/SCID mice bearing the MET-1 ATL leukemia was inhibited by daclizumab, depsipeptide, and the combination of daclizumab with depsipeptide. MET-1 ATL cells were transferred into mice. The groups (13 mice/group) included those receiving PBS, 2 weeks every other day 0.5 mg/kg per dose depsipeptide, 4 weekly doses of 100 μg of daclizumab, the combination of 2 weeks every other day 0.5 mg/kg per dose depsipeptide with 4 weekly doses of 100 μg daclizumab. (A) The mean concentration of sIL-2R-α in picograms per milliliter. The animals treated in the 2-week depsipeptide, 4-week daclizumab, and the combination of 2-week depsipeptide with 4-week daclizumab groups had significantly decreased values of sIL-2R-α compared with those of the PBS control group 8 weeks after therapy (P < .001). Furthermore, the animals receiving the combination of depsipeptide with daclizumab had significantly decreased levels of sIL-2R-α compared with those of the mice in the depsipetide alone group (P < .01) and daclizumab alone group (P < .05). (B) The mean concentration of β2μ in micrograms per milliliter 8 weeks after therapy. The serum levels of β2μ were significantly lower in the depsipetide treatment alone, daclizumab treatment alone, and combination group compared with PBS group (P < .001). The animals receiving the combination of depsipeptide with daclizumab had decreased levels of β2μ compared with those of the mice in the depsipetide alone group (P < .08) and daclizumab alone group (P < .1).
The growth of MET-1 ATL cells in NOD/SCID mice bearing the MET-1 ATL leukemia was inhibited by daclizumab, depsipeptide, and the combination of daclizumab with depsipeptide. MET-1 ATL cells were transferred into mice. The groups (13 mice/group) included those receiving PBS, 2 weeks every other day 0.5 mg/kg per dose depsipeptide, 4 weekly doses of 100 μg of daclizumab, the combination of 2 weeks every other day 0.5 mg/kg per dose depsipeptide with 4 weekly doses of 100 μg daclizumab. (A) The mean concentration of sIL-2R-α in picograms per milliliter. The animals treated in the 2-week depsipeptide, 4-week daclizumab, and the combination of 2-week depsipeptide with 4-week daclizumab groups had significantly decreased values of sIL-2R-α compared with those of the PBS control group 8 weeks after therapy (P < .001). Furthermore, the animals receiving the combination of depsipeptide with daclizumab had significantly decreased levels of sIL-2R-α compared with those of the mice in the depsipetide alone group (P < .01) and daclizumab alone group (P < .05). (B) The mean concentration of β2μ in micrograms per milliliter 8 weeks after therapy. The serum levels of β2μ were significantly lower in the depsipetide treatment alone, daclizumab treatment alone, and combination group compared with PBS group (P < .001). The animals receiving the combination of depsipeptide with daclizumab had decreased levels of β2μ compared with those of the mice in the depsipetide alone group (P < .08) and daclizumab alone group (P < .1).
Depsipeptide and its combination with daclizumab prolonged the survival of MET-1 leukemia-bearing SCID/NOD mice. At the time of the experiment, the mice had sIL-2R-α levels of 1000 to 10 000 pg/mL. The groups (13 mice/group) included those receiving intravenous PBS, 0.5 mg/kg per dose depsipeptide every other day for 2 weeks, 100 μg of daclizumab weekly for 4 weeks, and a combination of 0.5 mg/kg per dose depsipeptide every other day for 2 weeks with 100 μg of daclizumab weekly for 4 weeks. Another group receiving no tumor and no therapy was set up as a life span control for NOD/SCID mice. Event-free survival was followed for 200 days. The animals treated in depsipetide, daclizumab, and the combination of daclizumab with depsipeptide groups had significantly prolonged survivals compared with the PBS control group (P < .001). The combination of depsipeptide with daclizumab significantly prolonged the survival of leukemia-bearing mice compared with depsipeptide alone (P < .001) or daclizumab alone (P < .001).
Depsipeptide and its combination with daclizumab prolonged the survival of MET-1 leukemia-bearing SCID/NOD mice. At the time of the experiment, the mice had sIL-2R-α levels of 1000 to 10 000 pg/mL. The groups (13 mice/group) included those receiving intravenous PBS, 0.5 mg/kg per dose depsipeptide every other day for 2 weeks, 100 μg of daclizumab weekly for 4 weeks, and a combination of 0.5 mg/kg per dose depsipeptide every other day for 2 weeks with 100 μg of daclizumab weekly for 4 weeks. Another group receiving no tumor and no therapy was set up as a life span control for NOD/SCID mice. Event-free survival was followed for 200 days. The animals treated in depsipetide, daclizumab, and the combination of daclizumab with depsipeptide groups had significantly prolonged survivals compared with the PBS control group (P < .001). The combination of depsipeptide with daclizumab significantly prolonged the survival of leukemia-bearing mice compared with depsipeptide alone (P < .001) or daclizumab alone (P < .001).
The larger tumor burden treatment trial in the MET-1 model.
The tumor burden was triple that of the small tumor burden group (sIL-2R-α, 10 000-25 000 pg/mL). The treatment of depsipeptide and daclizumab (same dose and dosing schedule as the small tumor burden trial) alone and the combination of these 2 agents demonstrated therapeutic efficacy. The serum levels of tumor marker soluble IL-2Ra and β2μ in the depsipeptide group, daclizumab group, and the combination group was significantly lower than that of the PBS group at 4 weeks after therapy (data not shown). All 3 treatments significantly prolonged the survival of tumor-bearing mice (P < .001). Furthermore, the combination of depsipeptide with daclizumab significantly prolonged the survival of the tumor-bearing mice compared with depsipeptide alone (P < .01) or daclizumab alone (P < .05). Similar to the small tumor burden trial, the mice in the PBS group had extensive infiltrations of leukemic cells in the lungs, liver, and spleen, whereas the mice in the treatment group had much less infiltration in the lungs, liver, and spleen but had tumor outgrowth subcutaneously. The mice in the PBS group died before day 45 after therapy. However, at day 100 after therapy, 1 of 8 mice in the depsipeptide treatment alone group and 2 of 8 mice in the daclizumab treatment alone group were still alive. Furthermore, 5 of 8 mice in the combination therapy group survived more than 180 days (Figure 5).
Depsipeptide inhibited the tumor growth and prolonged the survival of leukemia-bearing mice in the large tumor burden therapeutic study. At the time of the initial therapy, the mice had sIL-2Ra levels of 10 000 to 25 000 pg/mL. Groups are the same as those described in Figure 4. The animals treated in depsipetide, daclizumab, and the combination of daclizumab with depsipeptide groups had significantly prolonged survivals compared with the PBS control group (P < .001). The combination of depsipeptide with daclizumab treatment significantly prolonged the survival of leukemia-bearing mice compared with depsipeptide alone (P < .01) or daclizumab alone treatment (P < .05).
Depsipeptide inhibited the tumor growth and prolonged the survival of leukemia-bearing mice in the large tumor burden therapeutic study. At the time of the initial therapy, the mice had sIL-2Ra levels of 10 000 to 25 000 pg/mL. Groups are the same as those described in Figure 4. The animals treated in depsipetide, daclizumab, and the combination of daclizumab with depsipeptide groups had significantly prolonged survivals compared with the PBS control group (P < .001). The combination of depsipeptide with daclizumab treatment significantly prolonged the survival of leukemia-bearing mice compared with depsipeptide alone (P < .01) or daclizumab alone treatment (P < .05).
Discussion
The MET-1 ATL model presents many features that parallel those observed in patients with adult T-cell leukemia and thus represents a valuable model for the evaluation of the efficacy of therapeutic agents directed toward ATL.8 In earlier studies, daclizumab showed efficacy both in the MET-1 ATL model and in human clinical trials. In the human study, therapy with the umodified murine version of anti-Tac provided effective therapy for 6 of 19 patients with ATL studied.33 There are 2 modes of tumor killing manifested by the monoclonal antibody daclizumab. One is the blockade of the binding of IL-2 to its receptor (IL-2R-α), as shown in patients with smoldering ATL, therefore leading to cytokine deprivation-induced apoptosis.33 Another mode is antibody-dependent cellular cytotoxicity, as shown in murine MET-1 model, which requires the expression of FcR-γIII on monocytes and granulocytes.34 Daclizumab provides no therapeutic efficacy in the murine ATL model when examined in FcR-γ knockout (FcR-γ−/−) mice that do not express FcR-γIII.34 Recently, another mechanism of action has been reported. In particular, in humans the interaction of daclizumab with the IL-2 receptor alpha subunit was associated with a 4- to 20-fold increase in the number of circulating CD56bright, CD25-expressing, IL-10–secreting natural killer cells that mediate negative immunoregulatory actions.35,36 Several approaches could be exploited to optimize the action of daclizumab in the therapy of leukemias and lymphomas. A paradigm is being established that monoclonal antibodies will not be used ultimately in monotherapy of human malignancy but rather will be used in association with an array of agents, including chemotherapeutic agents that manifest a different mode of action. In the MET-1 model, we have shown that the combination of daclizumab with the chemotherapeutic agent Velcade (bortezomib or PS-341, a proteasome inhibitor) or flavopiridol (a CDK inhibitor) had synergistic therapeutic effects in the treatment of ATL.10,37 To continue this theme, we are testing other chemotherapeutic agents, such as HDAC inhibitors (ie, depsipeptide) in combination with daclizumab in the MET-1 model.
The HDAC inhibitors are a new class of antineoplastic agents currently being evaluated in clinical trials. Several families of HDAC inhibitors have been characterized. These include the short-chain fatty acids, such as sodium butyrate and valproic acid; the organic hydroxamic acids, such as trichostatin A and suberanilohydroxamic acid; the benzamides, such as CI-994 and MS-27-275; the cyclic tetrapeptides, such as trapoxin A; and the bicyclic depsipeptides, such as depsipeptide. Among them, Vorinostat (suberanilohydroxamic acid) has been approved by the FDA as monotherapy for cutaneous T-cell lymphoma. In addition, depsipeptide has shown major responses in the treatment of leukemia and lymphomas. Similar to other HDAC inhibitors, depsipeptide has been shown to induce cell-cycle arrest, cellular differentiation, and apoptosis.16 Although the precise mechanism of cell growth arrest, cellular differentiation, and apoptosis is not clear, it is commonly accepted that depsipeptide exerts its antitumor effect via modulation of the expression and functions of cell-cycle regulators and apoptosis-related molecules.16 The molecular changes induced by depsipeptde include increased expression of p21,38 altered expression of cyclins, hyperphosphorylation of Rb,38 and decreased expression of c-myc in fibroblasts and in a T-cell hybridoma.39 Consistent with this, we observed that depsipeptide induced increased expression of p21, decreased expression of cyclin A, and decreased expression of antiapoptotic proteins Bcl-2 and Bcl-XL in HTLV-I–infected T-cell lines.
Besides its antitumor effects, depsipeptide has been shown to be able to induce the expression of IL-2R-α, the target of daclizumab, both in cell lines21 and in the patients with T-cell lymphoma.15 We reasoned that depsipeptide, which was reported to be an Il-2R α-inducing agent, might augment the acitivity of daclizumab, the combination of depsipeptide with daclizumab may improve the efficacy of daclizumab in the treatment of ATL. Indeed, in our Met-1 model of ATL, we observed greater efficacy for the combination of depsipeptide with daclizumab. When administered intraperitoneally at 0.5 mg/kg every other day for 2 weeks, the tumor growth was significantly inhibited and the survival of the mice was significantly prolonged in the combination group compared with those in either the daclizumab or depsipeptide groups alone (Figures 4, 5). However, in our study, depsipeptide treatment did not increase the expression of IL-2Ra in HTLV-1–infected cell lines (data not shown). This suggested that the synergistic effect between depsipeptide and daclizumab was probably the result of the combined effects of monoclonal antibody targeted therapy with a chemotherapeutic molecule, agents that act through different modes of tumor killing. As discussed in “Results,” the cytotoxicity of despeptide is induced by modulation of genes involved in cell-cycle regulation and apoptosis.40 On the other hand, the monoclonal antibody daclizumab does not act through blockade of the IL-2/IL2Ra autocrine loop in the MET-1 ATL model because the leukemic cells do not produce IL-2, nor do they express IL-2 mRNA. In addition, they do not proliferate in response to murine IL-2.8 Depsipeptide treatment did not induce the expression of IL-2 either (data not shown). The immunoglobulin Fc receptor does appear to be the major element involved in the action of daclizumab as daclizumab lost its therapeutic efficacy in the FcR-γIII–deficient mice.34 However, depsipeptide treatment had no effect on FcR-γIII expression in NOD/SCID mice (data not shown). This suggests that the synergistic effect of depsipeptide with daclizumab was not the result of increased FcR-γIII expression.
Currently, depsipeptide is in the phase 2 trial with cutaneous and peripheral T-cell lymphoma. Electrocardiogram abnormalities, thought to be a class effect associated with HADC inhibitors, were observed both in preclinical animal studies and in phase 1 testing of depsipeptide.41 However, extensive cardiac studies in the phase 2 trial of depsipetide in T-cell lymphoma concluded that the administration of depsipeptide was not associated with myocardial damage or impaired cardiac function.42 The potential effect of heart rate–corrected QT interval prolongation remains under study. With significant clinical benefit in patients with cutaneous and peripheral T-cell lymphoma, these safety data could further support the use of depsipeptide in patients with leukemias and lymphomas.
In conclusion, our data showed that depsipeptide had an antitumor effect both in vitro and in vivo. The combination therapy of depsipetide with daclizumab had significant synergistic therapeutic effect in the murine model of ATL. The results of our study support a trial of depsipeptide in ATL patients, preferably as an agent combined with daclizumab therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the intramural research program of the National Cancer Institute, National Institutes of Health (Bethesda, MD).
National Institutes of Health
Authorship
Contribution: J.C. designed and performed research; M.Z. and W.J. helped with the animal study; and T.A.W. designed the study and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas A. Waldmann, National Institutes of Health, Building 10, Room 4N115, 10 Center Drive, Bethesda, MD 20892-1374; e-mail: tawald@helix.nih.gov.