Abstract
Circulating platelets exhibit rapid signaling and adhesive responses to collagen that facilitate hemostasis at sites of vessel injury. Because platelets are anuclear, their collagen receptors must be expressed by megakaryocytes, platelet precursors that arise in the collagen-rich environment of the bone marrow. Whether and how megakaryocytes regulate collagen adhesion during their development in the bone marrow are unknown. We find that surface expression of activated, but not wild-type, α2 integrins in hematopoietic cells in vivo results in the generation of platelets that lack surface α2 receptors. Culture of hematopoietic progenitor cells ex vivo reveals that surface levels of activated, but not wild-type, α2 integrin receptors are rapidly down-regulated during cell growth on collagen but reach wild-type levels when cells are grown in the absence of collagen. Progenitor cells that express activated α2 integrins are normally distributed in the bone marrow in vivo and exhibit normal migration across a collagen-coated membrane ex vivo. This migration is accompanied by rapid down-regulation of activated surface integrins. These studies identify ligand-dependent removal of activated α2 receptors from the cell surface as a mechanism by which integrin function can be negatively regulated in hematopoietic cells during migration between the adhesive environment of the bone marrow and the nonadhesive environment of the circulating blood.
Introduction
Hematopoietic cells develop as adherent cells in contact with matrix proteins in the bone marrow until they are released as nonadherent cells into the circulating blood. Circulating platelets use collagen receptors to mediate platelet activation and firm adhesion in response to exposed vessel wall collagen as a primary hemostatic response.1,2 A critical platelet adhesive receptor for collagen that is expressed by mature megakaryocytes and platelets is integrin α2β1. Like other platelet integrins, α2β1 is held in a low-affinity conformation and unable to bind ligand until activating receptors on the platelet surface generate the “inside-out” intracellular signals that allosterically expose the ligand-binding α2 I domain.3,4 Platelet activation signals are generated either by soluble ligands such as thrombin or ADP or by the glycoprotein VI (GPVI) receptor, an immune receptor homolog that activates platelets directly in response to collagen.5-7 Affinity modulation of integrins is mediated by the interaction of talin with the integrin β subunit and subsequent induction of a conformational change in the α subunit from a low- to a high-affinity state. Once activated, integrin α2β1 mediates firm adhesion of circulating platelets to exposed vessel wall matrix and provides a foundation for platelet thrombus formation at sites of vessel injury.8-10
An outstanding question regarding platelet collagen responses is how platelets can be programmed to rapidly respond to minute amounts of collagen yet arise from megakaryocytes in the collagen-rich environment of the bone marrow. Megakaryocyte maturation is thought to require migration within the bone marrow from the osteoblastic stem cell niche to the bone marrow sinusoid where mature megakaryocytes and platelets are released into the blood.11-13 Thus restricting the expression of collagen receptors to mature precursors that have already entered the blood might prevent the generation of premature activation signals. Alternatively, negative regulation of collagen receptor function might be used during platelet generation within the bone marrow environment. Ex vivo studies of megakaryocyte maturation and proplatelet formation have suggested that the α2β1 collagen receptor might negatively regulate proplatelet formation,14,15 leading to the hypothesis that collagen receptors might be used by megakaryocytes to control the site of platelet formation and release. Whether and how collagen receptor activity is regulated during thrombopoiesis in vivo is not known.
To address the role and regulation of collagen receptors during thrombopoiesis, we have used retroviral vectors to express wild-type and constitutively active collagen receptors in hematopoietic precursors in mice. Our findings demonstrate that activated α2 integrins are dynamically down-regulated at the cell surface in a ligand-dependent fashion by hematopoietic progenitor cells ex vivo, and during platelet and leukocyte production in vivo. These findings reveal a mechanism for negative regulation of integrin function that may explain how circulating cells programmed to adhere to matrix proteins can arise from precursor cells in a matrix-rich environment.
Methods
Antibodies and reagents
Type-I fibrillar collagen (1 mg/mL) was purchased from Chronolog (Havertown, PA). Soluble fluorescein isothiocyanate (FITC)–labeled bovine type I collagen (1 mg/mL) and unlabeled bovine type I collagen were purchased from Chondrex (Redmond, WA). Rat anti–mouse integrin α2 antibody Dx5 was obtained from eBioscience (San Diego, CA). Anti-CD41 (integrin αIIb) monoclonal antibody and anti-β1 antibody 9EG76 were purchased from BD Biosciences (San Jose, CA). The anti-GPIbα antibody Xia.G5 was purchased from Emfret Analytics (Würzburg, Germany). Anti–human GPVI monoclonal antibody, HY101, was produced as previously described.7 Rat anti–mouse Flk-1 antibody was purchased from BD Biosciences (Franklin Lakes, NJ) and goat anti-GFP antibody was purchased from Abcam (Cambridge, MA). Cytokines and growth factors, MSCF, IL-3, IL-6, TPO, and SDF-1α were purchased from Peprotech (Rocky Hill, NJ). Alkaline phosphatase substrate 4-nitrophenyl phosphate was purchased from Sigma-Aldrich (St Louis, MO). Costar Transwell Permeable supports with 8.0-μm pores were purchased from Corning (Corning, NY)
Animals
α2-Deficient mice were generously provided by Dr Mary Zutter and Sam Santoro (Vanderbilt University). GPVI-FcRγ–deficient mice were purchased from Taconic Farms (Germantown, NY). α2;GPVI-FcRγ doubly deficient mice were generated by crossing α2-deficient mice to GPVI-FcRγ–deficient mice. Wild-type C57Bl/6 recipient mice were purchased from Charles River Laboratories (Wilmington, MA). All of the mice used for study were maintained in the animal facility of the University of Pennsylvania in accordance with National Institutes of Health guidelines and under Institutional Review Board–approved animal protocols.
Retroviral vector construction
The cDNA of murine integrin α2 was cloned by polymerase chain reaction (PCR) from C57BL/6 spleen total RNA. Mutations of integrin α2 in I-domain and membrane proximal region were generated using the Stratagene QuickChange mutagenesis kit (La Jolla, CA). Primers used were as follows: E318A forward: 5′TTCAATGTGGCCGACGCAGCGGCTCTTCTGGAG 3′, reverse: 5′CTCCAGAAGAGCCGCTGCGTCGGCCACATTGAA 3′; GFFKR del forward: 5′GCAGGTTTGTGGAAGCTCCAATATAAGAAAATGGG 3′, reverse: 5′ CCCATTTTCTTATATTGGAGCTTCCACAAACCTGC 3′. Wild-type and mutant α2 cDNAs were subcloned into the HFUW lentiviral vector (kind gift from Dr Eric Brown, Department of Cancer Biology, University of Pennsylvania School of Medicine) or murine stem cell virus, MigR1 retroviral vector as previously described.16 The lentivirusal and retrovirusal vectors were packed using HEK 293T cells.
Cell lines
RBL-2H3 cells were infected with HFUW lentiviruses encoding α2 integrins. Cells expressing wild or mutant α2 integrins were separated by cell sorting after being stained with Dx5 antibody, and the sorted cells were maintained in Dulbecco modified Eagle medium containing 10% fetal bovine serum and antibiotics. β1YA embryonic stem (ES) cells were generated as previously described6 and infected with HFUW lentiviruses encoding α2 integrins. Cells expressing wild or mutant α2 integrins were separated by cell sorting after being stained with Dx5 antibody, and the sorted cells were maintained on mouse embryonic fibroblast (MEF) feeder layer cells in ES cell media using a standard protocol.
Soluble collagen binding and static collagen adhesion assays
RBL cells or β1YA ES cells (105) expressing α2 integrins were harvested and resuspended in 100 μL Tyrode buffer containing 15 μg/mL FITC-labeled bovine type collagen, with or without the presence of 5 mM EDTA, and incubated for 10 minutes at 37°C. The reaction was stopped by adding 900 μL Tyrode buffer containing 20 μg/mL propidium iodine. FITC-soluble collagen binding in live cells was analyzed by fluorescence-activated cell sorting (FACS; FACSort; BD Biosciences, San Jose, CA). RBL cells (105) resuspended in Tyrode buffer with or without 5 mM EDTA were plated onto 96-well plates coated with 30 μg/mL type I fibrillar collagen and incubated at 37°C for 45 inutes. The plates were then rinsed 3 imes with PBS to remove nonadherent cells. The adherent cell number was determined by measuring intracellular alkaline phosphatase activity by adding 20 μg/mL of the substrate 4-nitrophenyl phosphate in 0.1% citrate buffer and measuring the OD405.
Fetal liver cell transplantation
Fetal liver cells were harvested from α2-deficient and a2/GPVI-FcRγ doubly deficient embryos at E14.5 to E18.5 and mononuclear cells purified with Lympholyte (Cedarlane Labs, Burlington, NC) gradient and cultured overnight in IMDM with a supplement of 10% fetal bovine serum, 100 ng/mL murine stem cell factor (MSCF), 20 ng/mL IL-3, and 10 ng/mL IL-6. The cells then were infected twice with α2 retroviruses at a multiplicity of infection (MOI) equal to 5 virions per cell. A total of 106 cells were transplanted by retro-orbital injection (250 μL per mouse) into recipient animals (8-10 weeks old) conditioned with a split lethal dose of 10 Gy irradiation. Mice were analyzed 8 to 9 weeks after the transplantation.
Blood collection and platelet count measurements
Blood (100 μL) was collected by retro-orbital bleeding using heparin as anticoagulant. Platelet-rich plasma (PRP) was obtained by diluting the whole blood 1:3 in Tyrode buffer and centrifuging at 100g for 4 minutes. The integrin α2 surface expressions in platelets were detected by PE-CD41 antibody and APC-Dx5 antibody using FACS analysis.
Bone marrow collection and integrin detection
Bone marrow cells from the reconstituted mice were flushed out of exposed femurs with Iscove modified Dulbecco medium containing 2% fetal bovine serum and 10 mM EDTA, rinsed, and resuspended in Iscove modified Dulbecco medium containing 2% fetal bovine serum and 5 mM EDTA. The integrin α2 surface expression in freshly harvested megakaryocytes was measured with PE-CD41 antibody and APC-Dx5 antibody using FACS analysis. The freshly harvested bone marrow cells were then cultured for 72 hours on plastic tissue culture plates that had been previously coated with 100 μg/mL fibrillar collagen or albumin (control) for 2 days in the presence of TPO 100 ng/mL and α2 expression detected using PE-CD41 and APC-Dx5 antibodies by FACS analysis.
Transwell migration studies
Bone marrow cells harvested from reconstituted mice were cultured with 100 ng/mL TPO for 7 days, washed once with Xvivo-20 (Invitrogen, Frederick, MD), and resuspended in Xvivo-20 at a density of 106 cells/mL. Cell suspension (200 μL) was applied to 8-μm pore transwell filters (Corning) coated with type I fibrillar collagen. Uncoated wells were used as controls. Xvivo-20 medium (600 μL) containing 200 ng/mL SDF-1α was added to the bottom chamber well. After incubation at 37°C for 24 hours, the cells that migrated through the transwell were counted and immunostained with anti-CD41 and anti-α2 antibodies and analyzed by flow cytometry. The chemotactic index is defined as the number of cells that passed through the transwell to the bottom chamber divided by the total input cell number.
Bone marrow staining
Femurs from reconstituted mice were collected, cut in half, fixed with 4% PFA for 24 hours, and decalcified with Tris-EDTA buffer for 72 hours. The decalcified bones were embedded in paraffin, sectioned longitudinally, and immunostained with antibodies recognizing Flk-1, VWF, and GFP. Detailed immunostaining protocols can be found at http://www.med.upenn.edu/mcrc/histology_core/. The bone marrow staining was observed using a Nikon Eclipse E600 fluorescence microscope (Nikon, Melville, NY) equipped with a 40×/0.75 objective lens. Images were taken using a Nikon DXM 1200 digital camera and Nikon ACT-1 software version 2.
Statistical analysis
P values were calculated using paired the Student t test where indicated.
Results
Expression of α2E318A and α2GFFKRΔ mutant integrins, but not wild-type α2 integrins, confers collagen binding in RBL-2H3 cells
Platelet α2β1 integrins are held in an “off” state and unable to bind ligand until activating signals confer a conformational change that opens the ligand-binding α2 I domain.3,17 To test regulation of integrin adhesion during thrombopoiesis, we wished to express α2 integrins that were uncoupled from affinity modulation and able to bind ligand constitutively. E318F α2 I domain mutant proteins have previously been shown to bind collagen with higher affinity than do wild-type I domain proteins.18 We have more recently observed in an independent mutagenesis study that the E318A mutation similarly results in a gain of function. Our analysis (S.K.D., T.P.S., and S.A.S., manuscript in preparation) suggests that the gain of function is a consequence of disrupting a salt bridge between E318 and R288 that acts as clasp to stabilize the closed or inactive conformation of the I domain.19 Therefore, E318A α2 integrins are held in their activated, high-affinity state, independent of inside-out signaling through the β subunit. Expression of α2E318A integrins provided a first approach to expressing a constitutively active collagen receptor. The α2 GFFKR juxtamembrane sequence has been postulated to interact with oppositely charged residues on the β1 juxtamembrane region within the cell to allosterically hold the integrin in an “off” state.20,21 Consistent with this model, mutations of the cognate region in the related αIIb and aL integrin subunits have been shown to confer increased ligand binding activity when expressed in cells22,23 and in vivo in mice.24 Thus in a second, structurally distinct strategy to study activated α2 integrins, we expressed α2GFFKRΔ, an α2 mutant in which the juxtamembrane GFFKR residues were deleted.
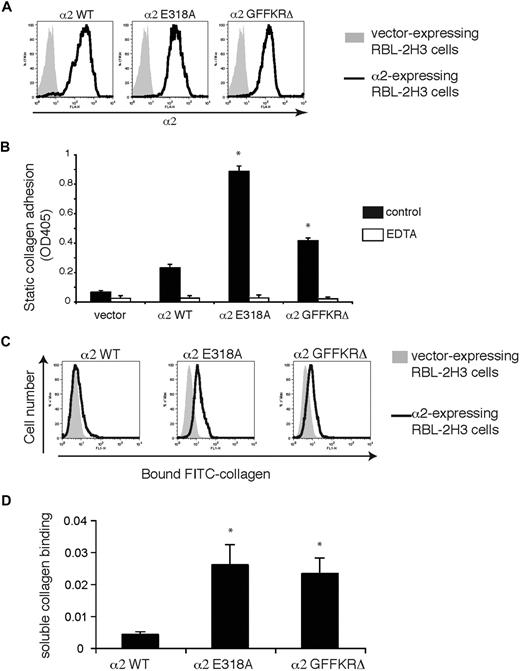
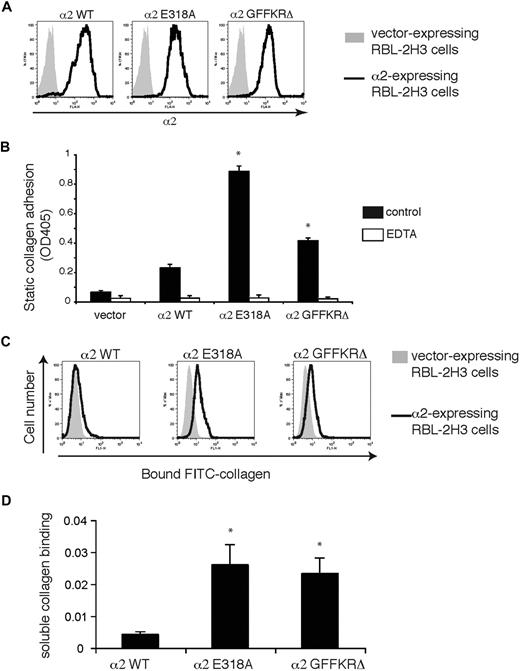
Wild-type α2, α2E318A, and α2GFFKRΔ mouse integrins were expressed in RBL-2H3 cells, a rat basophilic cell line that expresses β1 but not α2 integrins, to characterize the ligand binding of wild-type and mutant α2β1 integrins. Integrin subunits were stably expressed using lentiviral vectors, and cells were sorted to obtain populations with similar levels of receptor surface expression (Figure 1A). Cells expressing α2E318A and α2GFFKRΔ subunits exhibited increased adhesion to bound type I collagen (Figure 1B). A hallmark of activated integrins in platelets is their ability to bind soluble ligand, and we and others have previously shown that only activated platelets bind soluble FITC-collagen through the α2β1 integrin.5,6 To more rigorously test α2 receptor ligand binding, these cell populations were exposed to soluble FITC-collagen and bound collagen was detected using flow cytometry. Minimal soluble FITC-collagen was bound by wild-type α2-expressing RBL-2H3 cells, but significant soluble collagen binding was conferred by expression of both α2E318A and α2GFFKRΔ integrins (Figure 1C,D). Collagen binding was blocked by EDTA, a chelator that sequesters the cation essential for α2 I domain function4 and by Ha1/29, a monoclonal antibody that blocks the collagen binding site of the α2 I domain (Figure 1B and data not shown). These studies demonstrate that both the E318A α2 I domain mutant and the GFFKRΔ α2 juxtamembrane domain mutant receptors confer constitutive collagen binding when coupled to β1 integrin subunits in cells.
α2E318A and α2GFFKRΔ mutant integrins confer binding to immobilized and soluble collagen. (A) Surface expression of α2 integrins in RBL-2H3 cells infected with lentiviral vectors encoding wild-type (WT), E318A, and GFFKRΔ α2 integrin subunits. Cells were sorted to obtain populations with similar expression levels. (B) Static adhesion of RBL-2H3 cells expressing empty vector or the indicated α2 integrins to immobilized type I collagen in the presence and absence of 5 mM EDTA. N = 3; mean and SD are shown. *P < .05 compared with α2WT. (C) Activated α2 mutants confer soluble collagen binding. Binding of FITC-conjugated soluble collagen to RBL-2H3 cells expressing wild-type, E318A, or GFFKRΔ α2 subunits was measured using flow cytometry. (D) Quantitation of soluble collagen binding conferred by the E318A and GFFKRΔ α2 integrin mutants. N = 7; mean and SD are shown. *P < .05 compared with α2WT.
α2E318A and α2GFFKRΔ mutant integrins confer binding to immobilized and soluble collagen. (A) Surface expression of α2 integrins in RBL-2H3 cells infected with lentiviral vectors encoding wild-type (WT), E318A, and GFFKRΔ α2 integrin subunits. Cells were sorted to obtain populations with similar expression levels. (B) Static adhesion of RBL-2H3 cells expressing empty vector or the indicated α2 integrins to immobilized type I collagen in the presence and absence of 5 mM EDTA. N = 3; mean and SD are shown. *P < .05 compared with α2WT. (C) Activated α2 mutants confer soluble collagen binding. Binding of FITC-conjugated soluble collagen to RBL-2H3 cells expressing wild-type, E318A, or GFFKRΔ α2 subunits was measured using flow cytometry. (D) Quantitation of soluble collagen binding conferred by the E318A and GFFKRΔ α2 integrin mutants. N = 7; mean and SD are shown. *P < .05 compared with α2WT.
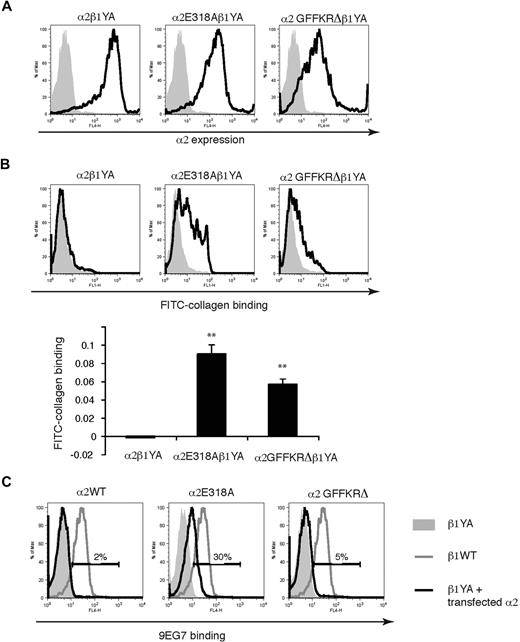
α2E318A and α2GFFKRΔ, but not wild-type α2 integrins, bind ligand when associated with activation-deficient β1YA integrin subunits
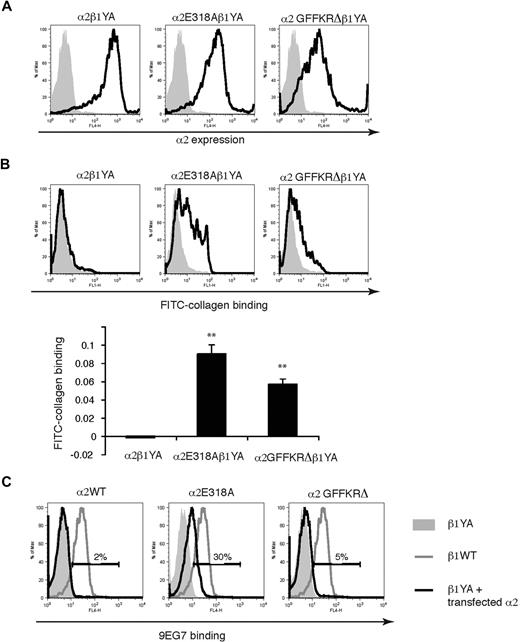
Integrin affinity state in platelets is positively regulated by the binding of talin to β subunit NPxY motifs.25-27 Previous studies of knockin mice that express β1 subunits in which the 2 intracellular tyrosine residues have been mutated to alanine (β1YA integrins) have demonstrated that α2β1YA receptors are unable to bind collagen even in activated platelets from β1YA homozygous knockin mice, a phenotype reproduced in embryonic stem (ES) cells.6 A more rigorous molecular test of ligand binding activity of these activated α2 subunits is therefore whether they can bind collagen when associated with the β1YA subunit. To further characterize α2E318A and α2GFFKRΔ integrins we used lentiviral vectors to express them in β1YA double knockin ES cells and tested collagen binding. In contrast to wild-type α2β1YA ES cells, α2E318Aβ1YA and, to a lesser extent, α2GFFKRΔβ1YA ES cells bound soluble FITC-collagen (Figure 2A,B). Previous studies of α2β1YA integrins have also demonstrated that these receptors fail to bind the affinity state–specific anti-β1 monoclonal antibody 9EG7 due to failure in β1 activation and subsequent exposure of an epitope by activated β1 receptors.6 To determine whether the α2E318A and α2GFFKRΔ can cause a conformational change in β1 independent of inside-out activation signals, we compared 9EG7 binding of wild-type α2β1YA ES cells, α2E318Aβ1YA ES cells, and α2GFFKRΔβ1YA ES cells. Expression of the α2E318A but not the α2GFFKRΔ mutant subunit partially rescued 9EG7 binding (Figure 2C), suggesting that the constitutively active α2 I domain can confer exposure of the 9EG7 binding motif in the neighboring β1 subunit. These findings are consistent with the prediction that the E318A mutation alters the conformation of the α2 extracellular domain independent of activation signaling from the β1 subunit, whereas the GFFKRΔ mutation does so through disruption of the α2-β1 interaction. These studies demonstrate that the α2E318A and α2GFFKRΔ mutant subunits confer ligand binding activity to α2β1 integrins through 2 structurally distinct mechanisms that do not rely on inside-out signals and β1-mediated allosteric changes.
α2E318A and α2GFFKRΔ integrin subunits rescue collagen binding when coupled to activation-deficient β1YA integrin subunits. (A) Lentiviral expression of wild-type and mutant α2 subunits in Itgβ1YA/YA embryonic stem cells in which both β1 intracellular tyrosines have been mutated to alanine. (B) The E318A and GFFKRΔ α2 mutants rescue soluble collagen binding of β1YA integrins. FITC-conjugated soluble collagen binding was measured using flow cytometry (above) and quantitated below. N = 3; mean and SD are shown. **P < .01 compared α2β1YA. (C) The activation state–specific monoclonal anti-β1 antibody 9EG7 binds wild-type β1 integrins (blue lines) but not β1YA integrins (gray shadow). Coupling of β1YA integrin subunits to α2E318A but not α2GFFKRΔ integrin subunits partially restores 9EG7 binding (red lines). Numbers indicate the percentage of 9EG7+ β1YA ES cells expressing wild-type or activated α2 integrins compared with untransfected β1YA ES cells.
α2E318A and α2GFFKRΔ integrin subunits rescue collagen binding when coupled to activation-deficient β1YA integrin subunits. (A) Lentiviral expression of wild-type and mutant α2 subunits in Itgβ1YA/YA embryonic stem cells in which both β1 intracellular tyrosines have been mutated to alanine. (B) The E318A and GFFKRΔ α2 mutants rescue soluble collagen binding of β1YA integrins. FITC-conjugated soluble collagen binding was measured using flow cytometry (above) and quantitated below. N = 3; mean and SD are shown. **P < .01 compared α2β1YA. (C) The activation state–specific monoclonal anti-β1 antibody 9EG7 binds wild-type β1 integrins (blue lines) but not β1YA integrins (gray shadow). Coupling of β1YA integrin subunits to α2E318A but not α2GFFKRΔ integrin subunits partially restores 9EG7 binding (red lines). Numbers indicate the percentage of 9EG7+ β1YA ES cells expressing wild-type or activated α2 integrins compared with untransfected β1YA ES cells.
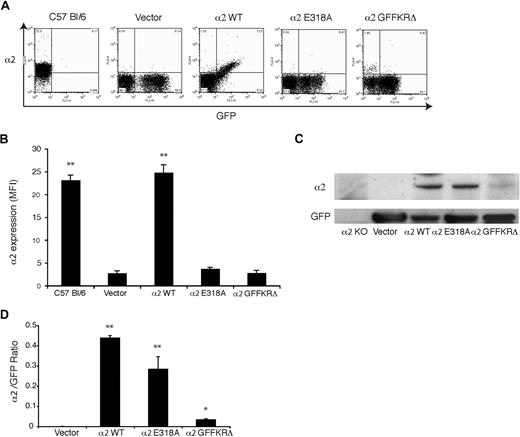
Surface expression of activated α2 integrins is lost during thrombopoiesis in vivo
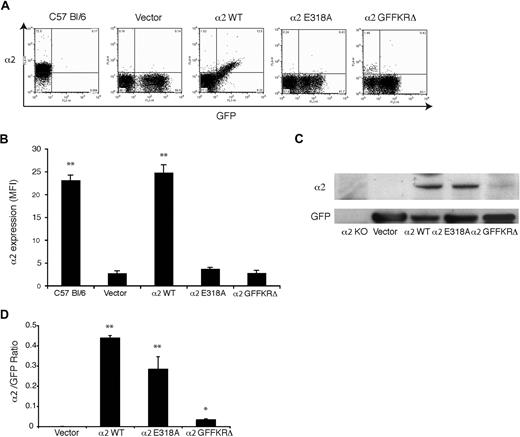
To test regulation of α2 integrins during thrombopoiesis, we used retroviral vectors to express wild-type α2, α2E318A, and α2GFFKRΔ integrins in α2-deficient and α2;GPVI doubly deficient hematopoietic progenitors. In this system, deficient fetal liver cells are exposed to retroviral vectors that drive coexpression of α2 subunits and GFP, and these cells are then used to reconstitute lethally irradiated host animals.16 After recovery, platelets derived from transfected precursors are identified by expression of GFP. In animals that were reconstituted with cells expressing wild-type mouse α2, GFP+ platelets expressed surface α2 integrins at a level indistinguishable from that of wild-type mouse platelets (Figure 3A). In contrast, GFP+ platelets in the circulating blood of animals reconstituted with vectors expressing the E318A and GFFKRΔ α2 mutants had no detectable surface expression of α2 integrins (Figure 3A,B). Similar results were obtained using α2-deficient and α2;GPVI doubly deficient hematopoietic donor cells (data not shown). To determine whether activated α2 receptors were completely absent or merely not expressed on the platelet surface, we performed immunoblots of total platelet lysate to detect cellular α2 and GFP proteins. Although these animals had various degrees of chimerism (ie, various fractions of platelets derived from stem cells expressing the retroviral transgene) there were no host-derived α2+;GFP− platelets and GFP levels were similar between groups (Figure 3A); thus the α2/GFP protein ratio can be used to measure α2 protein in GFP+ cells. Near normal levels of α2E318A integrins were detected in platelet lysate despite their absence on the platelet surface, but levels of GFFKRΔ integrin subunits were significantly reduced (Figure 3C,D). Previous studies of α2 integrin truncation mutants lacking the GFFKR domain have demonstrated that such receptors are unstable when heterologously expressed,20 suggesting that the observed lack of protein in α2GFFKRΔ platelets is the result of receptor instability and degradation. These findings demonstrate that surface expression of activated, but not wild-type, α2 integrins is lost during the generation of platelets in vivo.
Surface expression of activated but not wild-type α2 integrins is lost during platelet production in vivo. (A) Expression of surface α2 integrins and GFP in platelets from wild-type C57 Bl/6 mice and lethally irradiated mice reconstituted with hematopoietic stem cells lacking both α2 and GPVI that were transduced with GFP-expressing vector (vector) or vectors expressing the indicated α2 integrins and GFP. (B) Quantitation of platelet surface α2 integrin levels. Mean fluorescence intensity (MFI) was measured in the indicated platelets after staining with anti-α2 antibody. N = 3-5; mean and SD are shown. **P < .01 compared with vector control. (C) Immunoblot analysis of α2 integrins and GFP in total platelet lysate derived from the chimeric animals shown in panels A and B and in α2-deficient animals. (D) The ratio of platelet α2 integrin/GFP protein expression for the indicated animals is shown. N = 3; mean and SD are shown. *P < .05, **P < .01 compared with vector control.
Surface expression of activated but not wild-type α2 integrins is lost during platelet production in vivo. (A) Expression of surface α2 integrins and GFP in platelets from wild-type C57 Bl/6 mice and lethally irradiated mice reconstituted with hematopoietic stem cells lacking both α2 and GPVI that were transduced with GFP-expressing vector (vector) or vectors expressing the indicated α2 integrins and GFP. (B) Quantitation of platelet surface α2 integrin levels. Mean fluorescence intensity (MFI) was measured in the indicated platelets after staining with anti-α2 antibody. N = 3-5; mean and SD are shown. **P < .01 compared with vector control. (C) Immunoblot analysis of α2 integrins and GFP in total platelet lysate derived from the chimeric animals shown in panels A and B and in α2-deficient animals. (D) The ratio of platelet α2 integrin/GFP protein expression for the indicated animals is shown. N = 3; mean and SD are shown. *P < .05, **P < .01 compared with vector control.
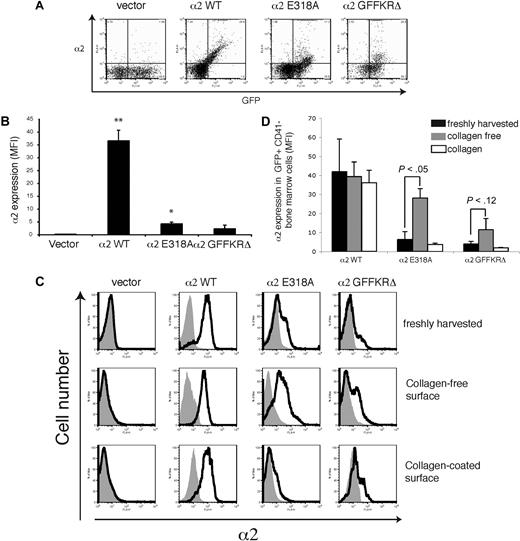
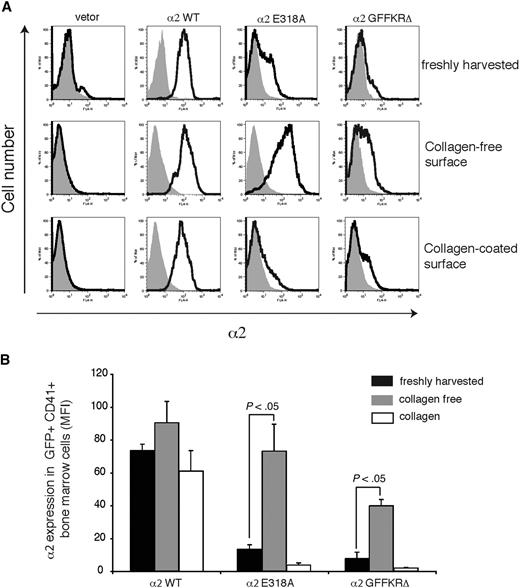
Surface expression of activated α2 integrins in platelet precursors is dynamically regulated by exposure to collagen
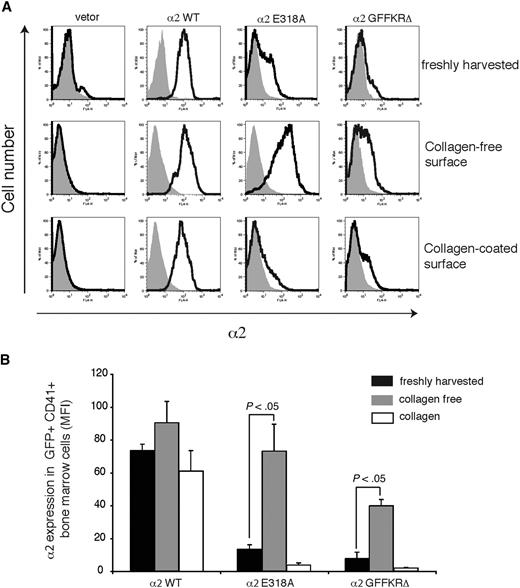
The failure to detect expression of 2 structurally distinct activated α2 integrins on the surface of circulating platelets could reflect either the inability of these integrin subunits to be stably expressed on the platelet surface in vivo or the down-regulation of activated surface receptors by megakaryocytes during platelet generation in a collagen-rich environment. To distinguish between these 2 possibilities, we examined the expression of wild-type and activated α2 subunits on the surface of CD41+ platelet precursor cells freshly harvested from the bone marrow of reconstituted animals. GFP+ platelet precursors from animals reconstituted with cells exposed to wild-type α2-expressing vector were uniformly positive for surface α2 expression, whereas those from animals reconstituted with cells exposed to empty vector lacked α2 integrin expression (Figure 4A,B). In contrast, GFP+ platelet precursors from animals reconstituted with cells exposed to vectors expressing either the E318A or the GFFKRΔ activated α2 mutants exhibited variable levels of cell surface expression, with cells expressing particularly low levels of α2GFFKRΔ integrins (Figure 4A,B). These findings suggested that activated α2 receptors can be expressed on the surface of platelet precursors in the bone marrow, but that some cells have low levels due to either negative selective pressure during thrombopoiesis or to dynamic down-regulation of receptor cell surface expression. To determine whether the level of surface expression of activated α2 integrins is dynamically regulated, freshly harvested CD41+ progenitors were cultured ex vivo in the absence or presence of type I collagen fibers for 72 hours. After culture in a collagen-free environment, cell surface expression of activated α2E318A integrins by platelet progenitors rose to a level similar to that observed for wild-type α2 integrins, whereas surface α2GFFKRΔ integrins also rose significantly, but to a lower level (Figure 4A,B). Conversely, after culture on collagen-coated plates, cell surface expression of activated α2 integrins was markedly reduced, approaching the null state observed in circulating platelets in vivo (Figure 4A,B). These findings demonstrate that the surface expression of activated α2 integrins is negatively regulated in response to collagen ligand.
Dynamic down-regulation of surface expression of activated α2 integrins in platelet precursors in response to collagen. (A) Expression of surface α2 in GFP+ CD41+ cells harvested from mice reconstituted with hematopoietic stem cells lacking both α2 and GPVI exposed to empty vector (vector) or vector expressing wild-type α2 integrins (α2WT), α2E318A integrins, and α2GFFKRΔ integrins are shown. The gray shadowed area represents α2 levels in GFP− nontransfected cells. Top panels show expression immediately after harvest from the bone marrow, middle panels show expression after 72 hours of ex vivo culture in the absence of collagen, and bottom panels show expression after 72 hours of ex vivo culture on collagen-coated plates. (B) Quantitation of α2 surface expression in the GFP+ CD41+ cells described in panel A. N = 5; mean and SD are shown.
Dynamic down-regulation of surface expression of activated α2 integrins in platelet precursors in response to collagen. (A) Expression of surface α2 in GFP+ CD41+ cells harvested from mice reconstituted with hematopoietic stem cells lacking both α2 and GPVI exposed to empty vector (vector) or vector expressing wild-type α2 integrins (α2WT), α2E318A integrins, and α2GFFKRΔ integrins are shown. The gray shadowed area represents α2 levels in GFP− nontransfected cells. Top panels show expression immediately after harvest from the bone marrow, middle panels show expression after 72 hours of ex vivo culture in the absence of collagen, and bottom panels show expression after 72 hours of ex vivo culture on collagen-coated plates. (B) Quantitation of α2 surface expression in the GFP+ CD41+ cells described in panel A. N = 5; mean and SD are shown.
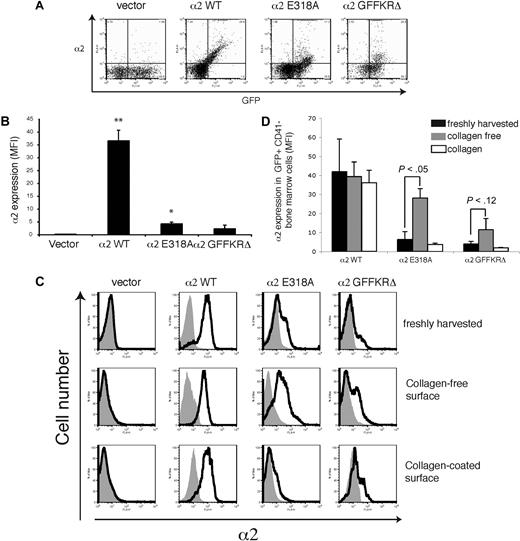
Surface expression of activated α2 integrins in leukocytes and nonplatelet precursors is also down-regulated by exposure to collagen
The retroviral vectors used to express wild-type and activated α2 integrins drive constitutive expression in hematopoietic stem cells and all their progeny. To determine whether the loss of surface activated α2 integrins is unique to platelets and megakaryocytes, we assayed surface levels of α2 on circulating leukocytes harvested from mice expressing wild-type and activated α2 integrins. Levels of surface α2E318A and α2GFFKRΔ integrins on circulating leukocytes were markedly reduced compared with wild-type α2 integrins, although some cells with higher levels were observed (Figure 5A,B). Studies of surface α2 levels in CD41− hematopoietic progenitors harvested from the bone marrow demonstrated dynamic, collagen-dependent down-regulation of receptors identical to that observed with CD41+ progenitors (Figure 5C,D). These studies reveal that surface levels of activated α2 integrins are reduced on all hematopoietic cells in vivo and dynamically down-regulated by collagen in all hematopoietic progenitors ex vivo. The fact that virtually all platelets derived from activated α2-expressing progenitors are devoid of surface α2 integrins, whereas some leukocytes express detectable levels of these receptors, is consistent with the fact that leukocytes are nucleated and able to synthesize new receptors in the collagen-free environment of circulating blood.
Surface expression of activated α2 integrins is down-regulated in leukocytes during hematopoiesis in vivo. (A) Expression of surface α2 integrins and GFP in white blood cells from lethally irradiated mice reconstituted with hematopoietic stem cells lacking both α2 and GPVI that were exposed to empty vector (vector) or vectors expressing the indicated α2 integrins. (B) Quantitation of leukocyte surface α2 integrin levels. Mean fluorescence intensity (MFI) was measured in the indicated cells stained with anti-α2 antibody. N = 3-5; mean and SD are shown. *P < .05, **P < .01 compared with vector control. (C) Expression of surface α2 in GFP+ CD41− bone marrow cells harvested from lethally irradiated mice reconstituted with α2-deficient hematopoietic cells exposed to empty vector (vector) or vector expressing wild-type α2 integrins (α2WT), α2E318A integrins, and α2GFFKRΔ integrins is shown. Top panels show expression immediately after harvest from the bone marrow, middle panels show expression after 72 hours of ex vivo culture in the absence of collagen, and bottom panels show expression after 72 hours of ex vivo culture on collagen-coated plates. (D) Quantitation of α2 surface expression in the GFP+ CD41− bone marrow cells described in panel A. N = 5; mean and SD are shown.
Surface expression of activated α2 integrins is down-regulated in leukocytes during hematopoiesis in vivo. (A) Expression of surface α2 integrins and GFP in white blood cells from lethally irradiated mice reconstituted with hematopoietic stem cells lacking both α2 and GPVI that were exposed to empty vector (vector) or vectors expressing the indicated α2 integrins. (B) Quantitation of leukocyte surface α2 integrin levels. Mean fluorescence intensity (MFI) was measured in the indicated cells stained with anti-α2 antibody. N = 3-5; mean and SD are shown. *P < .05, **P < .01 compared with vector control. (C) Expression of surface α2 in GFP+ CD41− bone marrow cells harvested from lethally irradiated mice reconstituted with α2-deficient hematopoietic cells exposed to empty vector (vector) or vector expressing wild-type α2 integrins (α2WT), α2E318A integrins, and α2GFFKRΔ integrins is shown. Top panels show expression immediately after harvest from the bone marrow, middle panels show expression after 72 hours of ex vivo culture in the absence of collagen, and bottom panels show expression after 72 hours of ex vivo culture on collagen-coated plates. (D) Quantitation of α2 surface expression in the GFP+ CD41− bone marrow cells described in panel A. N = 5; mean and SD are shown.
CD41+ progenitors expressing activated integrins demonstrate normal bone marrow localization in vivo and are able to migrate through collagen
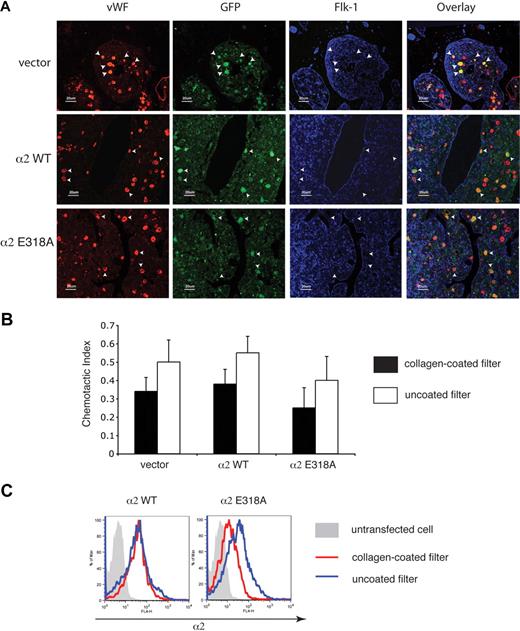
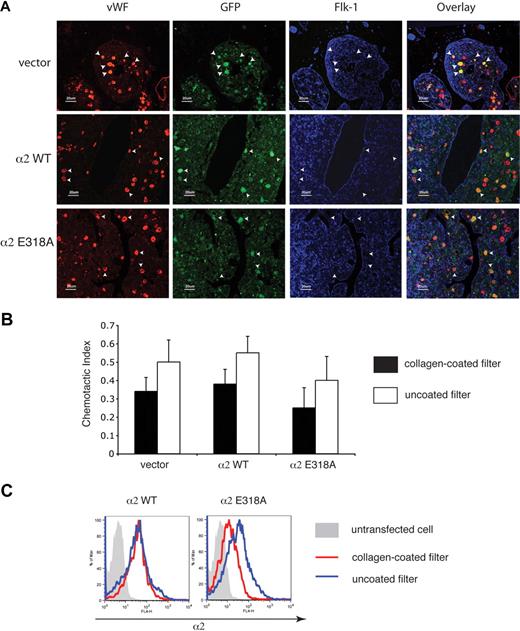
The facts that platelets derived from progenitor cells expressing activated α2 integrins lack such receptors on the cell surface and that progenitor cells expressing activated α2 integrins dynamically down-regulate their surface expression in response to collagen suggested that expression of activated α2 integrins might retard or even block the migration of megakaryocytes through the bone marrow during thrombopoiesis. To test the effect of activated α2 expression on megakaryocyte maturation and migration in the bone marrow, we used immunostaining to identify transduced megakaryocytes in the bone marrow in vivo. Transduced megakaryocytes, identified as large cells expressing both VWF and GFP, appeared in similar numbers and locations in the bone marrow of mice expressing wild-type and activated α2 integrins (Figure 6A). Significantly, transduced megakaryocytes expressing activated α2 integrins were neither excluded nor enriched in the regions around the bone marrow sinusoid (Figure 6A), suggesting that the migration of progenitors expressing activated α2 integrins during thrombopoiesis was unimpaired.
Expression of activated α2 integrins does not alter the location of megakaryocytes in the bone marrow in vivo or migration through collagen ex vivo. (A) Immunostaining for VWF, GFP, and Flk-1 in the bone marrow of mice expressing wild-type and activated α2 integrins. Femurs from mice expressing wild-type and activated α2 integrins were sectioned longitudinally and stained with anti-VWF antibody to identify megakaryocytes, with anti-GFP antibody to identify cells derived from hematopoietic stem cells that were transfected with retroviruses, and with anti–Flk-1 antibody to identify sinusoidal endothelial cells. White arrowheads indicate the location of 5 representative megakaryocytes that express the indicated α2 integrins in each panel. (B) Migration of bone marrow cells expressing wild-type or activated α2 integrins across a collagen-coated filter ex vivo. Freshly harvested cells were cultured ex vivo in the presence of thrombopoietin to induce megakaryocyte maturation and allowed to migrate across transwell filters coated or uncoated with collagen for 24 hours in response to an SDF-1 gradient. The chemotactic index is defined as the number of cells that migrated across the transwell filter divided by the total number of cell input. The error bars indicate SD of the chemotactic index from 3 independent experiments. No statistically significant difference was observed between groups (P > .05). (C) Migration across collagen is associated with loss of surface expression of activated but not wild-type α2 integrins. Surface expressions of α2 integrins on CD41+ cells that migrated through a collagen-coated filter are shown in red and those that migrated across a non–collagen-coated filter are shown in blue. The gray shadowed area represents the α2 levels of nontransfected cells.
Expression of activated α2 integrins does not alter the location of megakaryocytes in the bone marrow in vivo or migration through collagen ex vivo. (A) Immunostaining for VWF, GFP, and Flk-1 in the bone marrow of mice expressing wild-type and activated α2 integrins. Femurs from mice expressing wild-type and activated α2 integrins were sectioned longitudinally and stained with anti-VWF antibody to identify megakaryocytes, with anti-GFP antibody to identify cells derived from hematopoietic stem cells that were transfected with retroviruses, and with anti–Flk-1 antibody to identify sinusoidal endothelial cells. White arrowheads indicate the location of 5 representative megakaryocytes that express the indicated α2 integrins in each panel. (B) Migration of bone marrow cells expressing wild-type or activated α2 integrins across a collagen-coated filter ex vivo. Freshly harvested cells were cultured ex vivo in the presence of thrombopoietin to induce megakaryocyte maturation and allowed to migrate across transwell filters coated or uncoated with collagen for 24 hours in response to an SDF-1 gradient. The chemotactic index is defined as the number of cells that migrated across the transwell filter divided by the total number of cell input. The error bars indicate SD of the chemotactic index from 3 independent experiments. No statistically significant difference was observed between groups (P > .05). (C) Migration across collagen is associated with loss of surface expression of activated but not wild-type α2 integrins. Surface expressions of α2 integrins on CD41+ cells that migrated through a collagen-coated filter are shown in red and those that migrated across a non–collagen-coated filter are shown in blue. The gray shadowed area represents the α2 levels of nontransfected cells.
To directly test the ability of CD41+ progenitors expressing activated α2 integrins to migrate across a collagen-coated surface, we next assayed migration of these cells in a Boyden chamber assay ex vivo. After 24 hours, a similar number of CD41+ cells expressing activated α2 integrins and CD41+ cells expressing wild-type or no α2 integrins were observed to migrate across the collagen-coated filter (Figure 6B). However, in contrast to wild-type expressing CD41+ cells, α2E318A-expressing CD41+ cells exhibited reduced surface levels of α2 integrins after migration across collagen (Figure 6C). These findings suggest that expression of activated integrins does not block the migration of CD41+ progenitor cells across collagen in the bone marrow environment in vivo or in an experimental setting ex vivo, perhaps because activated α2 receptors are rapidly removed from the cell surface during migration across collagen.
Regulation of collagen receptor function during thrombopoiesis does not require restricting receptor expression to mature megakaryocytes
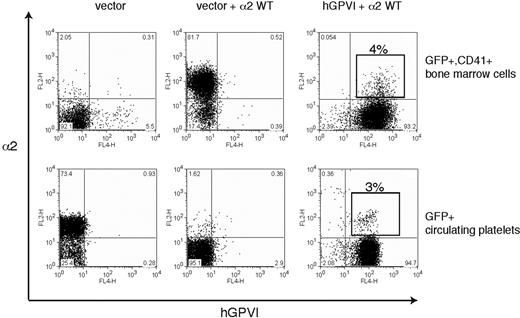
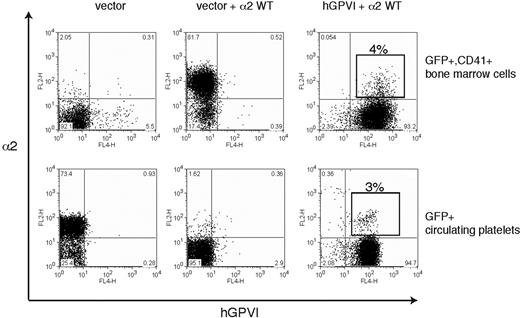
Ex vivo studies of megakaryocyte maturation have suggested that the expression of both GPVI and α2 is restricted to mature megakaryocytes.28-30 Thus a second mechanism by which collagen receptor function might be controlled in platelet precursors is by restricting receptor expression to mature megakaryocytes that have already migrated to the bone marrow sinusoid and are exiting the collagen-rich bone marrow environment. To determine whether restricting the expression of platelet collagen receptors to late-stage megakaryocytes is required for successful thrombopoiesis, we used retroviral vectors to constitutively coexpress GPVI and α2 during thrombopoiesis. To follow GPVI expression, we used human GPVI, a receptor that fully rescues collagen signaling in GPVI-deficient mouse platelets and confers collagen signals in the rat RBL-2H3 cell line (7 and data not shown). Analysis of GFP+ bone marrow cells harvested from reconstituted animals revealed surface coexpression of α2 and hGPVI in CD41+ cells (Figure 7 top), although competition between retroviruses resulted in a majority of cells that were transduced by only the GPVI-expressing vector (ie, GPVI+;α2− cells). Further analysis of GPVI+;α2+;CD41+ platelet precursors confirmed the expression of both collagen receptors on the surface of immature CD41+ megakaryocytes that lack GPIbα (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Analysis of circulating platelets revealed an identical fraction of GPVI+;α2+ platelets as that observed for CD41+ precursors in the bone marrow (4% vs 3%; Figure 7), indicating that constitutive coexpression of collagen receptors does not impair thrombopoiesis in vivo. These studies demonstrate that restricting the expression of collagen receptors to mature megakaryocytes is not necessary for normal thrombopoiesis.
Constitutive coexpression of wild-type GPVI and α2 collagen receptors does not impair platelet production. The expression of α2 integrins and human GPVI on CD41+ platelet precursors (top) from irradiated host animals reconstituted with α2-deficient bone marrow cells that were exposed to empty vector (left), empty vector + α2-expressing vector (middle), and α2-expressing vector + hGPVI-expressing vector (right) is shown. The boxed population indicates the population of CD41+ cells that express both α2 and hGPVI surface receptors. Expression of α2 and GPVI in the circulating blood platelets (bottom) of the animals shown in top panel is shown. Note that the fraction of α2+;GPVI+ double platelets is similar to that of double-positive CD41+ precursors in the bone marrow.
Constitutive coexpression of wild-type GPVI and α2 collagen receptors does not impair platelet production. The expression of α2 integrins and human GPVI on CD41+ platelet precursors (top) from irradiated host animals reconstituted with α2-deficient bone marrow cells that were exposed to empty vector (left), empty vector + α2-expressing vector (middle), and α2-expressing vector + hGPVI-expressing vector (right) is shown. The boxed population indicates the population of CD41+ cells that express both α2 and hGPVI surface receptors. Expression of α2 and GPVI in the circulating blood platelets (bottom) of the animals shown in top panel is shown. Note that the fraction of α2+;GPVI+ double platelets is similar to that of double-positive CD41+ precursors in the bone marrow.
Discussion
Hematopoietic cells develop in the bone marrow, an adhesive environment rich in matrix proteins, including collagen. Hematopoiesis requires the orchestrated movement of cells through the bone marrow in response to chemotactic and maturation signals before their release into the bloodstream, suggesting an important role for adhesive receptor function and regulation in this process. Our findings identify negative regulation of activated integrins through down-regulation of surface expression as a novel mechanism by which hematopoietic progenitors may control adhesive function in matrix-rich environments such as the bone marrow.
The primary high-affinity adhesive receptor for collagen expressed by mature megakaryocytes and platelets is the α2β1 integrin. α2β1 ligand binding is activated by the signals of a second collagen receptor, the immune receptor homologue GPVI.5,6 Because platelets are anuclear and the majority of platelet proteins are synthesized by megakaryocytes, these 2 collagen receptors must be expressed by megakaryocytes during maturation in the collagen-rich bone marrow. Two possible mechanisms may explain how megakaryocytes can express GPVI and α2β1 while developing amid abundant collagen in the bone marrow. Because recent studies have confirmed that platelets are formed exclusively in the blood,12,13 one way in which megakaryocytes might prevent the premature activation and function of platelet collagen receptors is by not expressing them on the cell surface until the cell has crossed into the collagen-free blood environment. Alternatively, megakaryocytes may use more active mechanisms to negatively regulate the function and/or expression of collagen receptors while maturing in the collagen-rich bone marrow environment.
To test a timed expression mechanism, we have constitutively expressed wild-type GPVI and α2 receptors in hematopoietic cells using retroviral vectors. We find that forced coexpression of GPVI and α2 in immature megakaryocytes is well tolerated and does not appear to confer a defect or selective disadvantage during thrombopoiesis in vivo. Thus tight coupling of the timing of collagen receptor expression to platelet release in the bloodstream is not required, supporting the existence of an active negative regulatory mechanism. To test for such a negative regulatory mechanism, we used a gain-of-function approach in which we expressed 2 distinct α2 integrin mutant receptors predicted to confer constitutive ligand binding in hematopoietic cells. We found that the E318A mutation in the collagen-binding I domain of the α2 integrin subunit confers strong, constitutive ligand binding even when coupled to β1YA integrins that have been shown to confer a complete loss of function to wild-type integrin alpha subunits,6,31 and that expression of α2E318A receptors is similar to that of wild-type receptors both in vivo and in vitro. In contrast, the α2 GFFKRΔ mutation conferred less robust ligand binding and exhibited lower expression levels in numerous cell types, results consistent with a more indirect gain-of-function mechanism20,21 and with previous studies demonstrating that this region may be required for stable receptor expression.20 Despite these limitations, the dynamic, ligand-dependent loss of activated α2 receptors from the cell surface observed with α2E318A mutant receptors was also observed with α2GFFKRΔ receptors, suggesting that loss of receptor surface expression is a response to increased adhesive function and not an unexpected consequence of the E318A mutation.
An important mechanistic question is how ligand-induced down-regulation of receptor expression is mediated in vivo in bone marrow progenitor cells. It has been shown that integrin receptors can be internalized after binding either endogenous ligands (eg, αIIbβ3 binding of fibrinogen32 ) or exogenous ligands (eg, bacteria33,34 ), and the α2β1 receptor on platelets has also been shown to internalize collagen fragments ex vivo.21 However, a role for integrin receptor internalization in vivo as a mechanism for dynamically regulating the adhesion of hematopoietic cells that need to migrate extensively has not been previously explored. Hematopoietic cells are highly mobile and pass freely between the bone marrow, blood, tissues, and lymph. Naive T cells must migrate constantly between the blood, lymph nodes, and lymph, whereas macrophages and dendritic cells move freely through the blood and tissues as part of immune surveillance. The importance of up-regulating integrin adhesion during hematopoietic cell movement in response to signals such as chemokines is well established,35-38 but whether and how hematopoietic cells subsequently down-regulate integrin adhesion and/or expression to remain mobile and perform this function repetitively is not established. The finding that activated α2 integrins on hematopoietic cells are rapidly internalized after exposure to collagen suggests that other integrins may be similarly regulated in vivo. Future studies will determine whether ligand-induced integrin internalization plays a broader role in hematopoietic cell migration as well as in thrombopoiesis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Eric Sebzda for help with experimental design and Joel Bennett, Skip Brass, Charles Abrams, and other members of the Kahn laboratory for their intellectual contributions.
This work was completed with support from the Department of Medicine at the University of Pennsylvania.
Authorship
Contribution: Z.Z. and A.A.S. designed and performed the experiments shown and wrote the paper; L.C. and P.M. helped perform the experiments; S.K.D., T.P.S., and S.A.S. characterized the E318A mutant protein; and M.L.K. designed the experiments performed and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark L. Kahn, Department of Medicine and Cardiovascular Institute, University of Pennsylvania, 952 BRB II/III, Curie Blvd, Philadelphia, PA 19104; e-mail: markkahn@mail.med.upenn.edu.