Abstract
We show that Indian Hedgehog (Ihh) regulates T-cell development and homeostasis in both fetal and adult thymus, controlling thymocyte number. Fetal Ihh−/− thymi had reduced differentiation to double-positive (DP) cell and reduced cell numbers compared with wild-type littermates. Surprisingly, fetal Ihh+/− thymi had increased thymocyte numbers and proportion of DP cells relative to wild type, indicating that Ihh also negatively regulates thymocyte development. In vitro treatment of thymus explants with exogenous recombinant Hedgehog protein promoted thymocyte development in Ihh−/− thymi but inhibited thymocyte development in Ihh+/−, confirming both positive and negative regulatory functions of Ihh. Analysis of Rag−/−Ihh+/− thymi showed that Ihh promotes T-cell development before pre–T-cell receptor (pre-TCR) signaling, but negatively regulates T-cell development only after pre-TCR signaling has taken place. We show that Ihh is most highly expressed by the DP population and that Ihh produced by DP cells feeds back to negatively regulate the differentiation and proliferation of their double-negative progenitors. Thus, differentiation from double-negative to DP cell, and hence the size of the DP population, is dependent on the concentration of Ihh in the thymus. Analysis of Ihh conditional knockout and heterozygote adult mice showed that Ihh also influences thymocyte number in the adult.
Introduction
Here we show that the intercellular signaling molecule, Indian Hedgehog (Ihh), regulates T-cell development, thereby restricting thymocyte number
Thymus size is tightly controlled by processes intrinsic to the thymus, about which little is known. The control of thymocyte number has been assumed to rely on competition between thymocyte precursors for limiting concentrations of mitogenic or survival factors, that by positively regulating the fate of the progenitor cell population control the number of thymocytes produced. Many factors have been described that promote the expansion of thymocyte progenitors and promote T-cell development, including cytokines,1 Notch signaling,2,3 the Wnt protein family,4 and Sonic Hh (Shh).5 Little, however, is understood about mechanisms that provide feedback, or a counting system, negatively regulating the upper limits of thymocyte differentiation and number. Here we show that Ihh provides such a counting system, negatively regulating the rate of differentiation from CD4−CD8− double-negative (DN) to CD4+CD8+ double-positive (DP) cell, and hence T-cell production and thymus size.
The Hh protein family (Shh, Ihh, and Desert Hh [Dhh]) signals for development, patterning, and organogenesis of many tissues during mammalian embryogenesis6,7 and is also involved in homeostasis and renewal of adult tissues, including blood and thymus.8-11 They can act as classic morphogens, giving concentration-dependent signals for position and patterning, and can regulate cell survival and proliferation.6,7 The 3 Hh proteins have distinct temporal and tissue-specific expression patterns and functions.12,13 Although Shh and Ihh are each essential during embryogenesis and have some overlapping functions,14 Shh is more pleiotropic and nonredundant in its actions, whereas Ihh has specialized functions in bone, cartilage, and gut.13-15 The Hh proteins share a common signaling pathway. They bind to the receptor Patched (Ptc), which releases the signal transduction protein Smoothened (Smo) to transmit a signal into the cell. In the absence of Hh, Ptc inhibits the activity of Smo. The downstream components of the signaling pathway are the glioblastoma-associated protein (Gli) family of transcription factors: Gli1, Gli2, and Gli3.7,16
During αβ T-cell development, CD4−CD8− DN cells give rise to the CD4+CD8+ DP population, which differentiate to mature CD8+CD4− or CD8−CD4+ single-positive (SP) cells. The DN population can be further subdivided by cell-surface expression of CD25 and CD44. CD44+CD25− (DN1) cells differentiate to become CD44+CD25+ (DN2) cells, which then differentiate to become CD44−CD25+ (DN3). The DN3 population gives rise to the CD44−CD25− (DN4) subset, which undergo a phase of rapid proliferation before differentiation into the DP population, in general via a cycling immature CD8+ intermediate single-positive (ISP) cell. T-cell lineage specification and T-cell receptor-β (TCR-β) chain rearrangements occur in the CD25+ (DN2 and DN3) population. Pre-TCR signaling is necessary for differentiation to DP cell,17 but other largely unidentified signals dependent on normal thymus architecture and cellular composition are also required.18
Shh, Ihh, and components of the Hh signaling pathway are expressed in the mouse thymus.10,19-23 In vitro studies first demonstrated that Hh signaling influences thymocyte development,10,24 and although they did not determine which Hh protein (Shh or Ihh) was physiologically significant, suggested that Hh signaling was predominantly a negative regulator of T-cell development. In contrast, subsequent ex vivo analysis of Shh−/− thymi revealed multiple positive-regulatory functions for Shh during fetal T-cell development. Absence of Shh caused reductions in thymocyte number, DN cell proliferation, differentiation from DN1 to DN2 cell, survival of DN4 cells, production of DP cells,5 and the ratio of mature CD8/CD4 SP cells.22,25,26 Conditional deletion of Smo from T-lineage cells has also shown that the Hh pathway provides essential positive signals for homeostasis of the earliest DN subsets and for differentiation from DN1 to DN2 but did not reveal any influence of Hh signaling on T-cell development after the DN2 stage.21
Here we take a genetic approach to assess the function of Ihh during thymocyte development, thereby reconciling conflicting data from earlier experimental systems.5,10,21,23,24 We demonstrate, by analysis of null and conditional-null mutants, that Ihh, produced by thymocytes themselves, regulates T-cell development and homeostasis in fetal and adult thymus. We show that Ihh and Shh have distinct but overlapping functions in the thymus, and that whereas Shh, secreted by the epithelium, is dominant in positively signaling for proliferation and differentiation of early DN progenitors, Ihh, produced by thymocytes, functions to control thymocyte numbers by negatively regulating the transition from icTCR-β+ DN3 to DP, providing concentration-dependent feedback on the production of DP cells.
Methods
Mice
C57BL/6 mice (B&K Universal, Hull, United Kingdom), Shh+/−12 and Ihh+/−13 mice, gifts from Philip Beachy (The Johns Hopkins University School of Medicine, Baltimore, MD) and Andrew McMahon (Harvard University, Cambridge, MA), respectively, were backcrossed onto C57BL/6 mice for more than 11 generations. Cre transgenic mice,27 a gift from Dimitris Kioussis (The National Institute for Medical Research, London, United Kingdom), and Floxed Ihh mice,15 were bred and maintained at Imperial or University College London, under United Kingdom Home Office regulations. Timed mates were as described.5 All animal experiments were approved by the institutional review board of University College London (London, United Kingdom).
Fetal thymus organ cultures
Fetal thymus organ cultures (FTOCs) were as described.5 Where stated, FTOCs were treated with recombinant mouse Sonic Hedgehog N-Terminus protein (catalog number 464-SH; R&D Systems, Minneapolis, MN) or 1 μg/mL azide-free anti-CD3 (BD Biosciences PharMingen, San Diego, CA).
Flow cytometry and antibodies
Thymi were dissected, and cell suspensions were prepared, stained, and analyzed as described,5,28 using directly conjugated antibodies from BD Biosciences PharMingen. Data are representative of more than 3 experiments. Statistical analysis was the unpaired Student t test (equal or unequal variance depending on data) and the F test. To allow comparison between litters, the number of cells recovered from each thymus, or the percentage of cells staining positive with a given antibody, were divided by the mean value from wild-type (WT) thymi from the same litter, to give a relative value or relative cell number. At least 3 different litters of any embryonic day (E) were analyzed.
Genotyping and PCR analysis
Ihh−/− embryos die around birth, and from E16.5 can be identified phenotypically by their shortened limbs and slightly small size, whereas Ihh+/− embryos and adults are healthy and phenotypically indistinguishable from WT.13 In addition to phenotypic identification, all embryos and animals were genotyped by polymerase chain reaction (PCR).
DNA extraction and PCR analysis were as described,5 using approximately 0.5 μg genomic DNA as template, on a Stratagene Robocycler (Stratagene, La Jolla, CA). Primers: Ihh/neo, forward: AGGAGGCAGGGACATGGATAGGGTG, reverse: TACCGGTGGATGTGGAATGTGTGCG. Shh/neo, forward: CTGTGCTCGACGTTGTACTG, reverse: AAGCCCG-AGACTTGTGTGGA.
Cre, forward: AGATGCCAGGACATCAGGAACCTG, reverse: TACCGGTGGATGTGGAATGTGTGCG; Ihh WT and Ihh fl/fl as described.13
Real-time RT-PCR
Quantitative PCR of VDJ TCR-β rearrangement
TCR-β VDJ rearrangement measurement was carried out as described.29
Results
Expression of Ihh and Gli1 in thymus populations
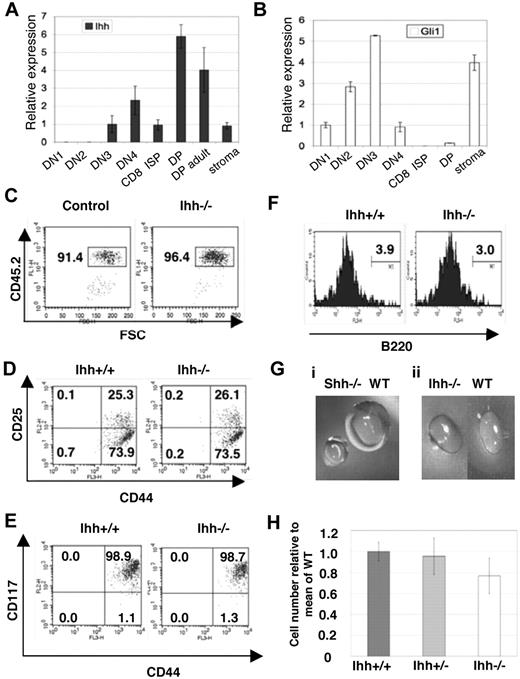
We have described Ihh expression in the whole thymus.10 Here we assess expression of Ihh in fluorescence-activated cell sorter (FACS)–sorted E16.5 fetal thymocyte populations and fetal thymus stroma. We found Ihh expression in both thymus stroma and thymocytes, with highest expression in the DP population. In the sorted thymocyte populations, low Ihh expression, equivalent to that found in the stroma, was detectable in the DN3 population, and relative expression increased 6-fold between the DN3 and DP populations (Figure 1A).
Early thymocyte development in Ihh−/− thymi. (A,B) Transcription of Ihh (A) and Gli1 (B) in sorted E16.5 fetal thymocyte populations and thymus stroma from C57BL/6 mice. Levels of Ihh and Gli1 transcription were normalized for HPRT mRNA content and are shown relative to HPRT-normalized transcription in the DN3 (Ihh) or DN1 (Gli1) subsets. cDNA samples were analyzed in triplicate by quantitative PCR on an iCycler (Bio-Rad, Hercules, CA) using iQ-SYBR Green Supermix (Bio-Rad). Thymocytes were sorted on a Modular Flow Cytometer (MoFlo; Dako North America, Carpinteria, CA), and purity was more than 98%. (C-F) Flow cytometry of E13.5 Ihh−/− and WT littermate thymi. (C) Dot plot of anti-CD45.2 staining versus forward scatter (FSC), showing the gate used for analysis of lymphocyte precursors. (D) The composition of DN subsets gated on CD45.2+ and stained with anti-CD44 and anti-CD25. (E) Dot plot of anti-CD117 staining versus anti-CD44 staining, gated on CD45.2+. (F) Histogram of anti-B220 staining, gated on CD45.2+CD44+ cells. Thymus size: control: 6.4 × 103, Ihh−/−: 4.8 × 103. (Gi) Photographs of E14.5 Shh−/− and WT littermate thymus lobes. (Gii) Photographs of E14.5 Ihh−/− and WT littermate thymus lobes. (H) The mean (± SE) relative cell number in WT (n = 7), Ihh+/− (n = 17), and Ihh−/− (n = 5) thymi on E14.5. To allow comparison between litters, the number of cells recovered from each thymus was divided by the mean number of cells recovered from WT thymi from the same litter, to give relative cell number from 4 E14.5 litters. Differences between WT, Ihh+/−, and Ihh−/− were not significant by Student t test.
Early thymocyte development in Ihh−/− thymi. (A,B) Transcription of Ihh (A) and Gli1 (B) in sorted E16.5 fetal thymocyte populations and thymus stroma from C57BL/6 mice. Levels of Ihh and Gli1 transcription were normalized for HPRT mRNA content and are shown relative to HPRT-normalized transcription in the DN3 (Ihh) or DN1 (Gli1) subsets. cDNA samples were analyzed in triplicate by quantitative PCR on an iCycler (Bio-Rad, Hercules, CA) using iQ-SYBR Green Supermix (Bio-Rad). Thymocytes were sorted on a Modular Flow Cytometer (MoFlo; Dako North America, Carpinteria, CA), and purity was more than 98%. (C-F) Flow cytometry of E13.5 Ihh−/− and WT littermate thymi. (C) Dot plot of anti-CD45.2 staining versus forward scatter (FSC), showing the gate used for analysis of lymphocyte precursors. (D) The composition of DN subsets gated on CD45.2+ and stained with anti-CD44 and anti-CD25. (E) Dot plot of anti-CD117 staining versus anti-CD44 staining, gated on CD45.2+. (F) Histogram of anti-B220 staining, gated on CD45.2+CD44+ cells. Thymus size: control: 6.4 × 103, Ihh−/−: 4.8 × 103. (Gi) Photographs of E14.5 Shh−/− and WT littermate thymus lobes. (Gii) Photographs of E14.5 Ihh−/− and WT littermate thymus lobes. (H) The mean (± SE) relative cell number in WT (n = 7), Ihh+/− (n = 17), and Ihh−/− (n = 5) thymi on E14.5. To allow comparison between litters, the number of cells recovered from each thymus was divided by the mean number of cells recovered from WT thymi from the same litter, to give relative cell number from 4 E14.5 litters. Differences between WT, Ihh+/−, and Ihh−/− were not significant by Student t test.
Both RT-PCR analysis and cell-surface staining have shown that Smo expression is highest in the CD25+ DN population, and down-regulated in the subsequent DN4 and DP populations, indicating that the DP cells are probably unable to respond to an Hh signal,10,21 and DP cells are not responsive to Shh treatment in vitro.10 The transcription factor Gli1 is a ubiquitous transcriptional target of Hh signaling but is not necessary to initiate the Hh signal, and measurement of its transcription is used as a readout of Hh signaling in a cell population.30 Therefore, to determine which fetal thymocyte populations responded to the Hh signal ex vivo, we assessed Gli1 transcription in sorted fetal thymocyte populations. As predicted by the pattern of Smo expression, we found highest Gli1 transcription in the CD25+ DN populations (DN2 and DN3), and Gli1 transcription was down-regulated in the subsequent DN4 and DP populations (Figure 1B). This pattern of expression correlates well with the expression of Gli3 and Gli2 in thymocytes, which are both expressed in DN populations but down-regulated at the DP stage, confirming that DP thymocytes cannot transduce the Hh signal.21-23 Thus, the DP population had the highest expression of Ihh but did not express significant levels of Gli1, implying that they were not themselves responding to an autocrine Hh signal, but rather signaling back to an earlier cell (Figure 1A,B).
Ihh has a redundant function at the transition from DN1 to DN2
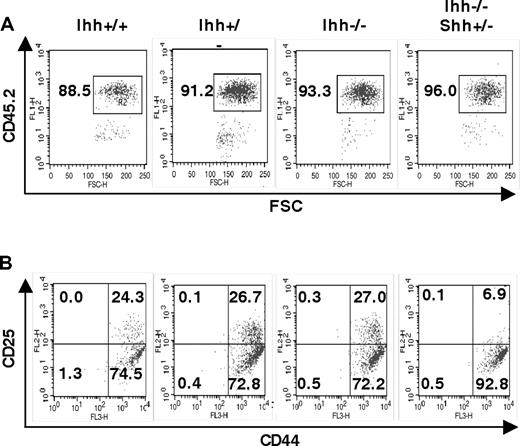
To assess the function of Ihh during fetal T-cell development, we studied thymocyte development in Ihh−/− embryos. Given that Smo, Shh, and Gli3 are important for differentiation from DN1 to DN2,5,21,23 we investigated a role for Ihh at the same developmental checkpoint. We analyzed E13.5 and E14.5 thymi, when the DN1 to DN2 transition first occurs. On E13.5, Shh−/− thymi had a reduction in cell number, proportion of CD45+ cells, and differentiation from DN1 to DN2.5 In contrast, we found no differences in the percentage of CD45+ cells or the proportion of DN1 and DN2 populations between Ihh−/− and littermate thymi (Figure 1C,D). We likewise found no differences in cell-surface expression of CD117 and B220 on the CD45+CD44+ thymocytes between Ihh−/− and littermate thymi (Figure 1E,F). On E14.5, the Shh−/− thymus contained approximately one tenth the number of thymocytes of its WT littermates, and the size of each thymus lobe was greatly reduced (Figure 1Gi). The E14.5 Ihh−/− thymi, however, were not obviously smaller than WT littermate thymi (Figure 1Gii,H). Given that we did not detect an essential function for Ihh at this stage of thymopoiesis and Shh and Ihh have overlapping functions in other tissues,6,7,14 we assessed redundancy between these 2 factors. We analyzed early thymocyte development and differentiation from DN1 to DN2 in double mutants. Shh−/−Ihh−/− double knockout embryos die in utero at E9.5,14 so we analyzed E13.5 Shh+/−Ihh−/− and littermate thymi. On E13.5, the Shh+/− thymus is phenotypically normal. Deletion of one copy of Shh in Ihh−/−(Shh+/−Ihh−/−) did not affect the proportion of CD45+ cells, but did, however, reduce the proportion of DN2 cells, from 27% in a Ihh−/−Shh+/+ thymus, to 6.9% in the Ihh−/−Shh+/− thymus (Figure 2). We found no differences in cell-surface expression of CD117 and B220 on the CD45+CD44+ DN1 thymocytes between Ihh−/−Shh+/− and littermate thymi (data not shown).
Redundancy between Shh and Ihh in early thymocyte development. (A,B) Flow cytometry of E13.5 Ihh+/+, Ihh+/−, Ihh−/−, and Ihh−/−Shh+/− thymi. (A) Dot plot of anti-CD45.2 staining versus FSC, showing the gate used for analysis of lymphocyte precursors. (B) CD44 and CD25 expression of thymocytes, gated on CD45.2+ cells. Thymus sizes were: Ihh+/+: 4.8 × 103; Ihh+/−: 6.4 × 103; Ihh−/−: 4.8 × 103; and Ihh−/− Shh+/−: 6.0 × 103.
Redundancy between Shh and Ihh in early thymocyte development. (A,B) Flow cytometry of E13.5 Ihh+/+, Ihh+/−, Ihh−/−, and Ihh−/−Shh+/− thymi. (A) Dot plot of anti-CD45.2 staining versus FSC, showing the gate used for analysis of lymphocyte precursors. (B) CD44 and CD25 expression of thymocytes, gated on CD45.2+ cells. Thymus sizes were: Ihh+/+: 4.8 × 103; Ihh+/−: 6.4 × 103; Ihh−/−: 4.8 × 103; and Ihh−/− Shh+/−: 6.0 × 103.
These data reveal a function for Ihh at the DN1 to DN2 transition and indicate that, in the E13.5 Ihh−/− thymus, Shh can compensate for absence of Ihh, but that overall concentration of Hh protein is limiting. As we did not detect Ihh transcription in the fetal DN1 and DN2 populations (Figure 1A), the function of Ihh on E13.5 is probably mediated by Ihh produced by the thymic stroma. Given that the proportion of CD45+ cells was not reduced by absence of one copy of Shh, we found no evidence that Ihh is involved in the seeding of the thymus or expansion of early progenitor cells. This function seems to be unique to Shh.
Ihh negatively regulates the transition from DN to DP
To determine whether Ihh is important at the transition from DN to DP cell, we compared thymocyte development in E16.5 Ihh−/−, +/−, and WT littermates. As E16.5 is the day of embryonic development on which DP cells first appear, the transition from DN to DP on E16.5 is largely synchronized. Thymocyte number was reduced in Ihh−/− thymi to less than half of WT littermate thymi, but surprisingly Ihh+/− thymi contained on average 1.4 times more thymocytes than WT thymi, and there was greater variation between individual Ihh+/− thymi (Figure 3A). The differences in thymocyte number relative to WT were statistically significant for both Ihh−/− and Ihh+/− embryos and were reflected in the size of the thymus lobes (Figure 3B).
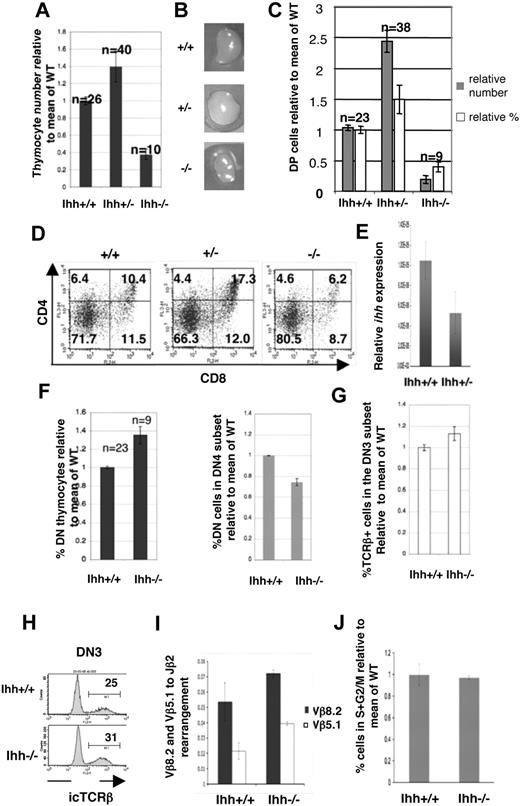
Thymocyte development in E16.5 Ihh−/− and Ihh+/− thymi. (A) Thymocyte number on E16.5. The mean relative cell number (± SE) in WT, Ihh+/−, and Ihh−/− thymi on E16.5. To allow comparison between litters, the number of cells recovered from each thymus was divided by the mean number of cells recovered from WT thymi from the same litter, to give relative cell number from different E16.5 litters. The differences in mean cell number between Ihh−/− and WT thymi (P < .001), Ihh+/− and WT thymi (P = .018), and Ihh−/− and Ihh+/− (P < .001) were statistically significant by Student t test. (B) Photographs of E16.5 WT, Ihh+/−, and Ihh−/− littermate thymus lobes. (C) The mean relative cell number (■) and mean relative percentages (□) of DP cells (± SE) in WT, Ihh+/−, and Ihh−/− thymi on E16.5. Differences in mean cell number and percentages between WT and Ihh+/− (P = .007, P = .02), WT and Ihh−/− (P < .001 for both), and Ihh+/− and Ihh−/− (P < .001 for both) thymi were statistically significant by Student t test. (D) Flow cytometry of E16.5 WT, +/−, and −/− littermate thymi stained with anti-CD4 and anti-CD8. Thymus size: WT 4.56 × 104, Ihh+/− 1.144 × 105, and Ihh−/− 1.68 × 104. (E) Transcription of Ihh in DP thymocytes from embryonic Ihh+/+ and Ihh+/− thymi. Relative levels of Ihh transcription were measured as described in Figure 1A and were normalized for HPRT mRNA content. (F) (Left panel) Mean percentages of DN thymocytes in WT and Ihh−/− thymi, relative to mean of WT littermates. The percentage of DN cells in the Ihh−/− thymus is significantly higher than in the Ihh+/+ thymi by Student t test (P = .002). (Right panel) Mean percentages of DN4 thymocytes in the DN subset relative to mean of WT littermates are shown for WT and Ihh−/− thymi. Differences between Ihh+/+ and Ihh−/− were statistically significant by Student t test (P < .001). (G) Mean percentages of icTCR-β+ cells in the DN3 subset are shown relative to mean of WT littermates for WT and Ihh−/− thymi. Differences between mean percentage icTCR-β+ in DN3 subset of Ihh+/+ and Ihh−/− were statistically significant by Student t test (P = .03). (H) Representative histograms of icTCR-β expression in the DN3 subsets of WT littermate (top) and Ihh−/− (bottom) thymi. (I) TCR-β locus rearrangement was measured in E15.5 DN thymocytes from WT and Ihh−/− littermates, according to the method of Gounari et al.29 DNA was amplified using primers 5′ to Vβ8.2 or Vβ5.1 and 3′ to Jβ2.7, and products were measured by quantitative PCR on an iCycler (Bio-Rad) using iQ-SYBR Green Supermix (Bio-Rad). DNA content was normalized relative to Thy1. Differences between Ihh−/− and WT were not significant by Student t test. (J) The bar chart shows the percentage of cells in S + G2/M, measured by PI staining of FACS-sorted E16.5 CD25+ DN cells from WT and Ihh−/− littermates, relative to the mean percentage in WT littermate populations. Differences in mean percentage between −/− and WT were not statistically significant.
Thymocyte development in E16.5 Ihh−/− and Ihh+/− thymi. (A) Thymocyte number on E16.5. The mean relative cell number (± SE) in WT, Ihh+/−, and Ihh−/− thymi on E16.5. To allow comparison between litters, the number of cells recovered from each thymus was divided by the mean number of cells recovered from WT thymi from the same litter, to give relative cell number from different E16.5 litters. The differences in mean cell number between Ihh−/− and WT thymi (P < .001), Ihh+/− and WT thymi (P = .018), and Ihh−/− and Ihh+/− (P < .001) were statistically significant by Student t test. (B) Photographs of E16.5 WT, Ihh+/−, and Ihh−/− littermate thymus lobes. (C) The mean relative cell number (■) and mean relative percentages (□) of DP cells (± SE) in WT, Ihh+/−, and Ihh−/− thymi on E16.5. Differences in mean cell number and percentages between WT and Ihh+/− (P = .007, P = .02), WT and Ihh−/− (P < .001 for both), and Ihh+/− and Ihh−/− (P < .001 for both) thymi were statistically significant by Student t test. (D) Flow cytometry of E16.5 WT, +/−, and −/− littermate thymi stained with anti-CD4 and anti-CD8. Thymus size: WT 4.56 × 104, Ihh+/− 1.144 × 105, and Ihh−/− 1.68 × 104. (E) Transcription of Ihh in DP thymocytes from embryonic Ihh+/+ and Ihh+/− thymi. Relative levels of Ihh transcription were measured as described in Figure 1A and were normalized for HPRT mRNA content. (F) (Left panel) Mean percentages of DN thymocytes in WT and Ihh−/− thymi, relative to mean of WT littermates. The percentage of DN cells in the Ihh−/− thymus is significantly higher than in the Ihh+/+ thymi by Student t test (P = .002). (Right panel) Mean percentages of DN4 thymocytes in the DN subset relative to mean of WT littermates are shown for WT and Ihh−/− thymi. Differences between Ihh+/+ and Ihh−/− were statistically significant by Student t test (P < .001). (G) Mean percentages of icTCR-β+ cells in the DN3 subset are shown relative to mean of WT littermates for WT and Ihh−/− thymi. Differences between mean percentage icTCR-β+ in DN3 subset of Ihh+/+ and Ihh−/− were statistically significant by Student t test (P = .03). (H) Representative histograms of icTCR-β expression in the DN3 subsets of WT littermate (top) and Ihh−/− (bottom) thymi. (I) TCR-β locus rearrangement was measured in E15.5 DN thymocytes from WT and Ihh−/− littermates, according to the method of Gounari et al.29 DNA was amplified using primers 5′ to Vβ8.2 or Vβ5.1 and 3′ to Jβ2.7, and products were measured by quantitative PCR on an iCycler (Bio-Rad) using iQ-SYBR Green Supermix (Bio-Rad). DNA content was normalized relative to Thy1. Differences between Ihh−/− and WT were not significant by Student t test. (J) The bar chart shows the percentage of cells in S + G2/M, measured by PI staining of FACS-sorted E16.5 CD25+ DN cells from WT and Ihh−/− littermates, relative to the mean percentage in WT littermate populations. Differences in mean percentage between −/− and WT were not statistically significant.
In Ihh−/− thymi, the proportion of DP cells was reduced (Figure 3C,D). In Ihh+/− thymi, however, both the proportion and absolute number of DP cells were increased, compared with WT, and on average the Ihh+/− thymus contained 2.4 times more DP cells than WT thymi (Figure 3C,D); 6.2% of cells were DP in Ihh−/− thymi compared with 10.4% and 17.3% in WT and Ihh+/− littermate thymi, respectively (Figure 3D). Given that the increase in production of DP cells in the Ihh+/− thymus was surprising, we used quantitative RT-PCR analysis of RNA from embryonic DP thymocytes to confirm expression levels of Ihh in WT, Ihh+/−, and Ihh−/−. As expected, Ihh transcription was reduced 2-fold from WT to Ihh+/− and was not detectable in Ihh−/− (Figure 3E).
Although the reduction in DP cells in the Ihh−/− thymus showed that Ihh promotes T-cell development (as seen in E13.5 Ihh−/−Shh+/− thymi, Figure 2B), the opposing phenotypes of the −/− and +/− mutants indicated that Ihh also negatively regulates the transition from DN to DP, as reducing the concentration of Ihh to one half of that of the WT thymus increased the production of DP thymocytes by more than 2-fold (Figure 3C,D).
Ihh is not required for TCR-β locus rearrangement
We found an overall increase in percentage of DN cells but a significant decrease in the proportion of DN4 cells in the Ihh−/− thymus (Figure 3F). Because transition to the DP stage requires pre-TCR signaling, the reduced differentiation from DN3 to DP observed in Ihh−/− thymus could be the result of failure to rearrange the TCR-β chain locus. To test this, we assessed the ability of DN3 thymocytes to produce functional TCR-β chain protein by measuring intracellular (ic) TCR-β chain expression. The Ihh−/− DN3 population expressed icTCR-β, and the percentage of icTCR-β+ cells in the DN3 population was indeed marginally higher in the Ihh−/− embryos compared with WT littermates (Figure 3G,H). We confirmed that TCR-β locus rearrangement was efficient in the Ihh−/− thymus, by quantitative PCR29 using 5′ primers to Vβ8.2 and Vβ5.1 and a 3′ primer to Jβ2.7 (Figure 3I). We found no evidence for reduction in efficiency of TCR-β locus rearrangement between Ihh−/− and WT. Because successful TCR-β locus rearrangement is associated with release from cell-cycle arrest,31 we also assessed cell-cycle status by propidium iodide (PI) staining of sorted DN3 cells from WT and Ihh−/− littermates. We found no significant difference in the proportion of cells in S + G2/M (Figure 3J). We therefore found no evidence for a reduction in TCR-β locus rearrangement.
Ihh is a negative regulator of DN3 cell proliferation
To identify the target cell of Ihh's negative regulation of cell number, we sorted CD25+ DN, CD8ISP, and DP populations from E16.5 Ihh+/− and WT thymi and assessed cell-cycle status by PI staining. There was no difference in the percentage of cells in cycle between the DP populations, but the Ihh+/− CD25+ DN population contained significantly more cells in S/G2 plus M than its WT counterpart, identifying it as a target of Ihh's negative regulation of expansion and differentiation (Figure 4A). In addition, a small but significant increase in the proportion of cells in cycle was observed in the Ihh+/− CD8ISP population, relative to WT (Figure 4A).
Ihh regulates thymus homeostasis. (A) Scatter plots show the percentage of cells in S + G2/M, measured by PI staining of FACS-sorted E16.5 CD25+ DN (left plot), CD8ISP (middle plot), and DP (right plot) populations from WT and Ihh+/− littermates, relative to the mean percentage in WT littermate populations. Differences in mean percentage between +/− and WT were statistically significant for CD25+ DN (P = .042) and CD8ISP (P = .040) populations, but not for DP cells (P = .981). (B) Scatter plots show relative thymocyte number from Ihh+/+Rag1−/− and Ihh+/−Rag1−/− thymi (relative to mean of Ihh+/+Rag1−/− littermates). Differences are significant by Student t test (P < .001). Mean thymocyte number: litter 1 Ihh+/+Rag1−/− 1.65 × 106, Ihh+/−Rag1−/− 1.33 × 106; litter 2 Ihh+/+Rag1−/− 1.7 × 106, Ihh+/−Rag1−/− 1.25 × 106; litter 3 Ihh+/+Rag1−/− 1.75 × 106, Ihh+/−Rag1−/− 1.31 × 106. (C) Bar chart shows fold increase in thymocyte number on induction of differentiation by treatment with 1 μg/mL anti-CD3 after 5 days of FTOCs for Rag1−/−Ihh+/+ and Rag1−/− Ihh+/− littermates. For each thymus, one lobe was cultured with anti-CD3 and the fold increase in cell number was calculated relative to the number of cells in the other untreated lobe. The fold increase in the Rag1−/−Ihh+/− was significantly different from that in the Rag1−/−Ihh+/+ by Student t test (P = .03). Mean thymocyte number: litter 1 Ihh+/+Rag1−/− untreated 1.65 × 106, Ihh+/+Rag1−/− + anti-CD3 1.425 × 107, Ihh+/−Rag1−/− untreated 1.33 × 106, Ihh+/−Rag1−/− + anti-CD3 1.725 × 107. Litter 2 Ihh+/+Rag1−/− untreated 1.7 × 106, Ihh+/+Rag1−/− + anti-CD3 1.49 × 107, Ihh+/−Rag1−/− untreated 1.245 × 106, Ihh+/+Rag1−/− + anti-CD3 1.689 × 107. (D) Dot plots show E17.5 WT, Ihh+/−, and Ihh−/− thymi stained with anti-CD4 and anti-CD8. Mean thymocyte number: litter 1 WT 2.05 × 106, Ihh+/− 3.3 × 106, Ihh−/− 2 × 106; litter 2 WT 3.5 × 106, Ihh+/− 2.865 × 106, Ihh−/− 8.5 × 105; litter 3 WT 3.35 × 106, Ihh+/− 2.63 × 106 Ihh−/− 1.675 × 106; litter 4 WT 3.35 × 106, Ihh+/− 2.336 × 106; litter 5 WT 3.868 × 106, Ihh+/− 3.69 × 106; litter 6 WT 4.8 × 106, Ihh+/− 4.756, Ihh−/− 3.8 × 106. (E) Scatter plot shows cell numbers (relative to mean of WT) from thymi from E17.5 WT, Ihh+/−, and Ihh−/− embryos. The difference in mean between WT and Ihh−/− is significant (P = .002, Student t test), and the difference in SD between WT and Ihh+/− is also significant (P < .001, F test). (F) Scatter plot shows  : thymocyte number (relative to mean of WT) from E17.5 WT littermate and Ihh+/− embryos. Ihh+/− thymi are grouped by relative size into 3 sets: I, more than 1.3; II, 0.9 to 1.1; and III, less than 0.9; ○: the percentage of cells in S + G2/M (relative to mean of WT), measured by PI staining of FACS-sorted E17.5 CD25+ from WT and Ihh+/− mice grouped into 3 groups I, II, and III as detailed in this figure legend.
: thymocyte number (relative to mean of WT) from E17.5 WT littermate and Ihh+/− embryos. Ihh+/− thymi are grouped by relative size into 3 sets: I, more than 1.3; II, 0.9 to 1.1; and III, less than 0.9; ○: the percentage of cells in S + G2/M (relative to mean of WT), measured by PI staining of FACS-sorted E17.5 CD25+ from WT and Ihh+/− mice grouped into 3 groups I, II, and III as detailed in this figure legend.
Ihh regulates thymus homeostasis. (A) Scatter plots show the percentage of cells in S + G2/M, measured by PI staining of FACS-sorted E16.5 CD25+ DN (left plot), CD8ISP (middle plot), and DP (right plot) populations from WT and Ihh+/− littermates, relative to the mean percentage in WT littermate populations. Differences in mean percentage between +/− and WT were statistically significant for CD25+ DN (P = .042) and CD8ISP (P = .040) populations, but not for DP cells (P = .981). (B) Scatter plots show relative thymocyte number from Ihh+/+Rag1−/− and Ihh+/−Rag1−/− thymi (relative to mean of Ihh+/+Rag1−/− littermates). Differences are significant by Student t test (P < .001). Mean thymocyte number: litter 1 Ihh+/+Rag1−/− 1.65 × 106, Ihh+/−Rag1−/− 1.33 × 106; litter 2 Ihh+/+Rag1−/− 1.7 × 106, Ihh+/−Rag1−/− 1.25 × 106; litter 3 Ihh+/+Rag1−/− 1.75 × 106, Ihh+/−Rag1−/− 1.31 × 106. (C) Bar chart shows fold increase in thymocyte number on induction of differentiation by treatment with 1 μg/mL anti-CD3 after 5 days of FTOCs for Rag1−/−Ihh+/+ and Rag1−/− Ihh+/− littermates. For each thymus, one lobe was cultured with anti-CD3 and the fold increase in cell number was calculated relative to the number of cells in the other untreated lobe. The fold increase in the Rag1−/−Ihh+/− was significantly different from that in the Rag1−/−Ihh+/+ by Student t test (P = .03). Mean thymocyte number: litter 1 Ihh+/+Rag1−/− untreated 1.65 × 106, Ihh+/+Rag1−/− + anti-CD3 1.425 × 107, Ihh+/−Rag1−/− untreated 1.33 × 106, Ihh+/−Rag1−/− + anti-CD3 1.725 × 107. Litter 2 Ihh+/+Rag1−/− untreated 1.7 × 106, Ihh+/+Rag1−/− + anti-CD3 1.49 × 107, Ihh+/−Rag1−/− untreated 1.245 × 106, Ihh+/+Rag1−/− + anti-CD3 1.689 × 107. (D) Dot plots show E17.5 WT, Ihh+/−, and Ihh−/− thymi stained with anti-CD4 and anti-CD8. Mean thymocyte number: litter 1 WT 2.05 × 106, Ihh+/− 3.3 × 106, Ihh−/− 2 × 106; litter 2 WT 3.5 × 106, Ihh+/− 2.865 × 106, Ihh−/− 8.5 × 105; litter 3 WT 3.35 × 106, Ihh+/− 2.63 × 106 Ihh−/− 1.675 × 106; litter 4 WT 3.35 × 106, Ihh+/− 2.336 × 106; litter 5 WT 3.868 × 106, Ihh+/− 3.69 × 106; litter 6 WT 4.8 × 106, Ihh+/− 4.756, Ihh−/− 3.8 × 106. (E) Scatter plot shows cell numbers (relative to mean of WT) from thymi from E17.5 WT, Ihh+/−, and Ihh−/− embryos. The difference in mean between WT and Ihh−/− is significant (P = .002, Student t test), and the difference in SD between WT and Ihh+/− is also significant (P < .001, F test). (F) Scatter plot shows  : thymocyte number (relative to mean of WT) from E17.5 WT littermate and Ihh+/− embryos. Ihh+/− thymi are grouped by relative size into 3 sets: I, more than 1.3; II, 0.9 to 1.1; and III, less than 0.9; ○: the percentage of cells in S + G2/M (relative to mean of WT), measured by PI staining of FACS-sorted E17.5 CD25+ from WT and Ihh+/− mice grouped into 3 groups I, II, and III as detailed in this figure legend.
: thymocyte number (relative to mean of WT) from E17.5 WT littermate and Ihh+/− embryos. Ihh+/− thymi are grouped by relative size into 3 sets: I, more than 1.3; II, 0.9 to 1.1; and III, less than 0.9; ○: the percentage of cells in S + G2/M (relative to mean of WT), measured by PI staining of FACS-sorted E17.5 CD25+ from WT and Ihh+/− mice grouped into 3 groups I, II, and III as detailed in this figure legend.
Ihh promotes DN thymocyte development before pre-TCR signal transduction but is a negative regulator after pre-TCR signal transduction
Because Ihh provides both positive and negative regulatory signals for the differentiation and proliferation of DN thymocytes, we asked when in thymocyte development the positive and negative signals occur with respect to pre-TCR signaling. We compared cell number between Ihh+/+Rag−/− and Ihh+/−Rag−/− thymi and found that the Ihh+/−Rag−/− thymi were significantly smaller than those of Ihh+/+Rag−/− littermates (Figure 4B; P < .001), demonstrating that Ihh transmits the positive signal before pre-TCR signal transduction. This is consistent with its early function at the transition from DN1 to DN2.
We then tested whether Ihh+/−Rag−/− thymocytes expand more efficiently than their Ihh+/+Rag−/− counterparts after FTOC treatment with anti-CD3, thereby mimicking a signal through the pre-TCR. For each thymus, we treated one lobe with anti-CD3 monoclonal antibody for 5 days and calculated the fold increase in thymocyte number relative to the number of cells in the other untreated lobe from the same thymus. The number of thymocytes in Ihh+/−Rag−/− FTOCs increased on average 13 times during the culture period, compared with a 9-fold expansion in the Ihh+/+Rag−/− FTOCs (Figure 4C). This difference was statistically significant (P = .03). Thus, Ihh promoted thymocyte development before pre-TCR signaling but negatively regulated thymocyte development after pre-TCR signaling has taken place.
Ihh regulates fetal thymus homeostasis
We have shown that reduced concentration of Ihh promotes differentiation from DN3 to DP stage and that the DN3 subset is the target population for this negative regulatory function of Ihh (Figures 3A-F, 4A-C). In addition, our expression analysis has shown that thymocytes, and in particular the DP population, provide most of the Ihh in the thymus (Figure 1A). Concentration of Ihh in the thymus will therefore depend largely on the number of DP thymocytes (ie, Ihh-producing cells). Taken together, our data suggest that Ihh produced by the thymocytes feeds back to negatively regulate the proliferation and differentiation of DN3 cells after pre-TCR signal transduction, in a concentration-dependent manner. Thus, Ihh signaling in the thymus provides a counting system for post-DN3 thymocyte number.
In the heterozygote fetal thymus, the Ihh+/− thymocytes make half the concentration of Ihh transcript (Figure 3E) compared with that of their WT counterparts; thus, the amount of Ihh signaling would be approximately half that observed in the WT thymus. Therefore, given this reduced negative regulatory signal in the Ihh+/− thymus, the target cells will proliferate and differentiate faster. This would result in a larger DP population, thus a larger thymus in total (as observed on E16.5, Figure 3A-E). Once the size of the DP population increases 2-fold, Ihh concentrations will reach WT levels, and so the negative regulatory signal will be fully restored, resulting in a slowdown of proliferation and differentiation. As a consequence, DP production would be reduced, which would then reduce Ihh signal, and the homeostatic cycle would begin again, leading to increased variation in thymocyte number in the heterozygotes.
This model would therefore predict that, on E17.5, the upper limit of size in the Ihh+/− thymus would be greater than in the WT and also that size would be more varied. To test this, we investigated thymocyte differentiation and proliferation on E17.5. As predicted, the Ihh+/− thymi were on average larger, the upper limits of thymus growth were increased 2-fold, and thymocyte number was much more variable (P < .001) than in the WT thymi (Figure 4D,E). The proportion of DP cells in the heterozygote thymus also mirrored the increased thymocyte number, with larger thymi containing more DP thymocytes (Figure 4D).
Because these data were consistent with concentration-dependent negative feedback on DP cell production, we assessed cell-cycle status of the target cell population of Ihh's negative regulatory action (CD25+ DN thymocytes, Figure 4A), from E17.5 Ihh+/− thymi, grouped according to size. PI staining was carried out on purified CD25+ DN thymocytes from E17.5 Ihh+/−, from 3 groups: I (thymus size > 1.3 relative to WT), II (thymus size 0.9-1.1 relative to WT), and III (thymus size < 0.9 relative to WT) (Figure 4F). In the larger Ihh+/− thymi (I), consistent with Ihh concentration being equivalent to that in WT, the percentage of cells in cycle in the CD25+ DN population was the same as that found in WT littermates. In contrast, in the smaller Ihh+/− thymi (III), where Ihh concentrations are lower than WT levels, the percentage of cells in cycle in the CD25+ DN population was higher. These data are consistent with the overall concentration of Ihh protein in the thymus influencing proliferation of the CD25+ DN cell, and with negative feedback restricting thymocyte number by restricting proliferation of an earlier target progenitor cell.
Analysis of E17.5 Ihh−/− thymi revealed partial recovery of thymocyte number and the DP population (Figure 4D,E), compatible with the positive regulatory function of Ihh being required early, before pre-TCR signal transduction.
Distinct and redundant functions for Ihh and Shh on E16.5
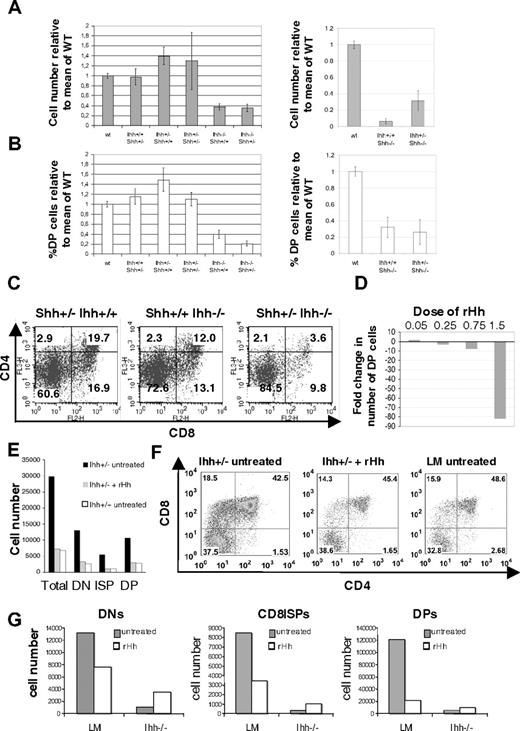
To assess redundancy and overlapping functions of Ihh and Shh on the production of DP cells, we studied the effect of removal of one copy of Ihh from the E16.5 Shh−/− thymus and vice versa. Removal of one copy of Ihh from Shh−/− (Shh−/−Ihh+/−) increased cell number 3-fold relative to Shh−/− littermate thymi (Figure 5A), indicating that the negative regulatory function of Ihh (revealed by reducing Ihh concentration) acts on a later stage of T-cell development than the positive Shh signal, so that reduction of Ihh signal allowed partial recovery of thymocyte number in the Shh−/− thymus. In the Shh+/−Ihh+/− thymus, thymocyte number was not greater than in the Shh+/+Ihh+/− but was increased relative to WT, and there was greater variability. As shown previously,5 the proportion of DP cells was reduced in Ihh−/− and in Shh−/− thymi, and there was an increase in the proportion of DN cells (Figures 3C-F, 4D). Deletion of one copy of Shh in Ihh−/− thymi (Shh+/−Ihh−/−) caused a greater reduction in the proportion of DP cells than seen in Ihh−/− thymi, in all litters examined (Figure 5B,C). The proportion of DP cells was 3.6% in the Shh+/−Ihh−/−, compared with 19.7% in the Shh+/−Ihh+/+ and 12% in the Shh+/+Ihh−/− littermate thymi (Figure 5C). Thus, both Ihh and Shh provide positive signals for DN cell expansion and differentiation, and it is the total amount of Hh signal that is critical at this stage.
Thymocyte development in Shh/Ihh double mutants and in FTOCs treated with exogenous recombinant Hedgehog protein. (A) Histograms show the mean relative cell number (± SE) in WT (n = 11), Ihh+/+Shh+/− (n = 5), Ihh+/−Shh+/+ (n = 8), Ihh+/− Shh+/− (n = 3), Ihh−/−Shh+/+ (n = 4), Ihh−/−Shh+/− (n = 3) (left), and in WT (n = 11), Ihh+/+Shh−/− (n = 3), and Ihh+/−Shh−/− (n = 3) thymi (right) on E16.5. (B) Histogram to show the mean relative percentage ( ± SE) of DP cells in the litters shown in panel A. (C) Expression of CD4 and CD8 on Shh+/− Ihh+/+, Shh+/+ Ihh−/−, and Shh+/− Ihh−/− thymi. Thymus sizes were: Shh+/− Ihh+/+: 2.6 × 105; Shh+/+ Ihh−/−: 1.28 × 105; and Shh+/− Ihh−/−: 5.2 × 104. (D) Bar chart shows the fold change in number of DP thymocytes recovered from WT E15 FTOCs treated for 3 days with different concentrations of r-mShh-N (R&D Systems), compared with the number of DP cells recovered from the untreated thymus lobe from the same embryos; 0.25 μg/mL r-mShh-N decreased the production of DP cells by approximately 2-fold. (E) Bar chart shows the number of cells from DN, CD8+ ISP, and DP thymocyte populations recovered from Ihh+/− and WT littermate E15 FTOCs cultured for 3 days, with or without treatment with 0.25 mg/mL r-mShh-N. Mean cell recovery per thymus lobe: Ihh+/− control 3.3 × 105; Ihh+/− + r-mShh-N 6.3 × 104; Ihh+/+ littermate control 7.0 × 104. In each experiment, the treated thymus lobe was compared with the untreated lobe from the same embryo. (F) Dot plots show anti-CD4 and anti-CD8 staining of individual thymus lobes from the experiment in panel E, in which treated thymus lobes were compared with the untreated lobe form the same embryo, or to a WT littermate lobe. (G) Bar charts show the number of cells from DN, CD8+ ISP, and DP thymocyte populations recovered from Ihh−/− and littermate (LM) E15 FTOC cultured for 5 days, with or without treatment with 0.25 μg/mL r-mShh-N. The treated lobe from one embryo was compared with the untreated lobe from the same embryo.
Thymocyte development in Shh/Ihh double mutants and in FTOCs treated with exogenous recombinant Hedgehog protein. (A) Histograms show the mean relative cell number (± SE) in WT (n = 11), Ihh+/+Shh+/− (n = 5), Ihh+/−Shh+/+ (n = 8), Ihh+/− Shh+/− (n = 3), Ihh−/−Shh+/+ (n = 4), Ihh−/−Shh+/− (n = 3) (left), and in WT (n = 11), Ihh+/+Shh−/− (n = 3), and Ihh+/−Shh−/− (n = 3) thymi (right) on E16.5. (B) Histogram to show the mean relative percentage ( ± SE) of DP cells in the litters shown in panel A. (C) Expression of CD4 and CD8 on Shh+/− Ihh+/+, Shh+/+ Ihh−/−, and Shh+/− Ihh−/− thymi. Thymus sizes were: Shh+/− Ihh+/+: 2.6 × 105; Shh+/+ Ihh−/−: 1.28 × 105; and Shh+/− Ihh−/−: 5.2 × 104. (D) Bar chart shows the fold change in number of DP thymocytes recovered from WT E15 FTOCs treated for 3 days with different concentrations of r-mShh-N (R&D Systems), compared with the number of DP cells recovered from the untreated thymus lobe from the same embryos; 0.25 μg/mL r-mShh-N decreased the production of DP cells by approximately 2-fold. (E) Bar chart shows the number of cells from DN, CD8+ ISP, and DP thymocyte populations recovered from Ihh+/− and WT littermate E15 FTOCs cultured for 3 days, with or without treatment with 0.25 mg/mL r-mShh-N. Mean cell recovery per thymus lobe: Ihh+/− control 3.3 × 105; Ihh+/− + r-mShh-N 6.3 × 104; Ihh+/+ littermate control 7.0 × 104. In each experiment, the treated thymus lobe was compared with the untreated lobe from the same embryo. (F) Dot plots show anti-CD4 and anti-CD8 staining of individual thymus lobes from the experiment in panel E, in which treated thymus lobes were compared with the untreated lobe form the same embryo, or to a WT littermate lobe. (G) Bar charts show the number of cells from DN, CD8+ ISP, and DP thymocyte populations recovered from Ihh−/− and littermate (LM) E15 FTOC cultured for 5 days, with or without treatment with 0.25 μg/mL r-mShh-N. The treated lobe from one embryo was compared with the untreated lobe from the same embryo.
The fact that the number of thymocytes was increased in the Ihh+/−Shh−/− thymus compared with Ihh+/+Shh−/− littermates allows a clear distinction to be made between the positive and negative regulatory roles for Hh signaling and indicates that Ihh concentration is important after Shh signaling, as rather than aggravating the phenotype, lowering the concentration of Ihh actually allowed partial recovery of DP thymocyte number.
Concentration-dependent regulation of thymocyte development by r-Hh treatment in FTOCs
To determine whether we could reconstitute the Ihh−/− and Ihh+/− thymus with exogenous Hedgehog protein, we treated FTOCs with recombinant Hedgehog (r-Hh). Mammalian Hh proteins are autocatalytically processed to form an active N-terminal fragment, which is highly homologous between Shh and Ihh and between species.6,7 We therefore treated FTOCs with recombinant mouse Shh N-terminus (r-mShh-N). We have previously shown that high-dose treatment of FTOCs with octylated recombinant human Shh N-terminus (oct-r-hShh-N) arrested thymocyte development at the DN stage,5,10 whereas treatment with low concentrations of this protein increased thymocyte production.5 We therefore titrated the r-mShh-N in WT FTOCs and measured DP cell production after 3 days (Figure 5D). The activity of the r-mShh-N was very similar in FTOCs to that of the oct-r-hShh-N we had used in previous studies, and at 1.5 μg/mL r-mShh-N arrested thymocyte development at the DN stage, decreasing the number of DP cells 80-fold, whereas, at the lowest concentration we assayed (0.05 μg/mL), we saw a modest increase in the production of DP cells (Figure 5D).
We then asked whether treatment of Ihh+/− FTOCs with r-Hh could restore the negative regulatory effect of Ihh on thymocyte development. Because Ihh+/− thymi contain approximately twice the number of thymocytes as WT thymi on E16.5, we chose a concentration of r-Hh that decreased DP production approximately 2-fold in our titration (Figure 5D). In FTOCs, we treated E15 Ihh+/− thymus lobes for 3 days with 0.25 μg/mL r-mShh-N and compared thymocyte development with that in the untreated lobe from the same thymus and to WT littermate thymus lobes. The untreated Ihh+/− FTOCs produced approximately 4 times more thymocytes than their WT counterparts, and this expansion was evident in DN, CD8ISP, and DP populations (Figure 5E,F). Treatment with 0.25 μg/mL r-mShh-N restored the negative regulatory signal and inhibited thymocyte development to WT levels. A reduction was seen not only in the number of DP cells produced but also in the expansion of the DN population (Figure 5E,F).
We then asked whether we could restore the positive regulatory function of Ihh on thymocyte development by treatment of Ihh−/− FTOCs with r-Hh. Given that treatment of Ihh+/− FTOCs with 0.25 μg/mL r-mShh-N restored the heterozygote thymus to WT thymocyte production, we reasoned simplistically that treatment of the Ihh−/− thymus with the same concentration of r-mShh-N should mirror the Ihh+/− thymus, in which Ihh protein concentrations are sufficient to provide the early positive regulatory signal for thymocyte development but not the later negative regulatory signal. We treated Ihh−/− and littermate FTOCs with 0.25 μg/mL r-mShh-N and compared thymocyte development in the treated thymus lobes with that in the untreated lobe from the same thymus. As seen previously (Figure 5D-F), r-mShh-N inhibited thymocyte development in the littermate FTOCs, but as predicted by our model, the treatment promoted thymocyte development in the Ihh−/− with increases in DN, ISP, and DP production (Figure 5G). Thus, treatment with r-Hh had opposing outcomes on Ihh−/− and Ihh+/− FTOCs, and we demonstrated both the positive and negative regulatory functions of Ihh in vitro.
Ihh signaling in the adult heterozygote thymus
To determine whether Ihh is important in the adult thymus, we confirmed Ihh expression in sorted adult thymocyte populations by quantitative RT-PCR. We detected Ihh transcription in all thymocyte populations, with a 6-fold up-regulation between the DN3 and DP populations (Figure 6A). Given that the DP population makes up more than 80% of adult thymocytes and expresses more Ihh than other thymocyte populations or the stroma, it produces most of the Ihh protein in the thymus. We also assessed Gli1 expression in sorted adult populations, and as reported previously,21,22 the pattern of Gli1 transcription in adult thymocytes was similar to that in fetal thymocytes, with expression peaking in the CD25+ (DN2/DN3) DN cells and virtually undetectable in the DP population (Figure 6B), indicating that adult DP cells, although the major producers of Ihh, are not themselves responding to an Hh signal.10,21
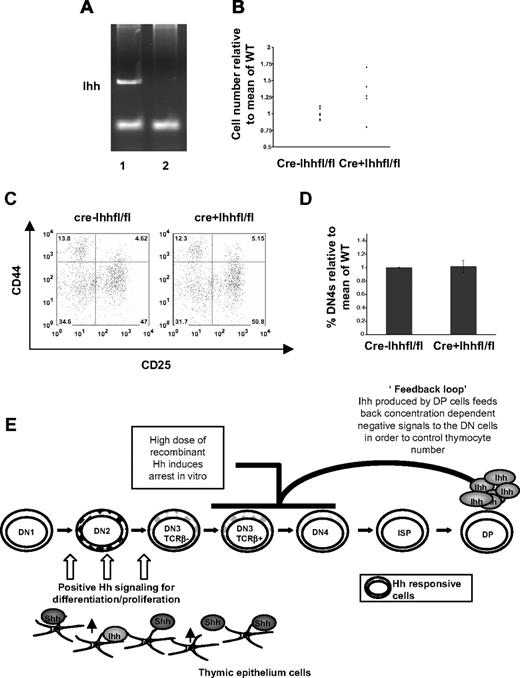
Thymocyte Ihh expression is required for adult thymocyte development. (A,B) Expression of Ihh (A) and Gli1 (B) was assessed by quantitative RT-PCR in FACS-sorted populations of adult and fetal murine tissue. Levels of Ihh and Gli1 transcription were normalized for HPRT mRNA content and are shown relative to HPRT normalized transcription in the CD25+ DN population (Ihh) and DN1 population (Gli1). Thymocytes were sorted on a Modular Flow Cytometer (MoFlo; Dako North America), and purity was more than 98%. (C) Relative thymocyte number (relative to the mean of WT littermates) of 3-week-old WT (n = 8) and Ihh+/− (n = 8) littermates revealed a significant difference in SD as judged by F test (P < .001). Mean thymocyte number: litter 1 WT 1.2 × 108, Ihh+/− 1.77 × 108; litter 2 WT 1.44 × 108, Ihh+/− 1.76 × 108; litter 3 WT 1.35 × 108, Ihh+/− 1.81 × 108. (D) Transcription of Ihh in DP thymocytes from Ihh+/+ and Ihh+/− thymi. Relative levels of Ihh transcription were measured as described in Figure 1A and were normalized for HPRT mRNA content. (E) Excision of the Ihh gene as assessed by PCR from vavCre− (lane1) and vavCre+ Ihhfl/fl mice (lane 2). (F) Relative cell number (calculated relative to the mean of the WT littermates) of the thymus of 4- to 6-week-old mice of littermates (all Cre−: nonknockout mice, n = 9) compared with knockout mice (vavCre+Ihhflnull, n = 4) showed a significant decrease in thymocyte cell number (P = .003). Mean thymocyte number: litter 1 littermate (LM) 1.7 × 108, vavCre+Ihhflnull 0.75 × 108; litter 2 LM 1.95 × 108, vavCre+Ihhflnull 0.47 × 108; litter 3 LM 1.48 × 108, vavCre+Ihhflnull 1.28 × 108; litter 4 LM 1.2 × 108, vavCre+Ihhflnull 9.2 × 107. (G) Relative cell number (calculated as in panel D) of the thymus isolated from 4- to 6-week-old mice of littermate vavCre−Ihhfl/fl mice (WT, n = 15) compared with vavCre+Ihhfl/fl mice (knockout mice, n = 5) showed a significant decrease in thymocyte number (P = .016). Mean thymocyte numbers: litter 1 vavCre−Ihhfl/fl 3 × 108, vavCre+Ihhfl/fl 1.925 × 108; litter 2 vavCre−Ihhfl/fl 3.24 × 108, vavCre+Ihhfl/fl 2.59 × 108; litter 3 vavCre−Ihhfl/fl 2.115 × 108, vavCre+Ihhfl/fl 1.3 × 108; litter 4 vavCre−Ihhfl/fl 2.8 × 108, vavCre+Ihhfl/fl 1.75 × 108. (H) FACS analysis of CD44 and CD25 expression on CD4−8−3− thymocytes from vavCre−Ihhfl/fl and vavCre+Ihhfl/fl mice revealed reduced transition to the DN4 stage in development. (I) Histogram shows the percentage of DN4 cells in the DN population, relative to the mean percentage in Cre− littermates for vavCre−Ihhfl/fl (n = 13) and vavCre+Ihhfl/fl knockout mice (n = 6). *Significant difference by Student t test (P = .028). (J) Intracellular TCR-β expression was analyzed in DN3 thymocytes from littermate vavCre−Ihhfl/fl WT (n = 14) and vavCre+Ihhfl/fl knockout mice (n = 6). (K) The bar chart shows the percentage of icTCR-β+ cells in the DN3 population, relative to the mean percentage of icTCR-β+ cells in the DN3 population in WT littermates, for vavCre−Ihhfl/fl WT (n = 14) and vavCre+ihh fl/fl knockout mice (n = 6). A significant increase in icTCR-β expression was observed in vavCre+Ihhfl/fl knockout DN3 population, as determined by Student t test (P = .012).
Thymocyte Ihh expression is required for adult thymocyte development. (A,B) Expression of Ihh (A) and Gli1 (B) was assessed by quantitative RT-PCR in FACS-sorted populations of adult and fetal murine tissue. Levels of Ihh and Gli1 transcription were normalized for HPRT mRNA content and are shown relative to HPRT normalized transcription in the CD25+ DN population (Ihh) and DN1 population (Gli1). Thymocytes were sorted on a Modular Flow Cytometer (MoFlo; Dako North America), and purity was more than 98%. (C) Relative thymocyte number (relative to the mean of WT littermates) of 3-week-old WT (n = 8) and Ihh+/− (n = 8) littermates revealed a significant difference in SD as judged by F test (P < .001). Mean thymocyte number: litter 1 WT 1.2 × 108, Ihh+/− 1.77 × 108; litter 2 WT 1.44 × 108, Ihh+/− 1.76 × 108; litter 3 WT 1.35 × 108, Ihh+/− 1.81 × 108. (D) Transcription of Ihh in DP thymocytes from Ihh+/+ and Ihh+/− thymi. Relative levels of Ihh transcription were measured as described in Figure 1A and were normalized for HPRT mRNA content. (E) Excision of the Ihh gene as assessed by PCR from vavCre− (lane1) and vavCre+ Ihhfl/fl mice (lane 2). (F) Relative cell number (calculated relative to the mean of the WT littermates) of the thymus of 4- to 6-week-old mice of littermates (all Cre−: nonknockout mice, n = 9) compared with knockout mice (vavCre+Ihhflnull, n = 4) showed a significant decrease in thymocyte cell number (P = .003). Mean thymocyte number: litter 1 littermate (LM) 1.7 × 108, vavCre+Ihhflnull 0.75 × 108; litter 2 LM 1.95 × 108, vavCre+Ihhflnull 0.47 × 108; litter 3 LM 1.48 × 108, vavCre+Ihhflnull 1.28 × 108; litter 4 LM 1.2 × 108, vavCre+Ihhflnull 9.2 × 107. (G) Relative cell number (calculated as in panel D) of the thymus isolated from 4- to 6-week-old mice of littermate vavCre−Ihhfl/fl mice (WT, n = 15) compared with vavCre+Ihhfl/fl mice (knockout mice, n = 5) showed a significant decrease in thymocyte number (P = .016). Mean thymocyte numbers: litter 1 vavCre−Ihhfl/fl 3 × 108, vavCre+Ihhfl/fl 1.925 × 108; litter 2 vavCre−Ihhfl/fl 3.24 × 108, vavCre+Ihhfl/fl 2.59 × 108; litter 3 vavCre−Ihhfl/fl 2.115 × 108, vavCre+Ihhfl/fl 1.3 × 108; litter 4 vavCre−Ihhfl/fl 2.8 × 108, vavCre+Ihhfl/fl 1.75 × 108. (H) FACS analysis of CD44 and CD25 expression on CD4−8−3− thymocytes from vavCre−Ihhfl/fl and vavCre+Ihhfl/fl mice revealed reduced transition to the DN4 stage in development. (I) Histogram shows the percentage of DN4 cells in the DN population, relative to the mean percentage in Cre− littermates for vavCre−Ihhfl/fl (n = 13) and vavCre+Ihhfl/fl knockout mice (n = 6). *Significant difference by Student t test (P = .028). (J) Intracellular TCR-β expression was analyzed in DN3 thymocytes from littermate vavCre−Ihhfl/fl WT (n = 14) and vavCre+Ihhfl/fl knockout mice (n = 6). (K) The bar chart shows the percentage of icTCR-β+ cells in the DN3 population, relative to the mean percentage of icTCR-β+ cells in the DN3 population in WT littermates, for vavCre−Ihhfl/fl WT (n = 14) and vavCre+ihh fl/fl knockout mice (n = 6). A significant increase in icTCR-β expression was observed in vavCre+Ihhfl/fl knockout DN3 population, as determined by Student t test (P = .012).
To determine whether Ihh is also a negative regulator of adult thymus homeostasis, we compared the thymus of adult inbred Ihh+/− and WT littermates. As seen in the fetal heterozygote thymus (Figures 3A, 4E), the upper limit to Ihh+/− thymocyte number was approximately 2-fold greater than in the WT littermates (Figure 6C), there was more variation between individual thymi, and Ihh transcription in Ihh+/− DP cells was one-half that in Ihh+/+ DP cells (Figure 6D), consistent with negative feedback of Ihh from the DP population on their DN progenitors.
Ihh signaling in the adult Ihh conditional knockout thymus
To further study the role of Ihh produced by the thymocytes, rather than the epithelium, on T-cell development in the adult thymus, we used 2 different conditional (“floxed”) Ihh mouse models.15 Excision was mediated by transgenic Cre in either the vav or CD2 transgenic cassettes.27 The vav cassette drives Cre expression in all hematopoietic cells, including thymocytes at all stages of their development, but not thymic epithelium. The CD2 transgenic cassette drives Cre expression in lymphocytes only, with partial expression in thymocytes starting at the DN2 stage and complete expression in all thymocytes only from the DN4 stage onward.27
In the case of the vavCre model, we confirmed efficient excision of the floxed Ihh allele by PCR amplification of the Ihh gene from thymocyte DNA from vavCre− Ihhfl/fl and vavCre+ Ihhfl/fl littermates (Figure 6E). We compared mice either in which both Ihh alleles were floxed and excised by vavCre expression (vavCre+Ihhfl/fl) or in which one allele was null and the other floxed and excised (vavCre+Ihhfl/−) and found a significant reduction in thymocyte number in both cases (Figure 6F,G). To demonstrate functionally that Ihh produced by thymocytes can mediate the early positive signal for differentiation, we assessed the DN populations in vavCre−Ihhfl/fl (littermate) and vavCre+Ihhfl/fl littermates (in which both alleles of Ihh are present in the thymus epithelium). In the vavCre+Ihhfl/fl thymus where Cre expression is complete at the DN1 stage,27 there was a significant reduction in the DN4 population (from 39.4% to 20.1%) and a concomitant increase in the proportion of DN3 cells (from 41.4% to 57.7%) (Figure 6H,I). As seen in the fetal Ihh−/− thymus, a slightly higher proportion of the DN3 population expressed icTCR-β than in the corresponding littermate DN3 population (Figure 6J,K), indicating that TCR-β locus rearrangement was not limiting DP cell production.
For the CD2Cre model, we confirmed efficient excision of the floxed Ihh allele by PCR amplification of the Ihh gene from thymocyte DNA from CD2Cre−Ihhfl/fl and CD2Cre+Ihhfl/fl littermates (Figure 7A). Analysis of the CD2Cre+Ihhfl/fl mice showed that, on average, thymi were larger and the upper limit of thymocyte number was increased relative to littermates (Figure 7B), indicating loss of negative regulation. However, there was no significant difference in the proportion of DN3 and DN4 cells (Figure 7C,D), indicating that the earlier positive signal was not affected. Thus, in the CD2Cre+Ihhfl/fl mice, where excision of Ihh happens at a later developmental stage than in the vavCre+Ihhfl/fl mice, Ihh's positive regulatory role before pre-TCR signaling remains intact, whereas its later negative regulatory role is compromised. The fact that, in contrast to the vavCre+Ihhfl/fl mice, DN populations were not affected in the CD2Cre+Ihhfl/fl is consistent with the later excision described in the CD2Cre model27 and indicates that Ihh production by the early DN populations provides an autocrine signal. Both conditional knockout models therefore demonstrate the functional importance of Ihh secreted by the thymocytes themselves. In both cases, some Ihh will still be produced by the thymic epithelium, but this is insufficient to provide either the full early positive signals or subsequent negative regulatory signals that control thymus homeostasis, hence the phenotypes observed.
Ihh produced by thymocytes signals to negatively regulate differentiation from DN to DP in the adult. (A) Excision of the Ihh gene as assessed by PCR from CD2Cre− (lane 1) and CD2Cre+ Ihhfl/fl (lane 2) thymocytes. (B) Relative thymocyte number (relative to the mean of WT littermates) of 4- to 6-week-old CD2Cre− and vavCre+ Ihhfl/fl littermates showed a significant difference in SD, by F test (P = .001). Mean thymocyte number: litter 1, CD2Cre−Ihhfl/fl 3.77 × 108, CD2Cre+Ihhfl/fl 4.225 × 108; litter 2, CD2Cre−Ihhfl/fl 1.03 × 108, CD2Cre+Ihhfl/fl 1.245 × 108; litter 3, CD2Cre−Ihhfl/fl 2.84 × 108, CD2Cre+Ihhfl/fl 3.575 × 108; litter 4, CD2Cre−Ihhfl/fl 1.08 × 108, CD2Cre+Ihhfl/fl1.34 × 108; litter 5, CD2Cre−Ihhfl/fl 2.67 × 108, CD2Cre+Ihhfl/fl 3.31 × 108. (C) FACS analysis of CD44 and CD25 expression on CD4−8−3− thymocytes from CD2Cre−Ihhfl/fl and CD2Cre+Ihhfl/fl mice. (D) Histogram shows the percentage of DN4 cells in the DN population, relative to the mean percentage in Cre− littermates for CD2Cre−Ihhfl/fl (n = 13) and CD2Cre+Ihhfl/fl knockout mice (n = 6). The differences were not significant by Student t test. (E) Model of functions of Shh and Ihh signaling in the control of thymocyte ho-meostasis.
Ihh produced by thymocytes signals to negatively regulate differentiation from DN to DP in the adult. (A) Excision of the Ihh gene as assessed by PCR from CD2Cre− (lane 1) and CD2Cre+ Ihhfl/fl (lane 2) thymocytes. (B) Relative thymocyte number (relative to the mean of WT littermates) of 4- to 6-week-old CD2Cre− and vavCre+ Ihhfl/fl littermates showed a significant difference in SD, by F test (P = .001). Mean thymocyte number: litter 1, CD2Cre−Ihhfl/fl 3.77 × 108, CD2Cre+Ihhfl/fl 4.225 × 108; litter 2, CD2Cre−Ihhfl/fl 1.03 × 108, CD2Cre+Ihhfl/fl 1.245 × 108; litter 3, CD2Cre−Ihhfl/fl 2.84 × 108, CD2Cre+Ihhfl/fl 3.575 × 108; litter 4, CD2Cre−Ihhfl/fl 1.08 × 108, CD2Cre+Ihhfl/fl1.34 × 108; litter 5, CD2Cre−Ihhfl/fl 2.67 × 108, CD2Cre+Ihhfl/fl 3.31 × 108. (C) FACS analysis of CD44 and CD25 expression on CD4−8−3− thymocytes from CD2Cre−Ihhfl/fl and CD2Cre+Ihhfl/fl mice. (D) Histogram shows the percentage of DN4 cells in the DN population, relative to the mean percentage in Cre− littermates for CD2Cre−Ihhfl/fl (n = 13) and CD2Cre+Ihhfl/fl knockout mice (n = 6). The differences were not significant by Student t test. (E) Model of functions of Shh and Ihh signaling in the control of thymocyte ho-meostasis.
Discussion
Here we show that the secreted signaling molecule Ihh regulates T-cell development, influencing thymus homeostasis and thymocyte number in both adult and fetus. Mice mutant in genes encoding several other secreted signaling molecules have reduced thymocyte numbers,4,5 but the phenotype of Ihh mutant mice is unusual in that, although thymocyte number and the transition to DP cell were reduced in the Ihh−/− thymus, they were actually increased in the Ihh+/− thymus, suggesting a novel role for Ihh in thymocyte homeostasis.
Analysis of both heterozygote and conditional Ihh knockout models showed that expression of Ihh by thymocytes is required to restrict the size of the thymus by transmitting negative signals to limit thymocyte number, and in vitro treatment of Ihh+/− FTOC with r-Hh protein restored this negative regulatory function.
The fact that Ihh was most highly expressed by the DP population that have down-regulated both Gli1 and Smo transcription10,21,22 and are not Hh-responsive10 suggests that Ihh regulates thymus homeostasis by providing a negative feedback loop on the production of DP cells (Figure 7C). Analysis of proliferation of the different thymocyte subsets indicated that this feedback targets the DN3 population. In addition, analysis of vavCre+Ihhfl/fl and CD2Cre+Ihhfl/fl thymi, and of Ihh+/−Rag−/− FTOCs showed that Ihh provides a positive signal for differentiation before pre-TCR signal transduction and a negative signal after pre-TCR signal transduction. Hh signaling is necessary for efficient generation of DP cells, as evidenced by the reduction in DP cells in the Shh−/−, Ihh−/−, and Ihh−/−Shh+/− thymi and by the increase in DP production in vitro by treatment of Ihh−/− FTOCs with exogenous r-Hh. Both Shh made by the thymus epithelium5,10,20 and Ihh made by thymocytes and epithelium promote early thymocyte differentiation before pre-TCR signal transduction. However, as the production of DP and SP cells increases (both of which express higher levels of Ihh than their DN progenitors), so would the concentration of Ihh, which could then, having reached a critical threshold, provide negative feedback on the DN population, preventing the thymus growing ad infinitum. In the Ihh+/− thymus, the concentration of Ihh produced would be one-half of the physiologic level produced in the WT thymus, allowing the thymus to grow approximately 2 times larger, before becoming subject to the “normal” (WT) levels of negative regulatory feedback. Analysis of the E16.5 fetal thymus facilitated detection of this negative feedback, as the first wave of production of DP thymocytes is synchronized.
Studies of conditional Smo knockout thymi showed that Hh pathway activation in thymocytes is essential for their survival, proliferation, and differentiation at the transition from DN1 to DN2.21 Our analysis of double mutants indicated that, although Shh secreted by the epithelium is dominant in providing this signal, Ihh, produced by thymocytes and stroma, has a redundant function to promote differentiation at the transition from DN1 to DN2. In addition, the double-mutant analysis on E16.5 enabled a clear distinction between the positive and negative regulatory functions to be made, as removal of one copy of Ihh from the Shh−/− alleviated, rather than aggravated, the Shh−/− phenotype and increased the production of DP cells, indicating that the negative regulatory action of Ihh is at a later developmental stage than the positive function of Shh. These different requirements for Shh and Ihh may also be the result of differences in concentrations of the 2 proteins. They are also probably the result of the different spatial and temporal expression patterns of Shh and Ihh,10,20 and are consistent with Ihh being expressed most highly by DP cells, after Shh is required.
The phenotype of the Ihh+/− and Ihh−/− mice provides an explanation for an apparent discrepancy between data obtained from in vitro experiments10 and from the ex vivo analysis of Shh−/−5 and conditional Smo knockout thymi.21 In FTOCs, partial removal of endogenous Hh activity by treatment with neutralizing anti-Hh antibody (which can bind both Shh and Ihh) increased differentiation from DN to DP cell.10 Likewise, the development of human CD34+ thymocyte progenitors in vitro was accelerated by neutralization of Hh signaling.24 These observations seemed to conflict with the phenotype of the Shh−/− thymus, which showed reduced differentiation from DN to DP cell.5 Our analysis of Ihh mutant embryos demonstrates that in vivo T-cell development is regulated by the overall concentration of Hh protein that the developing thymocyte receives and that, in WT thyme, Ihh protein can actually function as a brake on DP cell production (explaining the impact of the reduction in Hh signaling by treatment with neutralizing anti-Hh antibody in WT FTOCs). Clearly, the concentration of Ihh protein that a given thymocyte receives will depend on the architecture of the thymus and the position of that cell in the thymus, relative to Ihh-producing cells.
Conditional deletion of Smo from T-lineage cells, using transgenic Cre under the control of the lck promoter,21 did not show an influence of Hh signaling on T-cell development after the DN2 stage, whereas the data presented here demonstrate that Ihh regulates the rate of differentiation from DN3 to DP cell. It would not be possible to detect the negative regulatory function of Ihh on the DN to DP transition in the conditional Smo knockout, as the Smo-deficient DN3 population would be unable to transduce any Ihh signal. Consistent with our model, in which Ihh production by DP cells feeds back to signal to the DN population, deletion of Smo using transgenic Cre controlled by the CD4 promoter had no effect on T-cell development, confirming that the DP cells are not themselves responding to the Ihh they secrete.21
The fact that Hh pathway activation in thymocytes increases differentiation and expansion at the earliest stages of their development but reduces differentiation and proliferation at later developmental stages might seem surprising but has parallels in the development of other tissues, such as gut and retina.32,33 For example, in retinal development, Hh signaling has different effects at different stages of development and has been shown to push precursor cells out of the cell cycle, to signal for differentiation at a distinct stage, and can also promote proliferation.32,34-36 A cell's interpretation of the Hh signal will depend on many factors, including strength and duration of signal and the external and intracellular context of signal transduction,7,16,37 and so Hh signaling may affect stem, progenitor, and mature cells differently and have opposing effects on cellular processes, depending on the state of differentiation of the cell.
In the future, it will be important to identify the molecular mechanisms and transcriptional targets that account for the positive and negative regulatory functions of Hh signaling on thymocyte development. Both cell-cycle inhibitors, such as cdkn1 and cdkn2 family molecules, and factors required for cell-cycle progression, such as cyclin D2, are Hh target genes in other cell types.32,36,38,39 It is therefore possible that cell-cycle inhibitors are transcriptional targets of the Hh pathway accounting for the negative regulatory function, whereas cyclin D2 is an Hh target in the DN2 population, accounting for the earlier positive regulatory function. The Hedgehog signaling pathway interacts with bone morphogenetic protein (BMP) and Wnt signaling pathways in the development of other tissues.19,40 BMP 2/4 signaling has previously been shown to negatively regulate thymocyte development,41-43 but Wnt signaling promotes thymocyte development.4 It is therefore possible that the opposing positive and negative functions of Ihh in thymocyte development are mediated in part by Wnt and BMP 2/4 signaling, respectively.
In conclusion, we show that Ihh produced by thymocytes promotes T-cell development before pre-TCR signal transduction and limits T-cell development after pre-TCR signal transduction in a concentration-dependent manner, thereby restricting thymocyte production and thymus size.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Derek Davies (Cancer Research UK, London, United Kingdom) for cell sorting.
This work was supported by The Wellcome Trust, Medical Research Council, Biotechnology and Biological Sciences Research Council, and Leukemia Research Fund. N.J.R. was supported by a Foulkes Foundation Fellowship.
Wellcome Trust
Authorship
Contribution: S.V.O., A.L.H.-T., D.K.S., and T.C. prepared the manuscript and carried out experiments; and N.J.R., E.D., S.E.R., B.L., and J.T.D. contributed experimentally.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tessa Crompton, Immunobiology Unit, UCL Institute of Child Health, 30 Guilford Street, London WC1N 1EH, United Kingdom; e-mail: t.crompton@ich.ucl.ac.uk.
References
Author notes
*S.V.O., A.L.H.-T., and D.K.S. contributed equally to this study.










 : thymocyte number (relative to mean of WT) from E17.5 WT littermate and Ihh+/− embryos. Ihh+/− thymi are grouped by relative size into 3 sets: I, more than 1.3; II, 0.9 to 1.1; and III, less than 0.9; ○: the percentage of cells in S + G2/M (relative to mean of WT), measured by PI staining of FACS-sorted E17.5 CD25+ from WT and Ihh+/− mice grouped into 3 groups I, II, and III as detailed in this figure legend.
: thymocyte number (relative to mean of WT) from E17.5 WT littermate and Ihh+/− embryos. Ihh+/− thymi are grouped by relative size into 3 sets: I, more than 1.3; II, 0.9 to 1.1; and III, less than 0.9; ○: the percentage of cells in S + G2/M (relative to mean of WT), measured by PI staining of FACS-sorted E17.5 CD25+ from WT and Ihh+/− mice grouped into 3 groups I, II, and III as detailed in this figure legend.