Abstract
T cells possess a p38 activation alternative pathway in which stimulation via the antigen receptor (T-cell receptor [TCR]) induces phosphorylation of p38α and β on Tyr323. To assess the contribution of this pathway to normal T-cell function, we generated p38α knockin mice in which Tyr323 was replaced with Phe (p38αY323F). TCR-mediated stimulation failed to activate p38αY323F as measured by phosphorylation of the Thr-Glu-Tyr activation motif and p38α catalytic activity. Cell-cycle entry was delayed in TCR-stimulated p38αY323F T cells, which also produced less interferon (IFN)–γ than wild-type T cells in response to TCR-mediated but not TCR-independent stimuli. p38αY323F mice immunized with T-helper 1 (Th1)–inducing antigens generated normal Th1 effector cells, but these cells produced less IFN-γ than wild-type cells when stimulated through the TCR. Thus, the Tyr323-dependent pathway and not the classic mitogen-activated protein (MAP) kinase cascade is the physiologic means of p38α activation through the TCR and is necessary for normal Th1 function but not Th1 generation.
Introduction
There are 4 p38 mitogen-activated protein kinase (MAPK) isoforms, α, β, γ, and δ, each encoded by a separate gene. p38α and p38β are widely expressed and share 74% identity at the amino acid level.1 The expression of p38γ is restricted largely to skeletal muscle,2 whereas p38δ is found in salivary glands, the pituitary gland, and the adrenals.3 p38α is the major isoform in T cells, which express lesser amounts of p38β and p38δ.4 The authors of many studies have argued for an important role for p38, primarily the α isoform, in T-cell activation. For example, p38α is implicated as having a positive role in T-cell proliferation because p38α/p38β-specific inhibitors partially inhibit T-cell receptor (TCR)–mediated T-cell proliferation.5,6 Furthermore, mice lacking Gadd45α have constitutively active p38α, and their T cells are hyperproliferative when activated via the TCR.7,8 Treatment with p38 inhibitors or expression of a dominant negative p38 also have indicated that p38 is involved in signaling for interferon (IFN)–γ production by T-helper 1 (Th1) effector cells.9 The authors of studies with p38α-deficient T cells, however, found that these cells were able to develop and proliferate normally10 and that TCR-induced IFN-γ production was intact but interleukin (IL)–12 plus IL-18–induced IFN-γ was impaired.11 Hence, there is evidence for the importance of p38 in cytokine-driven IFN-γ expression, but its role in TCR-induced Th1-cell polarization and function, such as inflammatory cytokine production, needs to be clarified.
Like all MAPKs, p38α is activated by a kinase cascade that eventuates in the phosphorylation of 2 residues in its activation loop (in this case, Thr180 and Tyr182).12 This pathway can be initiated by many events, including stress (eg, osmotic shock, heat, ultraviolet radiation) and stimulation with cytokines such as tumor necrosis factor (TNF)–α and IL-1β.13 The most membrane-proximal kinases are MAPK kinase kinases (MAPKKKs), which phosphorylate and activate MAPK kinases (MKKs). The principle direct upstream activators of p38 are the dual-specificity kinases MKK3 and MKK6.14 Dual phosphorylation of p38 results in conformational changes that enhance its binding to substrate and catalytic activity.15 Two MAPK cascade-independent mechanisms leading to p38 activation recently have been described. TAK1-binding protein 1 (TAB1) binds p38α, but not other p38 isoforms, and induces autophosphorylation of the Thr180/Tyr182 motif.16 The physiologic relevance of this pathway is unclear. Another pathway leading to p38α and p38β activation was described in TCR-activated T cells, in which residue Tyr323 is phosphorylated by ZAP-70, leading to p38 autophosphorylation of the activation loop and increased activity toward substrates such as ATF-2.17 Moreover, Tyr323 phosphorylation was found to be the major mechanism of TCR-induced p38 activation in Jurkat T cells because activation of a p38 mutant in which a phenylalanine was substituted for Tyr323 was markedly impaired.
The relative importance of the TCR-mediated alternative activation pathway versus the MAPK cascade and the possible contribution of costimulatory signals are the subjects of this report. We generated p38α knockin mice in which Tyr323 was replaced with a mutant containing Phe323 (p38αY323F). We find that activation of p38α in TCR-stimulated T cells absolutely requires Tyr323 phosphorylation and that, in its absence, there is a delay in cell-cycle entry and impaired IFN-γ production in vitro and in response to Th1-skewing antigens in vivo.
Methods
Generation of p38αY323F mice
BAC-DNA (clone RP23-369C13), containing p38α gene segment, was obtained from BACPAC Resource Center (Oakland, CA). A 9.3-kb region from EcoRI site, upstream of exon 10, to AvaI site, downstream of exon 12, was used to construct a targeting vector consisting of neo-resistance cassette flanked by 2 loxP sites, into which Y323F mutation was inserted with the use of QuickChange PCR mutagenesis system from Stratagene (mutagenic primers were 5′-GCCTGTTGCTGACCCTTTTGACCAGTCCTTTGAAAG-3′ and 5′-CTTTCAAAGGACTGGTCAAAAGGGTCAGCAACAGGC-5′). Introduction of Y323F mutation generated new BclI-specific restriction site in p38αY323F knockin allele that was used for screening of correctly targeted clones. Long-template polymerase chain reaction (PCR) was used to amplify a product consisting of a part of targeted fragment downstream of Neo-resistance gene, the site of mutation and 1115 bp of endogenous p38α gene. PCR product was treated with BclI enzyme, yielding undigested wild-type allele (4172 bp) and digested knockin allele (3546 bp and 626 bp) that are specific for presence of Y323F mutation. Primers used for long-template PCR were forward primer 5′-AACGGGGAAGAGCTCAAGATGCCAAC-′3 and reverse primer 5′-ATAGAGAACGTGGTACCCAACCATCGTCCC-′3. p38αY323F mice were bred for at least 6 generations onto C57BL/6 background and wild-type, p38αY323F littermates were used in experiments. Mice were maintained in a National Institutes of Health (NIH, Bethesda, MD) animal facility and experiments followed approved NIH animal study protocols.
Reagents
Antibodies against p38α, phospho-p38, phospho-MKK3/6, phospho-MEK1/2, MEK1/2, MKK3/6, and phospho-ATF-2 were purchased from Cell Signaling Technology (Beverly, MA); anti-p38β, anti–T-bet, and anti–GATA-3 were from Santa Cruz Biotechnology (Santa Cruz, CA); CD4, CD8, B220, CD25, CD44, CD69, TNF-α, IL-2, IFN-γ, and IL-4 antibodies were from BD Pharmingen (Franklin Lakes, NJ); and phospho-Tyr323 p38 was detected with specific rabbit antiserum.17 Anti–rabbit and anti–mouse antibodies conjugated to AlexaFluor680 or JRDye800 were obtained from Molecular Probes-Invitrogen (Carlsbad, CA) or Rockland Chemicals (West Caldwell, NJ), respectively. Phorbol 12-myristate 13-acetate (PMA), ionomycin, sorbitol, and propidium iodide were purchased from Sigma-Aldrich (St Louis, MO). Recombinant IL-12 and IL-18 were purchased from BD Pharmingen. Hoechst 33 342 (DNA stain) was purchased from Molecular Probes, and pyronin Y (RNA stain) from Sigma-Aldrich.
Toxoplasma gondii immunization
Mice were immunized twice (day 0 and day 4) with 20 μg of soluble extracts from T gondii tachyzoites (STAg) that were prepared as described,18 and killed on day 8. Splenocytes were cultured for 3 days in complete medium supplemented with 10 μg/mL STAg, washed, rested overnight, and stimulated either with plate-bound anti-CD3 or PMA (10 ng/mL) plus ionomycin (1 μg/mL) for 6 hours, the last 3 hours in presence of monensin (3 μmol/L).
T-cell and B-cell isolation and activation
T cells or B cells were purified from spleens and lymph nodes of 6- to 8-week-old mice by negative selection with mouse T-cell or B-cell immunocolumns (Cedarlane Laboratories, Burlington, NC). Cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 250 μg/mL gentamicin, 100 U/mL penicillin, 4 mmol/L glutamine, and 50 μmol/L 2-ME (complete medium). T cells were stimulated for indicated times with 5 μg/mL plate-bound anti-CD3 (145-2C11; Pharmingen) and 3 μg/mL anti-CD28 (37.51; Pharmingen), PMA (20 ng/mL), ionomycin (1 μg/mL), IL-12 (5 ng/mL), IL-18 (50 ng/mL), or 0.6 mol/L sorbitol. B cells were activated for 10 minutes with 10 μg/mL goat F(ab′)2 anti–mouse immunoglobulin M (IgM) antibody (Jackson ImmunoResearch, West Grove, PA).
Immunoblotting and kinase assays
Protein extracts were prepared from equivalent numbers of cells, and the protein concentrations were measured with Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA) and equalized. Lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes (Invitrogen Life Technologies, Carlsbad, CA), and immunoblotted with indicated antibodies. For kinase assays and p38 analysis, signals were detected with anti–rabbit or anti–mouse secondary antibodies and developed with the use of chemiluminescence (Pierce Chemical, Rockford, IL). MEK1/2 and MKK3/6 proteins were visualized with an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). For kinase assays, p38α or p38β were immunoprecipitated from cell lysates by the use of specific antibodies and Protein G-Sepharose 4B Fast Flow Beads (Sigma-Aldrich). Beads were washed twice with lysis buffer (phosphate-buffered saline [PBS] with 0.5% Triton X-100) and once in kinase buffer (25 mmol/L Tris, 5 mmol/L β-glycerophosphate, 1 mmol/L Na3VO4, 1 mmol/L NaF, and 10 mmol/L MgCl2) and incubated for 30 minutes at 30°C in 30 μL with 1 μg of glutathione-S-transferase (GST)–ATF-2 (Cell Signaling Technology) and 100 μmol/L ATP, followed by immunoblotting with anti-phospho-ATF-2 antibody.
Cell-cycle analysis
To track the mitotic divisions, purified T cells (0.1 × 106 per well; 2 replicate wells per point) were labeled with 10 μmol/L carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) for 10 minutes at 37°C, stimulated with anti-CD3/CD28 or PMA/ionomycin, and CFSE staining analyzed at the indicated times by flow cytometry.
Enzyme-linked immunosorbent assay
Supernatants from T cells treated with plate-bound anti-CD3/CD28 for various times were collected and IFN-γ was analyzed by enzyme-linked immunosorbent assay (ELISA) with reagents purchased from BD Biosciences (San Jose, CA). Mouse recombinant IFN-γ was obtained from National Institute of Allergy and Infectious Diseases Reference Reagent Repository (Gaithersburg, MD) and used as a standard.
Flow cytometry
For detection of intracellular cytokines, cells were treated with monensin (3 μmol/L) for 3 hours before harvesting, stained with anti-CD4 antibody, fixed with BD Cytofix/Cytoperm kit (BD Biosciences), and stained with anti–IFN-γ and anti–IL-4 in the presence of 2.4G2 antibody to block FcR binding. Flow cytometry was conducted with a FACSCalibur with the use of CellQuest software (BD Biosciences). To analyze DNA and RNA expression, stimulated cells were fixed as above at indicated time points and stained with 10 μg/mL Hoechst 33342 (DNA) followed by staining with 4 μg/mL pyronin Y (RNA). Fluorescence signal was detected with the use of BD LSR II Flow Cytometer and BD FACSDiVa software. All flow cytometric data analysis was performed with FlowJo software (TreeStar, Ashland, OR).
Results
Generation of p38α knockin (p38αY323F) mice
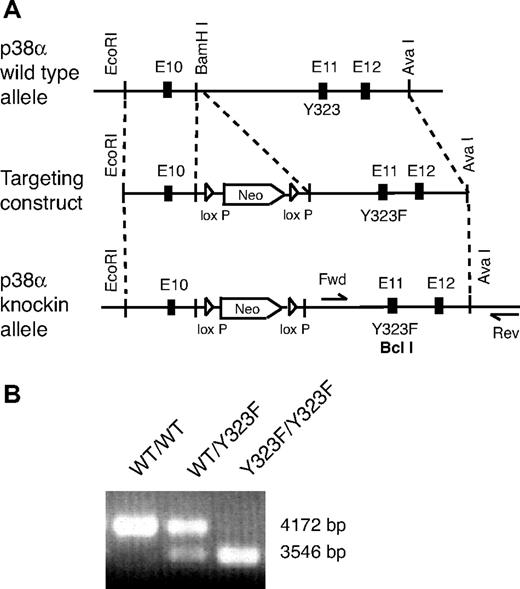
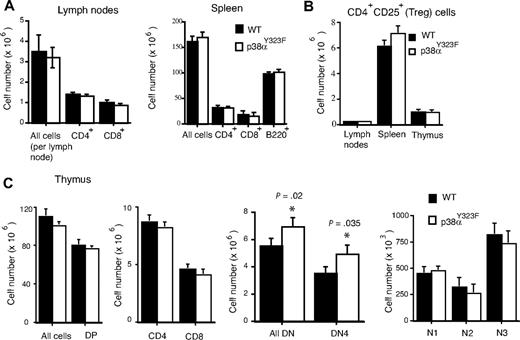
To investigate the importance of the Tyr323-dependent alternative pathway for T-cell activation in primary cells, we generated knockin (gene-targeted) mice in which Tyr323 of p38 was replaced with phenylalanine (Y323F). Tyr323 is encoded in p38α exon 11. A homologous recombinant targeting vector containing a neo-resistance cassette flanked by 2 loxP sites and the Y323F mutation was constructed. Y323F was introduced by generating a new BclI-specific restriction site in the knockin (p38αY323F) allele (Figure 1A). To obtain chimeric mice, (129 × C57BL/6)F1 ES cells were transfected with the targeting vector, and recombinant ES clones were injected into C57BL/6 blastocysts. The presence of the p38αY323F allele was determined by long-template PCR followed by digestion with the BclI restriction enzyme (Figure 1B). Both heterozygous and homozygous p38αY323F mice were viable, fertile, and of normal size and weight, which is in contrast to p38α knockout mice, which undergo perinatal demise as the result of placental insufficiency,19 and consistent with tissue-restricted use of the Tyr323 phosphorylation activation mechanism. The analysis of thymus, spleen, and lymph nodes revealed a normal complement of CD4+ and CD8+ cells (Figure 2A,C). The numbers of T regulatory cells (CD4+CD25+) in spleen, lymph nodes, and thymus also were comparable with those of wild-type mice (Figure 2B), as were the numbers of CD62LloCD44hi effector memory CD8+ T cells (data not shown). In mice expressing constitutively active MKK6 and therefore having active p38, there was a reduction in the progression of CD4−CD8− double negative (DN) CD44−CD25+ (DN3) to CD44−CD25− (DN4) thymocytes.20 Conversely, pharmacologic inhibition of p38 resulted in increased numbers of DN4 cells.21 We therefore performed a detailed analysis of CD44 and CD25 expression on DN thymocytes. We detected a small but statistically significant increase in the proportion of DN thymocytes in p38αY323F mice, which was entirely accounted for by the DN4 subset, with no statistically significant difference in other subsets (Figure 2C). These results are consistent with a role for the alternative p38 activation in pre-TCR signaling and inhibition of progression to the DN4 stage.
Generation of p38αY323F mice. (A) A 9.3-kb genomic fragment of p38α locus (top) was cloned with the use of AvaI- and EcoRI-specific restriction sites and used to create the targeting vector (middle), into which a Y323F mutation and LoxP-flanked neomycin resistance gene were inserted. Insertion of the Y323F mutation created a new BclI restriction site in the knockin allele. Positions of the forward (Fwd) and reverse (Rev) primers used in long template PCR are shown (bottom). (B) Genotyping of p38αY323F mice by long template PCR, followed by digestion with BclI restriction enzyme generated a nondigested 4172-bp wild-type (WT/WT) band and 3546-bp knockin (Y323F/Y323F) band together with a small 626-bp band (not shown).
Generation of p38αY323F mice. (A) A 9.3-kb genomic fragment of p38α locus (top) was cloned with the use of AvaI- and EcoRI-specific restriction sites and used to create the targeting vector (middle), into which a Y323F mutation and LoxP-flanked neomycin resistance gene were inserted. Insertion of the Y323F mutation created a new BclI restriction site in the knockin allele. Positions of the forward (Fwd) and reverse (Rev) primers used in long template PCR are shown (bottom). (B) Genotyping of p38αY323F mice by long template PCR, followed by digestion with BclI restriction enzyme generated a nondigested 4172-bp wild-type (WT/WT) band and 3546-bp knockin (Y323F/Y323F) band together with a small 626-bp band (not shown).
T-cell and thymocyte subsets. (A,B) Lymph nodes, spleen, and (C) thymus from wild-type and p38αY323F mice were homogenized. Cells were counted, then stained for CD4, CD8, and B220 (n = 10; bars indicate mean ± SEM). (C) Double-negative thymocytes were stained for CD44 and CD25 and the number of cells in different developmental stages are shown (n = 4; bars indicate mean ± SEM; P value was calculated with the Student t test). DN1 = CD44+CD25−, DN2 = CD44+CD25+, DN3 = CD44−CD25+, DN4 = CD44−CD25−.
T-cell and thymocyte subsets. (A,B) Lymph nodes, spleen, and (C) thymus from wild-type and p38αY323F mice were homogenized. Cells were counted, then stained for CD4, CD8, and B220 (n = 10; bars indicate mean ± SEM). (C) Double-negative thymocytes were stained for CD44 and CD25 and the number of cells in different developmental stages are shown (n = 4; bars indicate mean ± SEM; P value was calculated with the Student t test). DN1 = CD44+CD25−, DN2 = CD44+CD25+, DN3 = CD44−CD25+, DN4 = CD44−CD25−.
TCR- and costimulation-induced p38α activation require Tyr323 phosphorylation
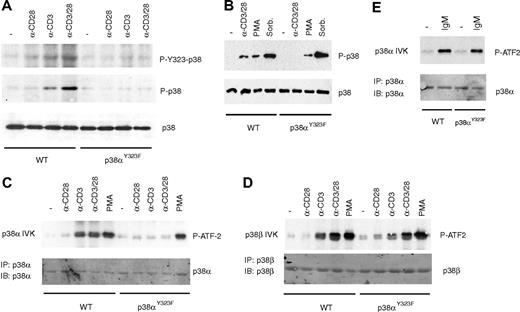
Experiments with Jurkat cells transiently expressing p38Y323F suggested that the alternative pathway is a major mechanism for activating p38α in response to TCR signaling.17 To determine its importance relative to the MAPK cascade in primary T cells, p38α phosphorylation and activation was assessed in p38αY323F T cells. T cells isolated from spleen and lymph nodes of wild-type or p38αY323F mice were stimulated with plate-bound anti-CD3 (α-CD3) and/or anti-CD28 antibodies or with stimuli that activate the MAPK cascade, such as PMA or hyperosmotic shock (Figure 3).22-24 Anti-CD3 induced Tyr323 as well as Thr180/Tyr182 phosphorylation, both of which were increased by costimulation with anti-CD28 (Figure 3A). In contrast, p38α was phosphorylated at none of these sites in TCR-stimulated p38αY323F T cells. Importantly, treatment with PMA or sorbitol caused p38 dual phosphorylation in both wild-type and p38αY323F T cells (Figure 3B), demonstrating that p38α activation via the MAPK cascade was unaffected by the Y323F substitution. T cells also express the Tyr323-containing p38β, albeit at lower levels than p38α.4
TCR stimulation with or without costimulation activates p38α through the alternative pathway. (A) T cells purified from spleen and lymph nodes of wild-type (WT) or p38αY323F mice were stimulated with plate-bound anti (α)-CD3 and/or α-CD28 antibodies (Abs) for 30 minutes. Whole lysates from 4 × 106 cells were immunoblotted with antibodies recognizing p38 phosphorylated on Tyr323 (P-Y323-p38), Thr180/Tyr182 (P-p38), or total p38. (B) WT and p38αY323F T cells were stimulated with α-CD3/CD28 Abs, PMA, or sorbitol, and phospho-p38 was detected in whole cell lysates. (C) p38α or (D) p38β were immunoprecipitated from WT and p38αY323F T cells stimulated with α-CD3/CD28 Abs or PMA, and in vitro kinase (IVK) assays of immune complexes with ATF-2 as a substrate were performed. (E) B cells purified from spleen of WT or p38αY323F mice were stimulated with anti-IgM antibody (10 μg/mL) for 10 minutes, p38α specifically immunoprecipitated from whole cell lysates, and kinase assays performed as in panel C.
TCR stimulation with or without costimulation activates p38α through the alternative pathway. (A) T cells purified from spleen and lymph nodes of wild-type (WT) or p38αY323F mice were stimulated with plate-bound anti (α)-CD3 and/or α-CD28 antibodies (Abs) for 30 minutes. Whole lysates from 4 × 106 cells were immunoblotted with antibodies recognizing p38 phosphorylated on Tyr323 (P-Y323-p38), Thr180/Tyr182 (P-p38), or total p38. (B) WT and p38αY323F T cells were stimulated with α-CD3/CD28 Abs, PMA, or sorbitol, and phospho-p38 was detected in whole cell lysates. (C) p38α or (D) p38β were immunoprecipitated from WT and p38αY323F T cells stimulated with α-CD3/CD28 Abs or PMA, and in vitro kinase (IVK) assays of immune complexes with ATF-2 as a substrate were performed. (E) B cells purified from spleen of WT or p38αY323F mice were stimulated with anti-IgM antibody (10 μg/mL) for 10 minutes, p38α specifically immunoprecipitated from whole cell lysates, and kinase assays performed as in panel C.
To determine whether the activation of this isoform was affected by expression of the p38α mutant, we immunoprecipitated p38α or p38β independently with specific antibodies, and then measured the phosphorylation of the substrate ATF-2. The absence of Tyr323 resulted in a lack of anti-TCR/CD28-induced but not PMA-induced p38α kinase activity (Figure 3C). In contrast, p38β kinase activity was increased to equal degrees in wild-type and p38αY323F T cells stimulated with anti-TCR/CD28 (Figure 3D). Despite the presence of Syk, a ZAP70 family member, p38 Tyr323 phosphorylation previously was found to be undetectable in antigen receptor–stimulated B cells.17 This issue was revisited with B cells purified from the spleens of wild-type and p38αY323F mice. The B cells were stimulated with anti-IgM and p38α immunoprecipitated from the lysates was assessed for kinase activity (Figure 3E). Wild-type p38α and p38αY323F were activated to a similar degree by BCR stimulation, confirming that p38α is not activated via the alternative pathway in antigen receptor–stimulated B cells.
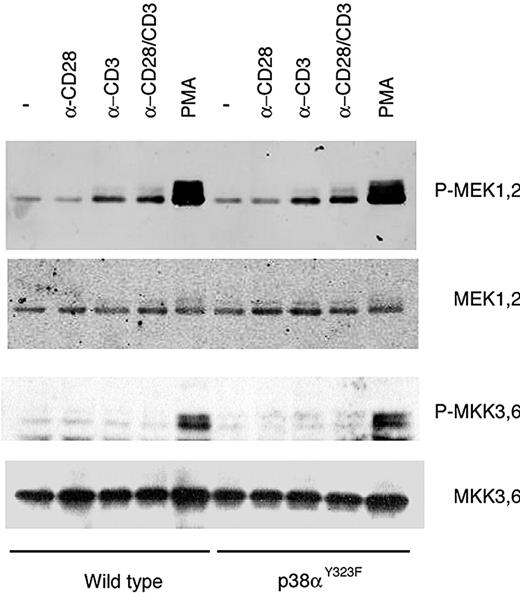
Although studies with Jurkat T leukemia cells have suggested that TCR plus costimulation through CD28 leads to induction of the MAPK cascade,25 dual phosphorylation of p38α was not detected in normal p38αY323F T cells after stimulation with anti-CD3/CD28 (Figure 3). To determine whether TCR and CD28 coengagement can initiate the MAPK cascade in primary T cells, the activation of MKKs was assessed. Stimulation of both wild-type and p38αY323F T cells resulted in the activation of MEK1/2, which are upstream of extracellular signal–regulated kinases (ERKs)26 although, unlike p38α, there was no difference between anti-CD3 and anti-CD3/CD28 stimulation (Figure 4). In contrast, TCR stimulation did not lead to detectable activation of MKK3/6, upstream regulators of p38.14 Thus, in normal cells, TCR engagement activates the MAPK cascade, leading to activation of ERK but not p38, which is consistent with the notion that the Tyr323-dependent alternative pathway is the major mechanism of p38α activation.
CD28 signaling does not stimulate p38 through the classic MAPK pathway. T cells purified from lymph nodes and spleen of wild-type or p38αY323F mice were stimulated with plate-bound α-CD3/CD28 for 5 minutes (MEK1/2 detection) or 30 minutes (MKK3/6). Phosphorylation of MEK1/2 and MKK3/6 was detected in whole cell lysates with the use of phosphospecific antibodies.
CD28 signaling does not stimulate p38 through the classic MAPK pathway. T cells purified from lymph nodes and spleen of wild-type or p38αY323F mice were stimulated with plate-bound α-CD3/CD28 for 5 minutes (MEK1/2 detection) or 30 minutes (MKK3/6). Phosphorylation of MEK1/2 and MKK3/6 was detected in whole cell lysates with the use of phosphospecific antibodies.
The onset of TCR-signaled proliferation is delayed in p38αY323F T cells
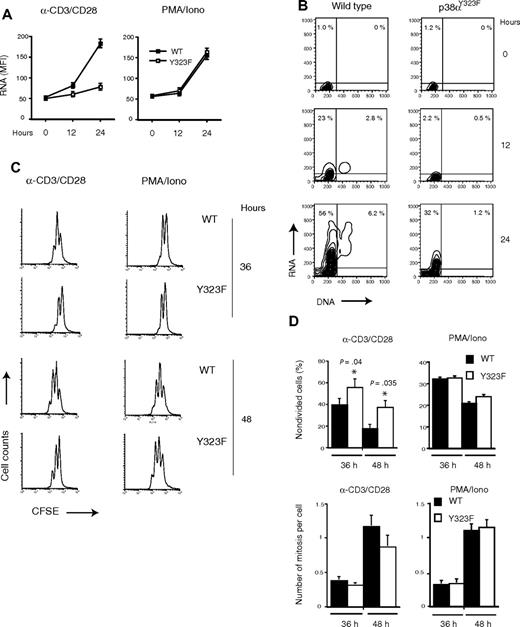
The majority of T cells isolated from secondary lymphoid tissues are in the G0 stage of the cell cycle. Mitogenic stimulation induces a transition from G0 to G1, followed by DNA synthesis (the G1/S transition).27 On the basis of pharmacologic inhibition and the presence of hyperactive p38 in Gadd45α-deficient mice, it has been suggested that p38 is a positive regulator of T-cell proliferation.4 However, p38α-deficient T cells in chimeric RAG-deficient mice were reported to proliferate normally as judged by [3H]thymidine incorporation.10 The fact that p38αY323F is not activated by TCR-mediated signaling allowed us to investigate its role in normal T-cell proliferation. The up-regulation of RNA levels is one of the events that define the movement of cells from G0 to G1.28 T cells purified from lymph nodes and spleen were stimulated with anti-CD3/CD28 or PMA and ionomycin. After paraformaldehyde fixation, RNA was stained with pyronin Y, and the cells were analyzed by flow cytometry (Figure 5A). A modest increase in intracellular RNA was detectable in wild-type cells after 12 hours of TCR stimulation but only minimal up-regulation in p38αY323F T cells. By 24 hours, RNA levels were markedly increased in the majority of wild-type T cells but only modestly so in p38αY323F T cells. By 48 hours, the RNA levels were comparable between T cells of each genotype (data not shown). Cells that were stimulated with PMA/ionomycin, however, exhibited similar increases in RNA content regardless of which p38α isoform they expressed (Figure 5A, right panel). Similar results were obtained when RNA and DNA levels were concomitantly measured: p38αY323F T cells had delayed entry into both G1 and S phase (Figure 5B). Cell division, as measured by CFSE dilution, was undetectable at 24 hours, but by 36 hours, anti-CD3/CD28 treated cells were found to divide (Figure 5C left panels). There was a reproducible lag for p38αY323F T cells that was reflected in a lower number of average divisions per cell even after 48 hours. In contrast, there was no difference in cell division between wild-type and p38αY323F T cells when the TCR-independent stimulus PMA and ionomycin was used (Figure 5C right panels). The proportion of nondivided cells and the average number of mitoses per cell are summarized in Figure 5D. A delay in RNA up-regulation and entry into cell cycle also was found when p38αY323F T cells that were stimulated with either alloantigen or superantigen, despite similar degrees of activation as assessed by up-regulation of the p38-independent CD69 (data not shown).29 No differences between TCR-stimulated wild-type and p38αY323F T cells in cell death, as assessed by propidium iodide staining, 7-amino-actinomycin D (7-AAD) staining, or accumulation of subdiploid DNA, were observed (data not shown). Therefore, the failure to activate T-cell p38α results in a modest delay in entry into the cell cycle, but once this checkpoint is past the cells undergo apparently normal division.
Onset of proliferation is delayed in p38αY323F T cells stimulated through TCR/CD28. (A) Wild-type (■; WT) and p38 αY323F (□; Y323F) T cells were stimulated with plate-bound anti-CD3/CD28 antibodies or with PMA plus ionomycin (PMA/Iono) for the indicated times, fixed, and RNA were stained with pyronin Y. Error bars represent SEM (3 replicates per condition). (B) WT and p38αY323F T cells were stimulated with plate-bound anti-CD3/CD28 antibodies for the indicated times, fixed, and DNA (Hoechst 33342) and RNA (Pyronin Y) were stained. The percentages of positive cells are indicated for each upper quadrant (results are representative of 2 independent experiments). (C) WT and p38αY323F (Y323F) T cells were purified from spleen and lymph nodes, labeled with CFSE, and stimulated with plate-bound α-CD3/CD28 antibodies or PMA/Iono for indicated times. One of 4 independent experiments is shown. (D) Summary of percentage of nondivided cells and mitotic indexes of WT and p38αY323F (Y323F) T cells from panel C, n = 4; bars indicate mean (± SEM); P value was calculated using the Student t test.
Onset of proliferation is delayed in p38αY323F T cells stimulated through TCR/CD28. (A) Wild-type (■; WT) and p38 αY323F (□; Y323F) T cells were stimulated with plate-bound anti-CD3/CD28 antibodies or with PMA plus ionomycin (PMA/Iono) for the indicated times, fixed, and RNA were stained with pyronin Y. Error bars represent SEM (3 replicates per condition). (B) WT and p38αY323F T cells were stimulated with plate-bound anti-CD3/CD28 antibodies for the indicated times, fixed, and DNA (Hoechst 33342) and RNA (Pyronin Y) were stained. The percentages of positive cells are indicated for each upper quadrant (results are representative of 2 independent experiments). (C) WT and p38αY323F (Y323F) T cells were purified from spleen and lymph nodes, labeled with CFSE, and stimulated with plate-bound α-CD3/CD28 antibodies or PMA/Iono for indicated times. One of 4 independent experiments is shown. (D) Summary of percentage of nondivided cells and mitotic indexes of WT and p38αY323F (Y323F) T cells from panel C, n = 4; bars indicate mean (± SEM); P value was calculated using the Student t test.
IFN-γ production is decreased in TCR-activated p38αY323F cells
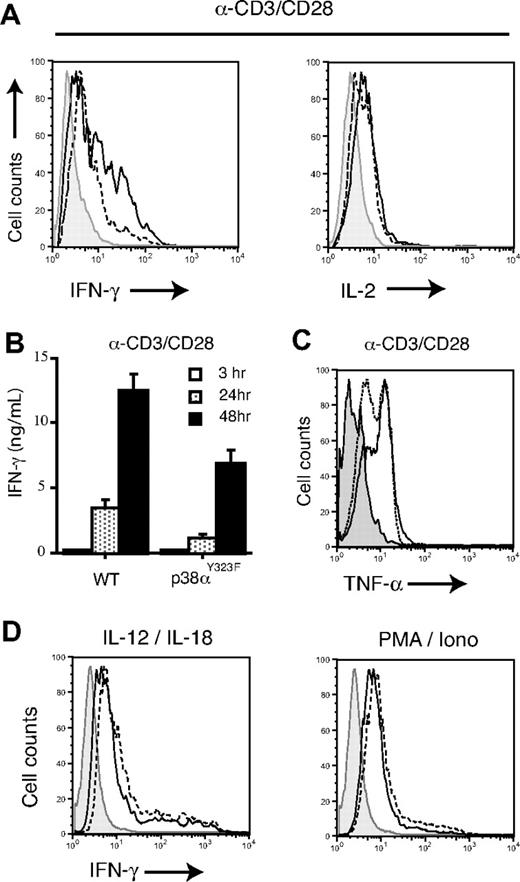
T-cell production of IFN-γ, but not IL-2, is dependent on p38 activity, as the former is suppressed by prevention of p38 activity with small molecule inhibitors or the expression of dominant negative p38α.9,30 To assess the role of the alternative p38 pathway, T cells from spleen and lymph nodes were stimulated with plate-bound anti-CD3/CD28 for 24 or 48 hours and cytokine levels quantitated. IL-2 production was similar between wild-type and p38αY323F T cells, but p38αY323F T-cell IFN-γ production was substantially less at 48 hours, both in terms of the number of IFN-γ–producing cells and the amount of IFN-γ produced per cell (Figure 6A). IFN-γ accumulation in the medium of stimulated p38αY323F T cells also was reduced (Figure 6B). Another p38-dependent cytokine, TNF-α,30 also was produced at lower levels by activated p38αY323F T cells (Figure 6C). Notably, 2 stimuli that induce T-cell IFN-γ production by stimulation of the classic MAPK cascade and MKK3/6 activation, IL-12 plus IL-18 and PMA plus ionomycin,11,22 induced equivalent IFN-γ production from wild-type and p38αY323F T cells (Figure 6D). Therefore, the T-cell alternative activation pathway is required for normal TCR-stimulated IFN-γ production.
TCR/CD28-induced IFN-γ expression is decreased in p38αY323F T cells. (A) Wild-type (solid line) and p38αY323F (dashed line) T cells were stimulated as indicated for 48 hours and IFN-γ and IL-2 expression were detected by intracellular staining (A) or ELISA (B). (C) TNF-α expression was detected by intracellular staining in T cells stimulated with α-CD3/CD28 for 48 hours. (D) T cells were treated with IL-12 plus IL-18 or PMA/Iono for 48 hours, and then IFN-γ production was analyzed by intracellular staining. Shaded histograms present stimulated wild-type cells stained with matched isotype control antibody only. One of 3 independent experiments is shown.
TCR/CD28-induced IFN-γ expression is decreased in p38αY323F T cells. (A) Wild-type (solid line) and p38αY323F (dashed line) T cells were stimulated as indicated for 48 hours and IFN-γ and IL-2 expression were detected by intracellular staining (A) or ELISA (B). (C) TNF-α expression was detected by intracellular staining in T cells stimulated with α-CD3/CD28 for 48 hours. (D) T cells were treated with IL-12 plus IL-18 or PMA/Iono for 48 hours, and then IFN-γ production was analyzed by intracellular staining. Shaded histograms present stimulated wild-type cells stained with matched isotype control antibody only. One of 3 independent experiments is shown.
In vivo Th1 function but not differentiation is dependent on p38α Tyr323 phosphorylation
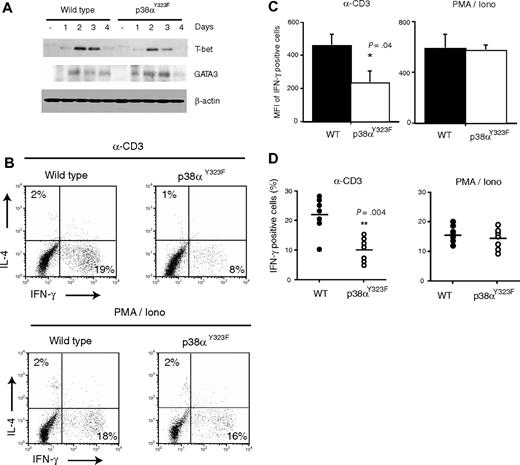
Whether p38 activity is required for T-cell differentiation to Th1 cells is controversial.9,10 IFN-γ–producing Th1-cell development is driven by transcription factors such as T-bet, whereas IL-4–producing Th2-cell development requires GATA-3.31,32 We analyzed the induction of these transcription factors by anti-CD3/CD28 in T cells cultured in the absence of skewing cytokines. Although T-bet levels were slightly lower in p38αY323F than wild-type T cells, the kinetics of expression were the same, with T-bet detectable on day 1, peaking at day 2, and decreasing thereafter (Figure 7A). GATA-3 expression followed similar kinetics, although its expression was slightly higher and more prolonged in p38αY323F compared with wild-type T cells. Despite the presence of GATA-3, we were unable to detect IL-4 protein on any day after stimulation (data not shown). Thus, both wild-type and p38αY323F cells express T-bet and GATA-3 upon TCR engagement, but in p38αY323F cells T-bet expression is modestly lower and GATA-3 correspondingly higher. This finding may reflect the lower levels of IFN-γ produced by p38αY323F T cells because IFN-γ up-regulates T-bet, which then suppresses GATA-3 activity.33
p38αY323F T cells polarize into Th1 cells in vivo but produce less IFN-γ upon TCR stimulation. (A) T-bet and GATA-3 expressions were analyzed in whole cell lysates of WT and p38αY323F T cells stimulated with plate-bound anti-CD3/CD28 Abs and harvested on the indicated days. (B) WT and p38αY323F mice were immunized with 20 μg of STAg and, 12 days later, splenocytes were restimulated with either anti-CD3 or PMA/Ion. IL-4 and IFN-γ in CD4+ T cells were analyzed by intracellular staining. The numbers indicate the frequency of positive cells in each quadrant. The data are representative for 1 of 2 independent experiments. (C) The expression of IFN-γ per responding cell (MFI) and (D) the percentage of CD4+ IFN-γ–positive T cells from individual immunized animals from 2 independent experiments are summarized (bars indicate the mean ± SEM; P value was calculated using the Student t test).
p38αY323F T cells polarize into Th1 cells in vivo but produce less IFN-γ upon TCR stimulation. (A) T-bet and GATA-3 expressions were analyzed in whole cell lysates of WT and p38αY323F T cells stimulated with plate-bound anti-CD3/CD28 Abs and harvested on the indicated days. (B) WT and p38αY323F mice were immunized with 20 μg of STAg and, 12 days later, splenocytes were restimulated with either anti-CD3 or PMA/Ion. IL-4 and IFN-γ in CD4+ T cells were analyzed by intracellular staining. The numbers indicate the frequency of positive cells in each quadrant. The data are representative for 1 of 2 independent experiments. (C) The expression of IFN-γ per responding cell (MFI) and (D) the percentage of CD4+ IFN-γ–positive T cells from individual immunized animals from 2 independent experiments are summarized (bars indicate the mean ± SEM; P value was calculated using the Student t test).
To determine whether the p38α alternative pathway is required for Th1 differentiation and function in vivo, wild-type and p38αY323F mice were immunized with soluble antigens isolated from T gondii tachyzoites (STAg), that induces a robust Th1 response in C57BL/6 mice.34 The animals were injected intraperitoneally with STAg on day 0, again on day 4, and killed on day 8. Splenocytes were cultured with STAg for 3 more days, washed, rested overnight, and restimulated either with plate-bound anti-CD3 or PMA plus ionomycin (Figure 7B). As expected, stimulation of wild-type T cells with anti-CD3 resulted in IFN-γ but little IL-4 expression. Notably, p38αY323F T cells consistently produced substantially less IFN-γ in response to stimulation via the TCR but equivalent amounts of IFN-γ when stimulated with the TCR-independent stimulus PMA and ionomycin. The levels of IFN-γ per responding cell and the percentage of IFN-γ positive cells from 2 independent experiments are shown in Figure 7C,D. Therefore, Th1 polarization occurred normally in p38αY323F mice as judged by the ability of the T cells to produce IFN-γ in response to PMA and ionomycin, but this manifestation of effector function was impaired in the Th1 stimulated through the TCR.
Discussion
The classic MAPK signaling cascade was long considered to be the only pathway leading to p38 activation. This pathway involves dual phosphorylation of a Thr-Glu-Tyr sequence located on a flexible loop termed the phosphorylation lip.35 Recently, 2 other pathways emerged, one involving an interaction with TAB1 and the other ZAP70-mediated phosphorylation of p38α and β on Tyr323 in TCR-signaled T cells. The structural bases of p38 activation via these newer pathways are largely unknown, although both TAB1- and ZAP70-induced activation seem to be dependent on autophosphorylation.17,36 Interestingly, dual phosphorylation of p38γ results in disordering of the same structural loop (L16) that contains Tyr323, and it has been suggested that disruption of this hydrophobic core and destabilization of L16 may be a general mechanism leading to p38 activation.37 Recently, the high-resolution crystal structures of p38α mutants that are spontaneously active revealed conformational changes in L16.38 Recently, several activating mutations were described in all p38 isoforms. Most of these mutations are located in L16 loop and induce conformational changes leading to substantial autophosphorylation activity.
These studies also suggest that the activation mechanism involves dimerization and subsequent trans autophosphorylation of Thr180.38 TCR-induced phosphorylation of p38α and β Tyr323 has been found in T cells but not in B cells, dendritic cells, fibroblasts, or keratinocytes,17,39-41 which is consistent with an obligatory role for ZAP70. Another factor implicated in the alternative pathway is that in TCR-activated cells the Dlgh1 scaffold protein seems to be necessary for bridging ZAP70 (and perhaps Lck) with p38 and subsequent phosphorylation of Tyr323.42 Whether regulated expression of Dlgh1 also contributes to the tissue specificity of the alternative pathway is not known.
The data on the importance of p38 signaling for T-cell proliferation are mixed. In studies in which p38 inhibitors were used, the authors found that TCR-mediated T-cell proliferation was reduced.5,6 However, experiments with p38α-deficient T cells suggested that proliferation is largely p38α independent.10,11 The results presented in this report demonstrate a subtle but clear role for p38α in the transition from G0 to G1. Once the G1 state was attained, entry into S phase and progression through mitosis appeared to be unimpaired. We propose that once a T cell has made the G0/G1 transition and is committed to proliferate, p38α activity is dispensable, which is consistent with normal production by IL-2 by stimulated p38αY323F T cells. Little is known about the mechanism of G0/G1 transition in T cells. However, because quiescent G0 T cells are in a state of low transcriptional activity, it is likely that transcriptional repressors are active. Several lines of evidence indicate that the products of the retinoblastoma tumor suppressor gene (Rb) may suppress the activity of E2F transcription factors and participate in the maintenance of G0.43 In G0 T cells, Rb is phosphorylated on Ser807/Ser811, and thereby inactivated, by cyclin C/cyclin-dependent kinase (Cdk) 3 and/or cyclin D/Cdk4,6 complexes within 5 hours of stimulation with anti-CD3/CD28, allowing exit from G0.44 p21, an inhibitor of Cdk3, Cdk4, as well as Cdk6 kinase activity in noncycling cells,45,46 can be directly phosphorylated on Ser130 by p38.47 This event has been proposed to relieve p21 inhibition of cyclin/Cdk,48,49 providing one possible mechanism for the ability of p38α to facilitate the G0/G1 transition. Whether direct p38α phosphorylation of molecules involved in regulation of cell-cycle entry or whether phosphorylation of intermediary molecules accounts for the delayed proliferation of p38αY323F T cells remains to be determined.
p38α and p38β (but not p38δ or p38γ) have a tyrosine at position 323, and both undergo activation via the alternative pathway.4,17 Consistent with this finding, we discovered that p38β immunoprecipitated from TCR-stimulated p38αY323F T cells was normally active. Nonetheless, we detected no dual-phosphorylated p38 species in lysates of TCR-activated p38αY323F T cells with a phosphospecific antibody that does not distinguish between the 2 p38 isoforms. Therefore, and consistent with published data on the relative levels in T cells,50 p38α is the major expressed isoform and p38β is present at low levels in T cells. It remains possible, however, that even low levels of p38β activity might contribute to the cellular response and that a p38αY323F/p38βY323F double-knockin mouse would have a more severe phenotype than the single p38α mutant. Efforts to produce such animals are under way.
The p38αY323F mice have been useful in distinguishing the role of T-cell p38α in Th1 differentiation versus IFN-γ production. It has been reported that the p38 inhibitors SB203580 and SC-409 blocked cytokine-mediated IFN-γ production by Th1 cells but did not prevent the activation or differentiation of new Th1 cells.30 p38α-deficient T and B cells developed and differentiated normally,10 and p38α-deficient CD4+ T cells polarized into Th1 effector cells in vitro although, in response to TCR-mediated stimulation, they expressed lower levels of IFN-γ, as manifested by a lower median fluorescence intensity of IFN-γ–positive cells by flow cytometry.11 We recently have shown that Th1 polarization in vivo is highly dependent on p38-dependent IL-12 production by dendritic cells and that the expression of constantly active p38α and β in T cells cannot restore Th1-cell differentiation in the absence of normal dendritic cell p38 activity.39 The findings in the present report demonstrate that TCR-mediated activation of p38α is dispensable for normal Th1 polarization in vivo. However, manifestation of this state, as defined by the secretion of IFN-γ in response to TCR signaling, is masked by the inability to activate p38α by the TCR-dependent alternative pathway. Therefore, p38α has positive and tissue-specific roles in Th1 generation (dendritic cells) and Th1 function (T cells).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Lino Tessarollo and Eilleen Southon (Gene Targeting Facility, National Cancer Institute–Frederick, Frederick, MD) for their assistance during generation of p38αY323F mice, and Remy Bosselut for critical review of the manuscript.
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH.
National Institutes of Health
Authorship
Contribution: L.J., D.N.S., D.J., P.R.M., and J.D.A. designed the research; L.J., D.N.S., and D.J. performed the research; D.J. and P.R.M. provided intellectual and technical expertise; L.J. and J.D.A. wrote the manuscript; and all authors reviewed the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan D. Ashwell, Laboratory of Immune Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892; e-mail: jda@pop.nci.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal